Published online Jul 24, 2022. doi: 10.5306/wjco.v13.i7.630
Peer-review started: March 31, 2022
First decision: April 28, 2022
Revised: May 19, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: July 24, 2022
Processing time: 112 Days and 16.2 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of death from cancer worldwide. Tumor markers like carbohydrate antigen 19-9 (CA 19-9) have been proven valuable as a diagnostic tool and a predictor for tumor staging and response to therapy.
To delineate the phenotype of normal CA 19-9 PDAC according to clinical features, disease staging and prognosis as compared with high CA 19-9 PDAC cases.
We performed a retrospective single-center analysis of all PDAC cases admitted in our Gastroenterology department over a period of 30 mo that were diagnosed by endoscopic ultrasound-guided tissue acquisition. Patients were divided into two groups according to CA 19-9 levels over a threshold of 37 U/mL. We performed a comparison between the two groups with regard to demographic and clinical data, biomarkers, tumor staging and 6-mo survival.
Altogether 111 patients were recruited with 29 having documented normal CA 19-9 (< 37 U/mL). In the CA 19-9 negative group of patients, 20.68% had elevated levels of both CEA and CA 125, 13.79% for CA 125 only whilst 17.24% for CEA only. The two groups had similar demographic characteristics. Abdominal pain was more frequently reported in positive vs negative CA 19-9 PDAC cases (76.83% vs 55.17%), while smoking was slightly more prevalent in the latter group (28.04% vs 31.03%). Tumors over 2 cm were more frequently seen in the positive CA 19-9 group, reflecting a higher proportion of locally advanced and metastatic neoplasia (87.7% vs 79.3%). Six-month survival was higher for the negative CA 19-9 group (58.62% vs 47.56%).
Elevated CA 19-9 at diagnosis seems to be associated with a more pronounced symptomatology, high tumor burden and poor prognosis compared to negative CA 19-9 PDAC cases. CEA and CA 125 can be adjunctive useful markers for PDAC, especially in CA 19-9 negative cases.
Core Tip: Given the large heterogeneity of pancreatic cancer, delineation of subgroups with different tumor biology is essential for personalized management. We outlined the phenotype of carbohydrate antigen 19-9 negative pancreatic cancer according to clinical features, disease staging and prognosis.
- Citation: Balaban DV, Marin FS, Manucu G, Zoican A, Ciochina M, Mina V, Patoni C, Vladut C, Bucurica S, Costache RS, Ionita-Radu F, Jinga M. Clinical characteristics and outcomes in carbohydrate antigen 19-9 negative pancreatic cancer. World J Clin Oncol 2022; 13(7): 630-640
- URL: https://www.wjgnet.com/2218-4333/full/v13/i7/630.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i7.630
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of death from cancer worldwide, mostly due to late-stage diagnosis and resistance to chemotherapy. According to Globocan statistics 2020, pancreatic cancer has an incidence rate of 4.9/100000 and mortality almost equal to its incidence of 4.5/100000[1]. In fact, while mortality rates from other types of cancer are decreasing, pancreatic cancer is the only malignancy with an unfavorable trend[2].
Because of its aggressive tumor biology, early diagnosis is very important in order to maximize outcomes. Several strategies have been considered for setting an early accurate diagnosis, from case-finding tools to surveillance of high-risk patients. Alongside the imaging evaluation, there is a great interest in the development of biomarkers for optimizing the management of pancreatic adenocarcinoma[3].
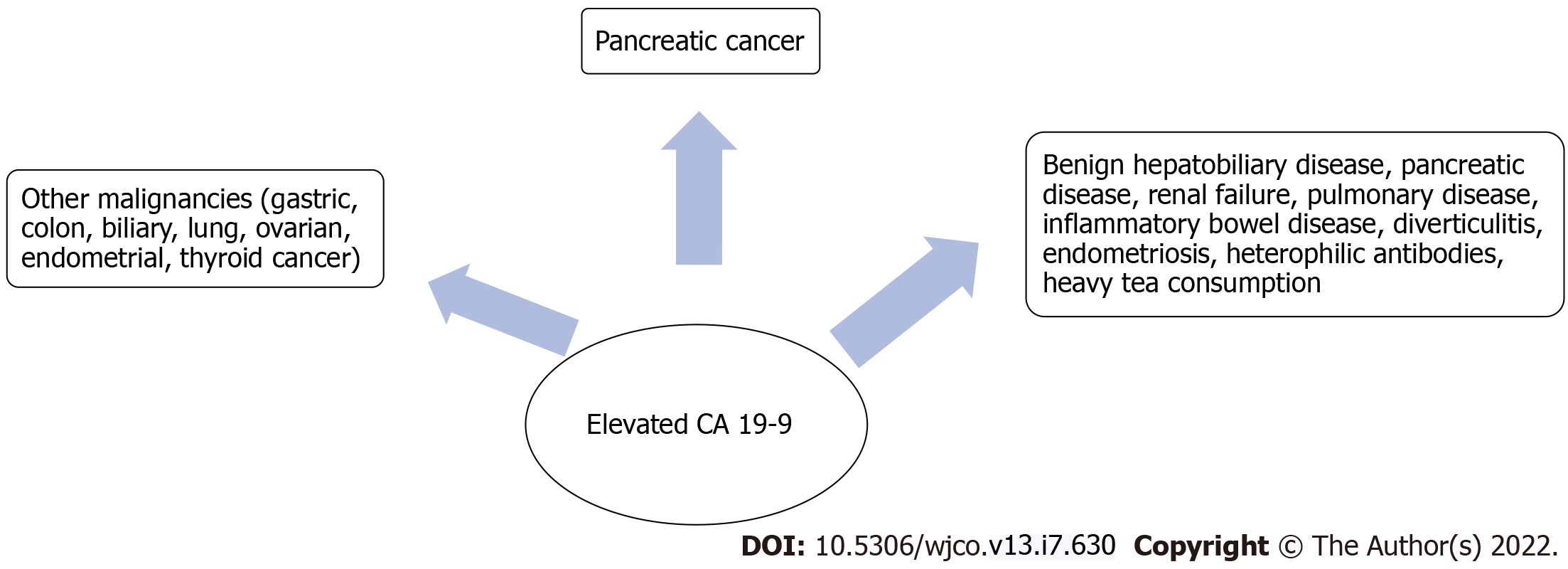
The most commonly used biomarker for PDAC is carbohydrate antigen 19-9 (CA 19-9), which is related to Lewis blood group antigens, and has been proven valuable as a diagnostic tool and in tumor staging, resectability and response to therapy[3]. CA 19-9, also called sialylated Lewis (a) antigen, is synthesized by pancreatic and biliary ductal cells and by other types of epithelium (stomach, colon, uterus, lung, salivary glands), which makes it a nonspecific biomarker for PDAC[4,5]. Elevated CA 19-9 has been reported in both benign and malignant pathology (Figure 1)[6,7]. Expression of CA 19-9 requires the presence of Lewis antigens A [Le(a+b-)] or B [Le(a-b+)], meaning that [Le(a-b-)] are theoretically non-producers of CA 19-9[8]. Lewis negative individuals ([Le(a-b-)]) lack the enzyme α1-3,4 fucosyltransferase, which is required for CA 19-9 biosynthesis. This dysfunction of the Lewis gene is associated with deficient protein fucosylation, which has been involved in cancer development[9].
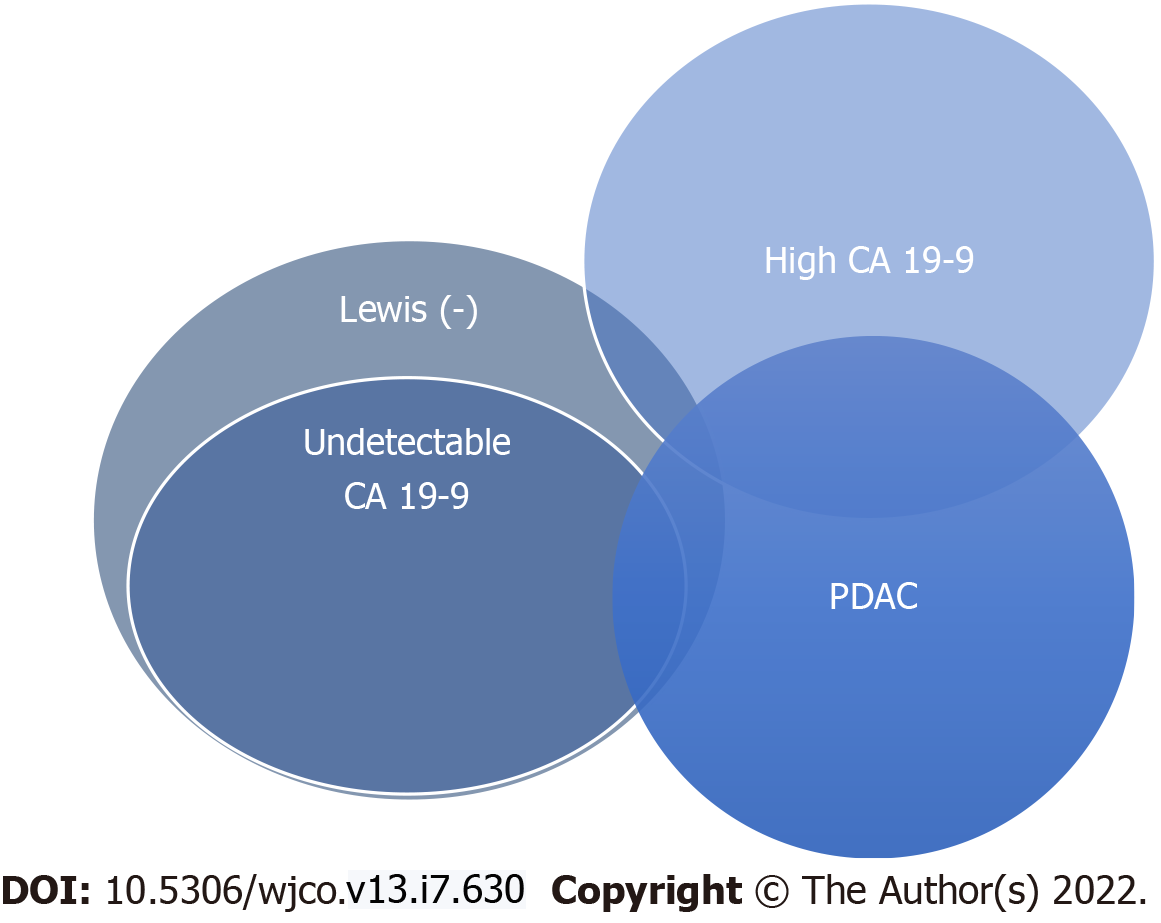
As CA 19-9 secretion is dependent on the Lewis antigen expression, undetectable false negative results can occur in Lewis antigen-negative individuals, meaning [Le(a−b−)] non-expressors[10]. This could represent a cause of delayed diagnosis in these patients and a pitfall in screening strategies based on CA 19-9. While red cell phenotyping for Lewis antigen status would provide insight in such situations, this is not routinely performed in clinical practice. However, despite the relationship between CA 19-9 secretion and Lewis antigen status, not all Lewis negative individuals with PDAC are non-secretors of CA 19-9, which makes CA 19-9 retain its diagnostic utility at least partially even in this patient category[11-13] (Figure 2).
Given the large heterogeneity of PDAC, delineation of subgroups with different tumor biology is considered of paramount importance for personalized management. Currently available literature is inconsistent regarding the clinical features and outcomes of patients with CA 19-9 or Lewis negative PDAC. Some authors have shown a better prognosis, while others have revealed worse outcomes compared to high CA 19-9 PDAC[14,15]. Our aim was to delineate the phenotype of CA 19-9 negative PDAC according to clinical features, disease staging and prognosis as compared with high CA 19-9 PDAC cases.
We performed a retrospective analysis of patients admitted to our Gastroenterology department during a period of 30 mo, from January 2019 to July 2021, who were diagnosed with PDAC by endoscopic ultrasound guided tissue acquisition. Demographic, clinical, laboratory work-up and imaging data were collected from patients’ medical records. Staging was carried out based on pancreatic-protocol computed tomography scan, according to the International Association of Pancreatology criteria for resectability-resectable, borderline resectable, locally advanced or metastatic disease[16]. Regarding tumor location, we grouped cases into lesions extended to head, uncinate and neck of the pancreas comprising one set and tumors of the body and tail representing another set. A 6-mo follow-up aimed at assessing survival was carried out either by reaching out to the general practitioner/oncologist or by contacting the patient/patient’s family by phone. Patients with missing data according to items assessed in this research were excluded from analysis. Also, patients lost from follow-up were excluded as survival could not be determined.
For the purpose of this study, we divided patients into two groups according to CA 19-9 levels. A threshold was set at 37 U/mL, and patients were classified as CA 19-9 negative or normal (for values < 37 U/mL)-group A and CA 19-9 positive (≥ 37 U/mL)-group B. We then compared the two groups according to demographic and clinical data, biomarkers, tumor staging and 6-mo survival.
Data analysis was carried out using SPSS Statistics 25 software (Armonk, NY, United States). Continuous variables were reported as mean, and categorical variables were reported as count and percentage. Comparison among the two groups was done using χ2 tests for categorical variable and a two-sample t-test for continuous variables at a significance of α = 0.05.
Altogether 111 patients were analyzed for the purpose of this study; 29 had documented normal CA 19-9 (< 37 U/mL) and 82 were CA 19-9 positive (≥ 37 U/mL). Demographic data, tumor characteristics and outcomes among the two groups was summarized in Table 1.
| Group A (n = 29) | Group B (n = 82) | P value | |
| Patient demographics | |||
| Age in yr, median | 64 | 67 | 0.241 |
| Male sex | 58.62 | 70.73 | 0.333 |
| At risk behaviors | |||
| Smoking | 31.03 | 28.04 | 0.946 |
| Drinker | 20.68 | 23.17 | 0.987 |
| Clinical findings | |||
| Abdominal pain | 55.17 | 76.83 | 0.048 |
| Jaundice | 27.58 | 29.26 | 0.946 |
| Weight loss | 62.06 | 63.41 | 0.924 |
| Diabetes mellitus | 34.48 | 34.14 | 0.845 |
| Tumor localization | |||
| Head, neck and uncinate | 62.06 | 57.31 | 0.820 |
| Body and tail | 37.93 | 42.68 | |
| Tumor size in cm | |||
| < 2 | 10.34 | 2.43 | 0.447 |
| 2-4 | 58.62 | 64.63 | |
| > 4 | 31.03 | 32.92 | |
| Staging | |||
| Resectable | 13.79 | 7.31 | 0.714 |
| Borderline resectable | 6.89 | 4.87 | |
| Locally advanced | 20.68 | 24.39 | |
| Metastatic | 58.62 | 63.31 | |
| Outcome | |||
| 6-mo survival | 58.62 | 47.56 | 0.308 |
With regard to sex distribution, a male predominance was seen in the study cohort (75/111, 67.5%), mostly owing to a higher male:female ratio in group B (2.4:1). Median age was similar between the two groups.
Considering at risk behavior among the patient population, a higher proportion of smokers was seen in group A (31.03% vs 28.04%), while heavy alcohol consumption was seen slightly more frequently in group B (23.17% vs 20.68%). Concerning the symptoms, abdominal pain was more prevalent in patients from group B (76.83% vs 55.17%), while weight loss and jaundice were noted in similar proportions in both patient groups. Also, diabetes mellitus was seen in about one-third of patients in both groups (34.48% vs 34.14%).
The average value of CA 19-9 was 16904.85 for group B compared with 8.48 for group A. In this latter group of patients, 20.68% had elevated levels for both CEA and CA 125, 13.79%for CA 125 only and 17.24% for CEA only. For both groups analyzed, most tumors (62.06%-group A, 57.31%-group B) were located in the head or uncinate process, while the remaining 37.93% and 42.68%, respectively, developed in the body or tail region. Regarding tumor size, there were no significant differences among the two groups in tumors over 4 cm. A higher proportion of lesions under 2 cm was reported in group A (10.34% vs 2.43%), while tumors sized 2-4 cm were more frequently seen in group B (64.63% vs 58.62%).
Analysis of tumor staging revealed there were more resectable (13.79% vs 7.31%) or borderline resectable tumors (6.89% vs 4.87%) in group A, while locally advanced and metastatic tumors were predominant in group B (24.39% vs 20.68%, 63.41% vs 48.62%). Six-month survival was higher in group A (58.62%) compared to group B (47.56%).
We further performed a subgroup analysis according to sex, taking into account the male predominance of our study cohort. While there were more men with elevated CA 19-9 than women (77.33% vs 66.67%), the proportion of locally advanced or metastatic tumors was higher in subgroup B females than males (95.83% vs 84.48%). Regarding symptomatology, abdominal pain was more frequent in group B for both sexes, but the difference seen with group A was higher for males (72.41% vs 47.06%) than females (87.50% vs 66.67%) without being statistically significant. We also conducted an analysis according to an age threshold set at 65 years. While advanced tumors were seen more in subgroup A less than 65 years of age compared to over 65 (86.7% vs 71.4%), in group B 90.5% of elderly patients had locally advanced or metastatic neoplasia compared to 83.9% in those under 65 years. Six-month survival was similar in subgroups A and B according to the 65-year threshold (57.1% and 49.0% for patients under 65 years and 60.0% and 45.2%, respectively, for those 65 years or older).
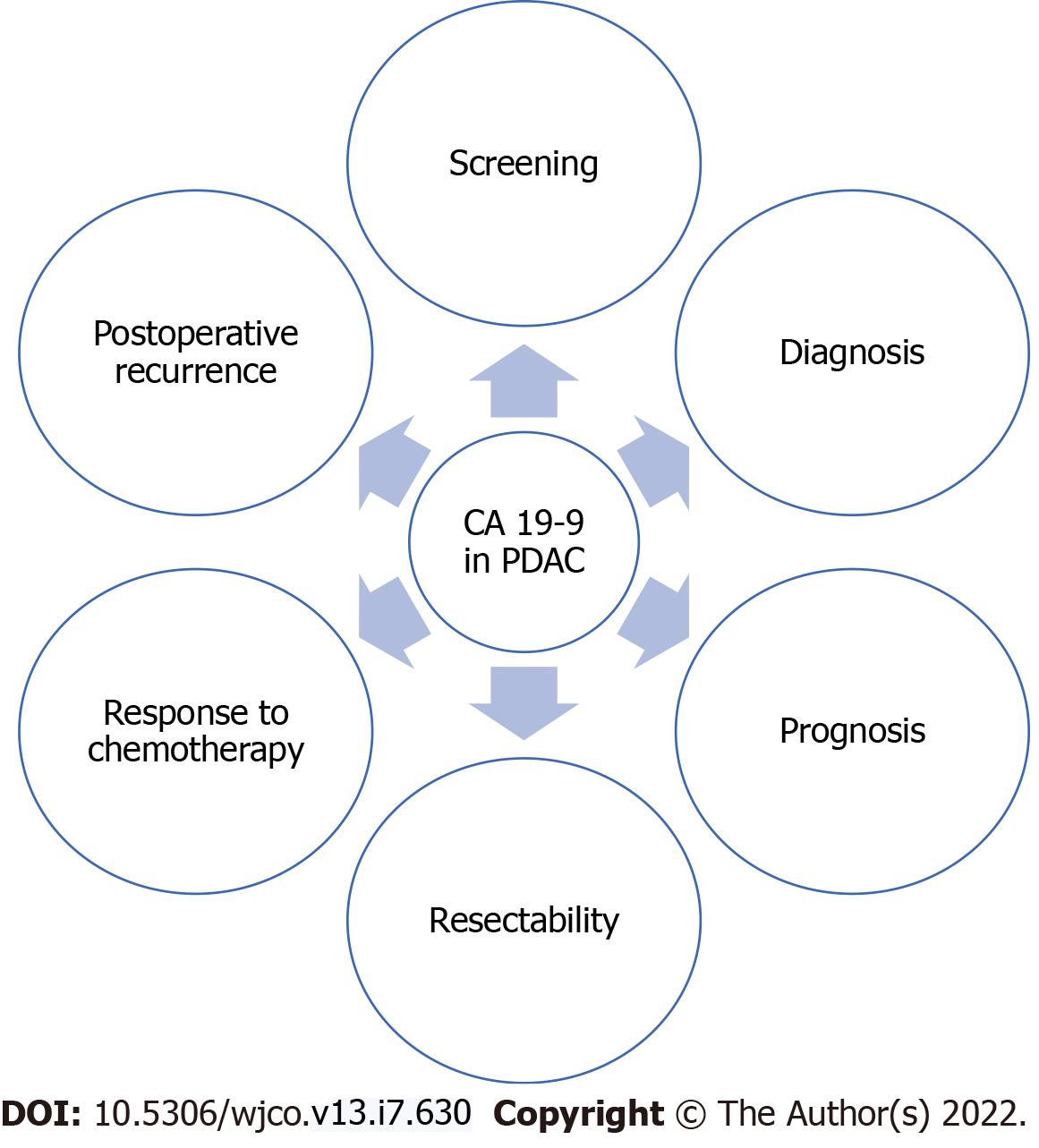
CA 19-9 is the most widely used biomarker for PDAC, but its major drawbacks are represented by false positive results in benign inflammatory conditions and extra-pancreatic neoplasms and by false negative results in Lewis negative individuals, which comprise about 10% of the Caucasian population[5]. However, in several aspects CA 19-9 remains a valuable biomarker for PDAC management, from screening and diagnosis to treatment response, prognosis and recurrence (Figure 3)[9,17-21].
In our study, we enrolled PDAC patients and divided them into two groups: CA 19-9 positive (n = 82) and CA 19-9 negative (n = 29), according to a threshold of 37 U/mL. Six-month survival was better in the CA 19-9 negative patients (58.62% vs 47.56%), reflecting a lower proportion of locally advanced and metastatic disease in this group. This could be explained by triggering of imaging studies in patients with elevated CA 19-9, leading to an early stage diagnosis and thus a better prognosis, while in patients with negative CA 19-9 further investigations are often deferred due to lack of concern, leading to delayed diagnosis in advanced stages and poorer prognosis.
Some authors have proposed genotyping Lewis antigen along with CA 19-9 dosing in order to improve its diagnostic accuracy[22,23], but recent studies have shown that CA 19-9 retains its utility even in Lewis negative individuals[11]. CA 19-9 values over 37 U/mL were seen in 27.4% of Lewis negative patients, and areas under the receiver operating characteristic curve for the diagnostic accuracy of CA 19-9 were similar in Lewis negative PDAC patients compared to all PDAC patients (0.842 vs 0.898). This was also shown by Kwon et al[14], who also found that not all Lewis negative PDAC patients are non-secretors of CA 19-9. In this study, 172/375 (45.87%) of patients in the Le(a−b−) group had a serum CA 19-9 over 37 U/mL. The paradoxical elevation of CA 19-9 in Lewis negative individuals might be explained by partial secretion of the protein, which can be detected by enzymatic immunoassays or by cross-reactivity of the antibodies used for CA 19-9 dosing; treating the collected specimen with blocking agents has been proposed as a method to eliminate interference with heterophilic antibodies[5,13,24]. Therefore, PDAC prognosis is different if patients are stratified according to either CA 19-9 or to Lewis antigen.
A literature search of studies assessing PDAC outcomes according to CA 19-9 and Lewis antigen status has shown inconsistent results (Table 2)[11,14,25-40]. While low CA 19-9 PDAC has been associated with better prognosis, some have shown that Lewis negative PDAC harbors a more aggressive tumor biology and has a poorer outcome[15]. Discordant results might be due to different patient populations and different timeframes of studies, and not least to overlap of Lewis-negative with detectable CA 19-9 PDAC patients. Some authors have concluded that the usefulness of the 37 U/mL threshold for CA 19-9 is more appropriate for PDAC diagnosis than predicting prognosis. However, others have shown a strong correlation of CA 19-9 with tumor burden, survival and recurrence[41,42].
| Survival analysis according to CA 19-9 values | |||
| Ref. | n | CA 19-9 in U/mL | Survival |
| Overall median survival in mo | |||
| Berger et al[30], 2004 | 7 | Undetectable | 32 |
| 21 | ≤ 37 | 35 | |
| 44 | 38-200 | 22 | |
| 57 | > 200 | 16 | |
| Ferrone et al[33], 2006 | Mean survival time in yr | ||
| 29 | < 37 | 2.3 | |
| 82 | ≥ 37 | 1.6 | |
| Waraya et al[28], 2009 | Disease-specific survival in mo | ||
| 23 | ≤ 37 | 30.6 | |
| 66 | > 37 | 12.7 | |
| Hirakawa et al[29], 2011 | Median survival in mo | ||
| 41 | Normal | 39.0 | |
| 84 | Elevated | 16.9 | |
| Hartwig et al[32], 2011 | Median survival in mo | ||
| 232 | < 37 | 28.0 | |
| 418 | 37-399 | 23.5 | |
| 239 | ≥ 400 | 14.5 | |
| Turrini et al[40], 2009 | Median survival in mo | ||
| 50 | < 37 | 22 | |
| 53 | 400-900 (n = 27), > 900 (n = 26) | 15 | |
| Katz et al[34], 2010 | Median survival in mo | ||
| 21 | < 37 | 52.8 | |
| 78 | > 37 | 21.2 | |
| Kondo et al[35], 2010 | Preoperative 3-yr survival (%) | ||
| 32 | < 37 | 57% | |
| 77 | > 37 | 30% | |
| Hata et al[36], 2012 | Preoperative median survival in mo | ||
| 51 | < 37 | 16.2 | |
| 218 | > 37 | 16.4 | |
| Bergquist et al[37], 2016 | Median OS in mo | ||
| 3666 | < 37 | 19.1 | |
| 7140 | > 37 | 14 | |
| Jia et al[38], 2019 | Median OS in mo | ||
| 13 | < 35 | 21 | |
| 107 | ≥ 35 | 11 | |
| Mattiucci et al[25], 2019 | Median OS in mo | ||
| 39 | 0-5.0 | 25 | |
| 167 | 5.1-37.0 | 38 | |
| 139 | 37.1-100.0 | 32 | |
| 178 | 100.1-353.0 | 22 | |
| 177 | > 353.1 | 20 | |
| Kondo et al[26], 2017 | Median survival in mo | ||
| 65 | < 37 | 52.0 | |
| 84 | ≥ 37 | 23.7 | |
| 88 | < 50 | 52.0 | |
| 61 | ≥ 150 | 20.9 | |
| 101 | < 300 | 46.7 | |
| 48 | ≥ 300 | 18.8 | |
| Dong et al[27], 2014 | Median OS in mo | ||
| 18 | < 37 | 21.6 | |
| 102 | ≥ 37 | 14.2 | |
| Kang et al[31], 2007 | Disease free survival in mo | ||
| 18 | < 50 | 22.20 | |
| 43 | ≥ 50 | 19.31 | |
| Kwon et al[14], 2020 | Median survival in d | ||
| 408 | < 37 | 644 | |
| 779 | > 37 | 340 | |
| Survival analysis according to Lewis antigen status | |||
| Luo et al[39], 2017 | Median survival in mo | ||
| 682 | 137 CA 19-9 (-) | Stage I, II: 16.6 in Lewis (-), 17.6 in Lewis (+) | |
| 47 Lewis (-) | Stage III, IV: 6.0 in Lewis (-), 7.8 in Lewis (+) | ||
| Luo et al[11], 2018 | Median survival in mo | ||
| 1482 | 19.8% CA 19-9 (-) | 8.0 in Lewis (-) | |
| 8.4% Lewis (-) | 10.0 in Lewis (+) | ||
| Kwon et al[14], 2020 | Median survival | ||
| 1187 | 203 CA 19-9 (-) | 356 d in Lewis (-) | |
| 375 Lewis (-) | 477 d in Lewis (+) | ||
In order to better predict outcomes, some have proposed measuring other markers such as CA 242, CA 50, CEA, CA 125 or periostin complementary to CA 19-9 for PDAC[43-48]. Additional markers, such as CEMIP, apolipoprotein A-I and transferrin[49,50], were shown to be useful especially in PDAC with normal CA 19-9 levels. Lee et al[49] showed that CEMIP (also called KIAA1199) had a diagnostic yield of 86.1% in CA 19-9 negative PDAC, and the combination of CEMIP + CA 19-9 had a significantly improved area under the receiver operating characteristic curve over CA 19-9 alone (0.94 vs 0.89, P < 0.0001). In our study, 34.47% of CA 19-9 negative PDAC cases had elevated levels of CA 125, 37.92% for CEA and 20.68% for both. Concerning the patients with negative CA 19-9 and positive CA 125 and CEA, 83.33% had metastatic disease at the time of the diagnosis and only 50.00% survived at 6 mo.
Similar results were seen in the paper by Luo et al[39]. In Lewis negative patients, high values of CEA were seen in 63.8% of patients, and CA 125 was seen in 51.1%. They concluded that CEA and CA 125 should be routinely measured for PDAC. Considering the metastatic burden and survival among 853 pancreatic cancer patients, Liu et al[15] observed that Lewis negative PDAC constitutes an aggressive tumor subtype, with low secretion of CA 19-9 and high secretion of CA125. In line with Luo et al[39], others have highlighted the fact that CEA and CA 125, similar to CA 19-9, can also be used to monitor therapeutic response[51].
Interestingly, several papers have shown that CA 19-9 and the other biomarkers are upregulated early in the course of PDAC development-up to 2 years before clinical diagnosis and can be used to detect preclinical pancreatic cancer[52,53]. This could be useful for screening strategies of high-risk groups, keeping in mind that Lewis negative individuals might be missed by this approach. Moreover, clinicians should take note that CA 19-9 is also of limited value in the follow-up of Lewis negative patients, in order to avoid erroneous decisions in PDAC management.
The current study has several limitations. Patients recruited in this study were from a hospital-based setting, which had either an acute presentation (jaundice, pancreatitis) or were referred for diagnostic procedures. Also, we acknowledge the lack of Lewis antigen genotyping in our study population, which might have provided further insight into PDAC outcomes according to both CA 19-9 and Lewis antigen status. Another important limitation is the sample size, which makes it very difficult to obtain a statistically significant analysis.
In our study, patients with negative CA 19-9 had a better prognosis than those with values over 37 U/mL. Elevated CA 19-9 at diagnosis seems to be associated with a more pronounced symptomatology and higher tumor burden. CEA and CA 125 can be adjunctive useful markers for PDAC, especially in CA 19-9 negative cases.
Carbohydrate antigen 19-9 (CA 19-9) is the most widely used biomarker for pancreatic ductal adenocarcinoma (PDAC), but its use is hindered by both false-positive and false-negative results.
There are inconsistent results regarding the outcome of CA 19-9 negative PDAC cases.
To delineate the phenotype of negative CA 19-9 PDAC according to clinical features, disease staging and outcome.
Retrospective single-center analysis of PDAC cases over a period of 30 mo.
Among 111 recruited patients, 29 had normal CA 19-9. Patients with elevated CA 19-9 had higher tumor burden and more advanced staging. Six-month survival was higher for the negative CA 19-9 group (58.62% vs 47.56%).
Negative CA 19-9 PDAC has a better prognosis than PDAC with high CA 19-9 values. CEA and CA 125 can be adjunctive useful markers for PDAC, especially in CA 19-9 negative cases.
Negative CA 19-9 PDAC cases warrant in-depth analysis of tumor biology to assess if there is indeed a different phenotype of neoplasia.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalli K, United Kingdom; Singh I, United States; Zimmitti G, Italy S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64539] [Article Influence: 16134.8] [Reference Citation Analysis (176)] |
| 2. | Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, Negri E, Malvezzi M. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann Oncol. 2020;31:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 3. | Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, Qian Y, Huang Q, Ni Q, Liu C, Yu X. Roles of CA 19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875:188409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 196] [Article Influence: 49.0] [Reference Citation Analysis (1)] |
| 4. | Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19-9-tumor marker: Past, present, and future. World J Gastrointest Surg. 2020;12:468-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (4)] |
| 5. | Dasgupta A, Wahed A. Chapter 13-Tumor Markers. In: Dasgupta A, Wahed A, editors. Clinical Chemistry, Immunology and Laboratory Quality Control [Internet]. San Diego: Elsevier, 2014. [DOI] [Full Text] |
| 6. | Kim S, Park BK, Seo JH, Choi J, Choi JW, Lee CK, Chung JB, Park Y, Kim DW. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci Rep. 2020;10:8820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (2)] |
| 7. | Al-Janabi AAHS, Tawfeeq EF. Interfering Effect of Black Tea Consumption on Diagnosis of Pancreatic Cancer by CA 19-9. J Gastrointest Cancer. 2017;48:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 8. | Scarà S, Bottoni P, Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 9. | Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 352] [Reference Citation Analysis (3)] |
| 10. | Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501-5503. [PubMed] |
| 11. | Luo G, Fan Z, Cheng H, Jin K, Guo M, Lu Y, Yang C, Fan K, Huang Q, Long J, Liu L, Xu J, Lu R, Ni Q, Warshaw AL, Liu C, Yu X. New observations on the utility of CA 19-9 as a biomarker in Lewis negative patients with pancreatic cancer. Pancreatology. 2018;18:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Hamada E, Taniguchi T, Baba S, Maekawa M. Investigation of unexpected serum CA 19-9 elevation in Lewis-negative cancer patients. Ann Clin Biochem. 2012;49:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Parra-Robert M, Santos VM, Canis SM, Pla XF, Fradera JMA, Porto RM. Relationship Between CA 19.9 and the Lewis Phenotype: Options to Improve Diagnostic Efficiency. Anticancer Res. 2018;38:5883-5888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Kwon S, Kim S, Giovannucci EL, Hidalgo M, Markey MK, Bovik AC, Kwon MJ, Kim KJ, Im H, Park JY, Bang S, Park SW, Song SY, Chung MJ. Lewis Antigen Phenotype and Survival of Patients with Pancreatic Cancer. Pancreas. 2020;49:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Liu C, Deng S, Jin K, Gong Y, Cheng H, Fan Z, Qian Y, Huang Q, Ni Q, Luo G, Yu X. Lewis antigennegative pancreatic cancer: An aggressive subgroup. Int J Oncol. 2020;56:900-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW, Kishiwada M, Kitagawa H, Michalski CW, Wolfgang CL. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 510] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 17. | Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Choe JW, Kim HJ, Kim JS, Cha J, Joo MK, Lee BJ, et al. Usefulness of CA 19–9 for pancreatic cancer screening in patients with new-onset diabetes. Hepatobiliary & Pancreatic Diseases International.. 2018;17:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 20. | Alexakis N, Gomatos IP, Sbarounis S, Toutouzas K, Katsaragakis S, Zografos G. High serum CA 19-9 but not tumor size should select patients for staging laparoscopy in radiological resectable pancreas head and peri-ampullary cancer. European Journal of Surgical Oncology (EJSO). 2015;41:265-269. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Goh SK, Gold G, Christophi C, Muralidharan V. Serum carbohydrate antigen 19-9 in pancreatic adenocarcinoma: a mini review for surgeons. ANZ J Surg. 2017;87:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Luo G, Guo M, Jin K, Liu Z, Liu C, Cheng H, Lu Y, Long J, Liu L, Xu J, Ni Q, Yu X. Optimize CA 19-9 in detecting pancreatic cancer by Lewis and Secretor genotyping. Pancreatology. 2016;16:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Indellicato R, Zulueta A, Caretti A, Trinchera M. Complementary Use of Carbohydrate Antigens Lewis a, Lewis b, and Sialyl-Lewis a (CA19.9 Epitope) in Gastrointestinal Cancers: Biological Rationale Towards A Personalized Clinical Application. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Passerini R, Cassatella MC, Boveri S, Salvatici M, Radice D, Zorzino L. The pitfalls of CA 19-9: routine testing and comparison of two automated immunoassays in a reference oncology center. Am J Clin Pathol. 2012;138:281-287. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Mattiucci GC, Morganti AG, Cellini F, Buwenge M, Casadei R, Farioli A. Prognostic Impact of Presurgical CA 19-9 Level in Pancreatic Adenocarcinoma: A Pooled Analysis. Transl Oncol. 2018;12:1-7. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kondo N, Murakami Y, Uemura K, Nakagawa N, Takahashi S, Ohge H, Sueda T. Comparison of the prognostic impact of pre- and post-operative CA 19-9, SPan-1, and DUPAN-II levels in patients with pancreatic carcinoma. Pancreatology. 2017;17:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Dong Q, Yang XH, Zhang Y, Jing W, Zheng LQ, Liu YP, Qu XJ. Elevated serum CA 19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Waraya M, Yamashita K, Katagiri H, Ishii K, Takahashi Y, Furuta K, Watanabe M. Preoperative serum CA 19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann Surg Oncol. 2009;16:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Hirakawa T, Nakata B, Amano R, Kimura K, Shimizu S, Ohira G, Yamada N, Ohira M, Hirakawa K. HER3 overexpression as an independent indicator of poor prognosis for patients with curatively resected pancreatic cancer. Oncology. 2011;81:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 31. | Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Büchler MW, Werner J. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 33. | Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA 19-9 Levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897-2902. [RCA] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 34. | Katz MHG, Varadhachary GR, Fleming JB, Wolff RA, Lee JE, Pisters PWT. Serum CA 19-9 as a Marker of Resectability and Survival in Patients with Potentially Resectable Pancreatic Cancer Treated with Neoadjuvant Chemoradiation. Ann Surg Oncol. 2010;17:1794-1801. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y, Ohge H, Sueda T. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Hata S, Sakamoto Y, Yamamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. Prognostic impact of postoperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2012;19:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. Journal of the American College of Surgeons. 2016;223:52-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Jia F, Liu M, Li X, Zhang F, Yue S, Liu J. Relationship between S100A4 protein expression and pre-operative serum CA19.9 levels in pancreatic carcinoma and its prognostic significance. World J Surg Oncol. 2019;17:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Luo G, Liu C, Guo M, Cheng H, Lu Y, Jin K, Liu L, Long J, Xu J, Lu R, Ni Q, Yu X. Potential Biomarkers in Lewis Negative Patients With Pancreatic Cancer. Ann Surg. 2017;265:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 40. | Turrini O, Schmidt CM, Moreno J, Parikh P, Matos JM, House MG, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD. Very high serum CA 19-9 levels: a contraindication to pancreaticoduodenectomy? J Gastrointest Surg. 2009;13:1791-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951-5; discussion 955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | van Manen L, Groen JV, Putter H, Pichler M, Vahrmeijer AL, Bonsing BA. Stage-Specific Value of Carbohydrate Antigen 19-9 and Carcinoembryonic Antigen Serum Levels on Survival and Recurrence in Pancreatic Cancer: A Single Center Study and Meta-Analysis. Cancers. 2020;12:2970. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Dong D, Jia L, Zhang L, Ma N, Zhang A, Zhou Y, Ren L. Periostin and CA242 as potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer. Cancer Sci. 2018;109:2841-2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA 19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:11683-11691. [PubMed] |
| 45. | Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, Xu DK, Zhao P. The clinical value of serum CEA, CA 19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 46. | Gui J-C, Yan W-L, Liu X-D. CA 19-9 and CA242 as tumor markers for the diagnosis of pancreatic cancer: a meta-analysis. Clin Exp Med. 2014;14:225-233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Coppin L, Benomar K, Corfiotti F, Cattan S, Renaud F, Lapere C, Leteurtre E, Vantyghem MC, Truant S, Pigny P. CA-125, but not galectin-3, complements CA 19-9 for discriminating ductal adenocarcinoma versus non-malignant pancreatic diseases. Pancreatology. 2016;16:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Ballehaninna UK, Chamberlain RS. Biomarkers for pancreatic cancer: promising new markers and options beyond CA 19-9. Tumour Biol. 2013;34:3279-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 49. | Lee HS, Jang CY, Kim SA, Park SB, Jung DE, Kim BO, et al. Combined use of CEMIP and CA 19-9 enhances diagnostic accuracy for pancreatic cancer. Sci Rep.. 8:3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Lin C, Wu WC, Zhao GC, Wang DS, Lou WH, Jin DY. ITRAQ-based quantitative proteomics reveals apolipoprotein A-I and transferrin as potential serum markers in CA 19-9 negative pancreatic ductal adenocarcinoma. Medicine (Baltimore). 2016;95:e4527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Xu HX, Liu L, Xiang JF, Wang WQ, Qi ZH, Wu CT, Liu C, Long J, Xu J, Ni QX, Yu XJ. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA 19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9(4):e94928.. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | O'Brien DP, Sandanayake NS, Jenkinson C, Gentry-Maharaj A, Apostolidou S, Fourkala EO, Camuzeaux S, Blyuss O, Gunu R, Dawnay A, Zaikin A, Smith RC, Jacobs IJ, Menon U, Costello E, Pereira SP, Timms JF. Serum CA 19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2015;21:622-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |