Published online Mar 24, 2022. doi: 10.5306/wjco.v13.i3.186
Peer-review started: March 14, 2021
First decision: July 18, 2021
Revised: July 31, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 24, 2022
Processing time: 374 Days and 17.4 Hours
Gastric cancer (GC) is the result of a multifactorial process whose main compon
Core Tip: Understanding the factors related to the host, infection by Helicobacter pylori and the mechanisms of tumor evasion are fundamental to understand the development of gastric cancer (GC). However, in the face of a complex immune environment, there are still many questions to be answered. Thus, we highlight in this work the main aspects related to GC, from infection and gastric microenvironment to immune response.
- Citation: Lima de Souza Gonçalves V, Cordeiro Santos ML, Silva Luz M, Santos Marques H, de Brito BB, França da Silva FA, Souza CL, Oliveira MV, de Melo FF. From Helicobacter pylori infection to gastric cancer: Current evidence on the immune response. World J Clin Oncol 2022; 13(3): 186-199
- URL: https://www.wjgnet.com/2218-4333/full/v13/i3/186.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i3.186
Helicobacter pylori (H. pylori) infection is acquired mainly in early childhood and if not treated properly can remain for life. Because of this, the infection is highly present around the world and has been linked to a wide spectrum of gastrointestinal diseases[1]. The prevalence of the bacteria varies according to geographic regions, age of the patient, socioeconomic status, education, living environment and profession. In developing countries, such as those in Latin America, they can have a prevalence of up to 80% of infected adults[2]. Recent work by Elzouki et al[3] evaluated 114 patients with gastric cancer (GC) and indicated that the infection rate per H. pylori was 63.2%. Through its virulence factors, it damages the gastric mucosa and the hormonal release, changing the stomach environment, often asymptomatically[4].
Thus, after a chronic inflammatory process, the host may develop GC in the long term, with adenocarcinoma as the most common type. GC is a worldwide public health problem since approximately one million new people are diagnosed each year with this pathology. Among the types of neoplasms, gastric adenocarcinoma is the fifth most common and is the third cause of cancer-related death worldwide. Although there is a strong association between H. pylori infection and gastric neoplasms, only 1%-3% of those infected end up developing GC[5]. Also in this sense, in 1994, the World Health Organization identified H. pylori as a group 1 carcinogen, which means a certain relationship to carcinogenesis, confirming the role of this bacterium in the process of GC development.
The mechanisms involved in this process are complex and not well known, but it is known that the tumor evasion mechanism of the immune response has a fundamental role in this process[5]. The development of this neoplastic microenvironment results from genetic and epigenetic changes in oncogenes and tumor suppressor genes, which results in deregulation of cell signaling pathways and the process of apoptosis[6]. It is known that the prognosis of patients with GC is not good, with an average 5-year survival rate of less than 20%[7]. The prognosis of patients with GC can also vary according to their classification, which can be based from anatomical location to recent molecular discoveries.
This work aims to provide an updated review on the main characteristics that lead to the development of gastric neoplasms from gastric infection by H. pylori in order to provide solid data that help in the knowledge about GC.
H. pylori has a lot of virulence factors that favor its maintenance in the hostile gastric environment. The recognition of these factors can even determine how serious the infection with the bacteria can be. Here, the main factors will be listed.
Of primary importance for the pathogenic action of H. pylori, the flagella play a crucial role in colonization by this pathogen[8]. About four to eight flagella make up the H. pylori flagellar group, and each of these unitary flagella is made up of three structures: the basal body, the hook and the filament[9]. Such composition allows this bacterium not only the motility through gastrointestinal fluids, known as “swimming” but also in solid or semi-solid media, known as “spreading” and “swarming” movements, which are crucial for entry into gastrointestinal epithelial cells[8].
In addition, still in the mobility and fixation of this bacterium in the gastric environment, the chemotaxis process guarantees the interaction with molecules such as mucins, sodium bicarbonate, urea and sodium chloride, facilitating the effectiveness of the infection[10,11]. Another factor of paramount importance in the role of chemotaxis occurs in the process of continuity of infection, causing it to become chronic. In this sense, the chemotaxis ability allows H. pylori to circumvent the host’s immune responses, achieving chronic infection[12].
Various adhesion molecules are described as important for the colonization process by H. pylori, in addition to helping to protect this pathogen against mucin activity and contributing to access to important nutrients, such as nickel, which are essential for effective infection[13]. An important adherence factor that has been described in the literature is BabA, responsible for the connection with Lewis H-1 type antigens. In addition to this adhesion function, this molecule also appears to be related to the type of clinical manifestation presented by the host in the face of infection[14-16].
Another molecule of importance for the adhesion of this pathogen to gastric tissue is the outer inflammatory protein, which is also linked to the production of interleukin (IL)-8, mucosal damage and duodenal ulcer[17]. In addition, studies also argue that there is a possible relationship between outer inflammatory protein expression and greater chances of developing GC[18]. Still on the adhesion molecules of this pathogen, 33 proteins form the H. pylori outer membrane proteins (Hop)[19,20]. Even though most of them still do not have their activity well described or understood, some of them already have their role in the pathogenicity of H. pylori highlighted, such as BabA (HopS), SabA (HopP), HopQ and HopZ. BabA is related to the specific link to the b and H-1 Lewis antigens from the surface of the gastric epithelial cells, and SabA is associated with binding to Lex and the adherence of the bacterium to laminin[21-23]. Meanwhile, the HopQ and HopZ proteins are relatively consolidated as to their importance for pathogen adherence, and the former also appears to be related to gene A associated with cytotoxins (CagA) gene expression[24].
Several molecules are listed as important for the pathogenesis of H. pylori. Among them, the role of urease stands out, which is activated even before the bacterium adheres to the gastric tissue, making an adequate acclimation of the pH of the gastric environment, regulating it to protect this bacterium[25]. In addition, urease is related to the production process of ammonia derived from urea, due to the urea channels that allow the entry of this substance in the pathogen and the intrabacterial action of this enzyme[26]. Furthermore, in addition to its role in colonization, urease seems to be important for regulating the immune response, controlling a macrophage-pathogen interaction, modulating the pH of the phagosome and ensuring the survival of H. pylori[27].
One of the proteins most expressed by H. pylori is the catalase that converts hydrogen peroxide into water and molecular oxygen[28]. Of paramount importance for the protection of the pathogen against the host’s immune responses, prevention of death mediated by the complement system and avoidance against the action of phagocytes, catalase seems to be related to the clinic of gastric tumors and cancers[29,30]. Apparently, this process occurs through chronic inflammation, prevention of apoptosis and induction of mutagenesis (processes related to the action of this enzyme)[31].
H. pylori strains can be classified as CagA positive or CagA negative. Apparently, CagA is the main virulence factor of this pathogen, and its greatest expression seems to be directly related to more aggressive clinical manifestations, such as acute gastritis, gastric ulcer and GC[32-35]. This process is related to its ability to affect cellular motility, proliferation and apoptosis, affecting the entire conformation of gastric tissue and predisposing inflammatory pathways that facilitate these clinical presentations[35]. Still on the CagA gene, different forms of phosphorylation that occur (EPIYA A, B, C or D) are related to different results, with types C and D (Western and Eastern strains) more related to the outcome of GC[36].
Vacuolating cytotoxin A is an essential cytotoxin for the pathogenesis of H. pylori, promoting autophagic processes during the acute phase of infection, in addition to promoting the appearance of impaired autophagosomes and unbalancing cell proliferation and death during a chronic phase of infection[30]. Present in all strains of this pathogen, vacuolating cytotoxin A can be encoded by different genopatterns, being the strains s1 and m1 more related to higher levels of inflammation and consequently less indolent clinical presentations, such as gastric atrophy and carcinoma[37-40].
Another determinant factor in the pathogenesis of H. pylori is the cag-pathogenicity island, which is composed of approximately 32 genes[41]. The cag-pathogenicity island is responsible for the encoding of a type 4 secretion system that helps modulate the cellular metabolism of the host cell, translocate virulence factors such as CagA to the gastric epithelial cells and upregulate proinflammatory cytokine secretion[42,43]. Also, strains that present cag-pathogenicity island are more related to peptic ulcer and GC[44,45]. Moreover, the interaction of CagA with the SH2 containing protein tyrosine phosphatase-2 is extremely relevant. The CagA/SH2 containing protein tyrosine phosphatase-2 link happens through a tyrosine phosphorylation-dependent process, which promotes activation of the SH2 containing protein tyrosine phosphatase-2/extracellular signal-regulated kinase/mitogen-activated protein kinase pathway and consequently causes cytoskeleton alterations known as the “hummingbird” phenotype[46,47]. These changes interfere in cellular growth and motility, which may predispose the host to genetic mutations and further GC[48]. In addition, H. pylori lipopolysaccharides also play an important role in the pathogenesis of this bacterium. The lipopolysaccharides are capable of binding laminin and as a consequence promote a gastric leakiness and further cellular apoptosis[49]. Furthermore, H. pylori lipopolysaccharides might be related to the development of GC, given that it upregulates toll-like receptor 4 and enhances cell proliferation, both via activation of the mitogen-activated protein kinase kinase 1/2-extracellular signal-regulated kinase 1/2-mitogen-activated protein kinase pathway[50].
Still in the virulence factors of H. pylori, several others can be mentioned, such as heat shock proteins, superoxide dismutase and degrading enzymes (proteases and phospholipases), being listed in this article.
H. pylori induces a significant immune response in the gastric environment of infected individuals. The onset of the inflammatory processes related to the infection occurs with the promotion of innate immunity mechanisms, involving the triggering of pattern recognition receptors of gastric epithelial cells by bacterial components such as lipopolysaccharide, NapA and nucleic acids[51]. The aforementioned recognition of foreign antigens by immune system receptors leads to the activation of intracellular signaling pathways that culminate in the release of proinflammatory cytokines, which promote the activation and recruitment of CD4+ and CD8+ T cells to the gastric environment[52]. Subsequently, a chronic inflammation against H. pylori infection is established, being characterized by a polarization of T helper (Th) 1/Th17 responses, which is followed by the action of regulatory T (Treg) cells responsible for controlling the inflammatory process.
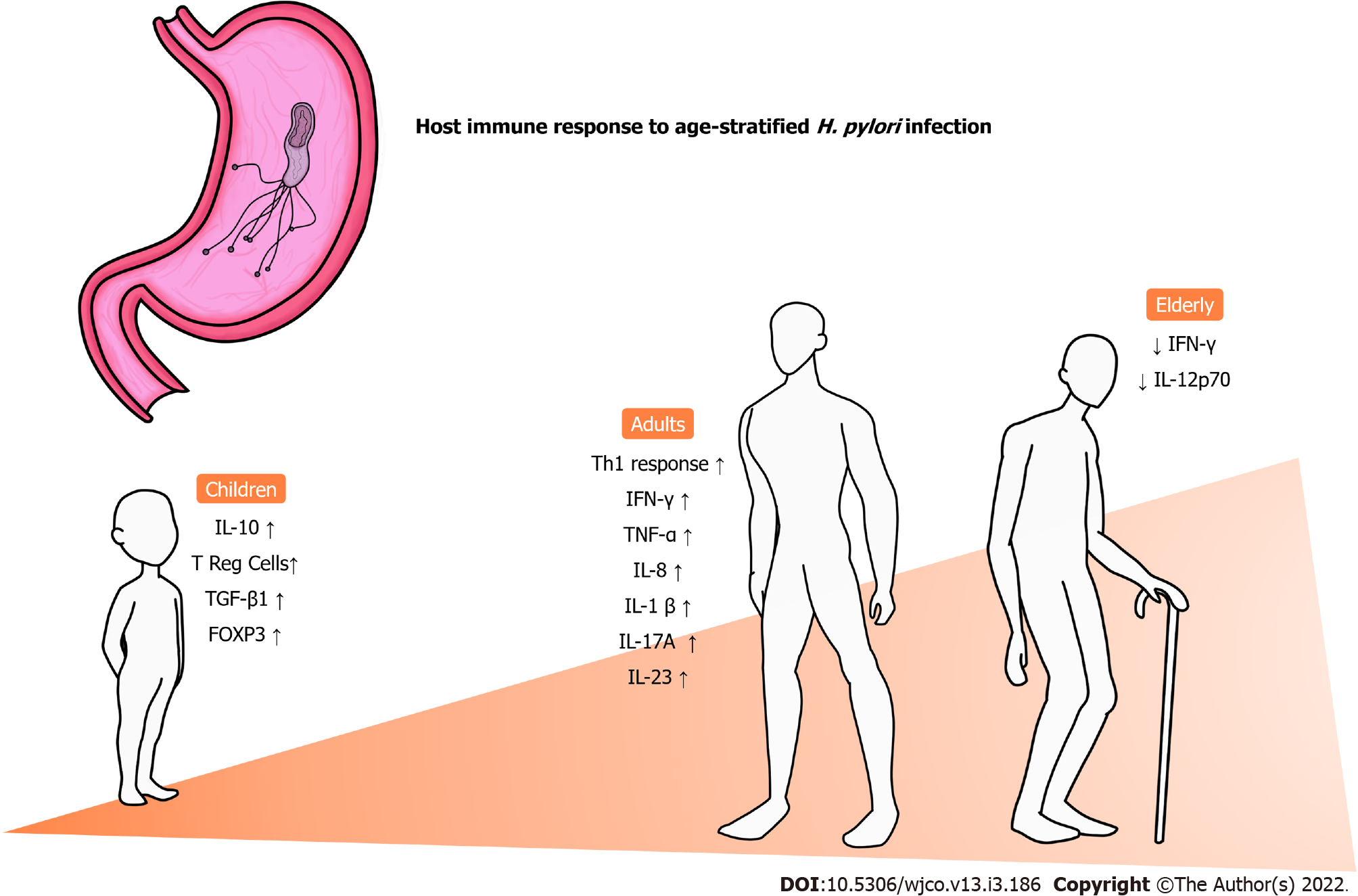
The inflammatory pattern varies between groups of patients and seems to be strongly influenced by age[53]. Figure 1 summarizes the main changes in the immune response to infection by H. pylori according to age. In general, a predominance of the Th1 response is commonly observed in adults, along with high levels of interferon γ (IFN-γ), tumor necrosis factor α, IL-1β and IL-8[54-56], which is mostly responsible for the recruitment of neutrophils and further setup of an inflammatory environment[57]. However, when it comes to children, this pattern of cytokine release and consequent responses are not presented in the same way as adults. In a previous investigation with H. pylori-positive adults and children evaluating Treg and Th17 responses in the gastric mucosa, our group observed that children have higher expression of Treg-related cytokines such as IL-10 and transforming growth factor beta 1 (TGF-β1) than adults. On the other hand, adults had a prominent expression of cytokines associated with Th17 responses (IL-1β, IL-17A and IL-23) compared to children. Moreover, that study found that the expression of FoxP3+ Treg cells in the gastric mucosa was significantly higher in infants than in adults, and more intense infiltration of mononuclear and polymorphonuclear cells was observed in the latter group[58]. In another investigation evaluating the levels of cytokines associated with innate and Th1 responses in the gastric mucosa of infected individuals, we demonstrated that children express significantly higher concentrations of tumor necrosis factor α and IL-1α than adults, whereas the contrary was observed with regard to the expression of IL-2, IL-12p70 and IFN-γ. In addition, a progressive reduction in the levels of IFN-γ and IL-12p70 was observed with aging among adults, including elderly individuals, whereas a similar process was observed with the expression of IL-1, IL-2, IL-12p70 and IFN-γ in children[59].
H. pylori-induced Th1 responses have been associated with the development of corpus gastritis, which can result in gastric atrophy and intestinal metaplasia, important in precancerous lesions[60]. Moreover, Treg cells have been associated with various relevant protumor mechanisms in the setting of GC. Enhanced tumor infiltration of FoxP3+ Treg cells have been positively correlated with poor outcomes among patients with gastric adenocarcinoma[61].
Although the aforementioned immune profiles play important roles in the GC onset and progression, growing evidence have emphasized the remarkable protumoral activities associated with Th17 cells. High levels of IL-17 in the tumor environment have been related to increased concentrations of vascular endothelial growth factor and enhanced tumor vascularization. In addition, cytokines promote IL-6 production in tumor cells, and it is a protein that induces vascular endothelial growth factor release as well but also stimulates STAT3, which suppresses apoptosis and prolongs the survival of malignant cells[62]. In a recent study enrolling patients with H. pylori-related diseases, our group demonstrated that GC patients lack IL-27 production both in the gastric environment and peripheral blood[63]. This cytokine is an important inhibitor of Th17 responses by impairing the expression of RORγT, the main IL-17A transcription factor[64].
The classification of GC can be useful for determining a more effective diagnosis as well as a more targeted treatment and better prognosis for cancer patients. The classification system can use anatomical location, degree of invasion, lymphatic involvement, histological type and molecular subtypes[65,66]. Among several classification options, there are older ones that have fallen out of use or continue to be used today (such as Lauren’s), and there are recent updates from renowned institutions such as the World Health Organization.
The anatomical classification can be divided into: (1) Cardial; and (2) Distal. The location of tumors of origin at the gastroesophageal junction, whether esophageal or gastric, may not be identified until the tumor has already reached an expressive size. The literature has shown that the tumor originating in the cardia usually presents a more aggressive behavior in relation to the distal ones, frequently invading the gastric walls[67]. In addition, the occurrence of tumors in the distal region has decreased to the detriment of those in the proximal region[68].
Classification according to the degree of invasion can be done in early or advanced cancer. The early type, limited to the mucosa and submucosa, has a lower degree of development and injury and has a 5-year survival rate of 85% to 90%, while patients with the advanced type have a 5% to 20% survival rate. Furthermore, the advanced type can be evaluated by the Borrmann classification: polypoid (type 1), ulcerated with defined edges (type 2), ulcerated with ill-defined edges (type 3) and plastic linitis, characterized by diffuse infiltrate without evidence of ulceration (type 4)[69].
Lauren’s Histological Classification, widely used since its publication in 1965, has been useful in the discussion of GC. This classification divides gastric adenocarcinoma into two histomorphologic types, intestinal (well, moderately or poorly differentiated) and diffuse (undifferentiated, with or without signet ring cells). The intestinal type is more common in males and older patients, with a better prognosis. It is characterized by tumor cells that unite and organize into glandular formations, just as it occurs in intestinal adenocarcinomas. In addition, the intestinal type usually develops in an environment of atrophic gastritis and presents greater expression of the e-cadherin adhesion molecule[70]. The diffuse type, more prevalent in young individuals and more easily identified in early stages, is characterized by tumor cells that invade neighboring tissues, with little cohesion, loss of e-cadherin expression, without gland formation and with marked fibrosis. In addition, this type presents endocrine markers more frequently and has a higher production of basic fibroblast growth factor[71-73].
Among the most recent classifications, in 2019 the World Health Organization updated the classification of tumors of the digestive system, including GC. In this new approach, histogenesis and the degree of differentiation were not considered, but it recognizes several types of malignant epithelial tumors (tubular, papillary, poorly cohesive signet ring phenotype, another type of poorly cohesive, mucinous, mixed cell) as well as rare variants[74].
The National Institute of Health Cancer Genome Atlas project helped to redefine the molecular classification of GC into four subtypes: (1) GC with Epstein-Barr virus infection; (2) Microsatellite unstable tumors; (3) Genetically stable tumors; and (4) Chromosomally unstable GCs[75,76]. The first constitutes about 9% of GCs and is more common in males, has lesions in the bottom and gastric body with a lower mortality rate and has hypermethylated DNA[77]. The second type represents 22% of GC cases and has a high mutation rate (with high frequency in the KRAS pathway), generally related to an epigenetic event[75,78]. The third type is usually aneuploid and diagnosed early, representing about 20% of GC cases, in addition to having a predominance of diffuse histology and located in the distal region of the stomach[75]. The latter type represents 50% of CG and has histology of the intestinal type, and its frequency is high in cancers of the esophagogastric junction. Chromosomal instability is the result of DNA aneuploidy and mutations in various proto-oncogenes and tumor suppressor genes[75,79].
Atrophic gastritis: In atrophic gastritis (AG), there is an inflammatory process that promotes gland loss and decreased secretory function, modifying the gastric environment, which may be associated with a state of achlorhydria or hypochlorhydria[80]. A recent study showed that the relative risk for GC was 1.7 in moderate AG and 4.9 in severe AG compared to none or mild AG (control)[81]. However, it seems to be possible to identify the evolution of this risk early. In the study by Miki et al[82], it was reported that the progression of AG is closely linked with progressive reductions in the levels of pepsinogen I and II. Therefore, measuring these levels can be an opportunity to assess the progression of gastritis[82]. Another way to assess the risk of progression is through the location and extent of atrophy. A staging system based on the degree of atrophy and the topography of atrophy, called Operative Link for Gastritis Assessment was created for this purpose. In this system, stages 3 and 4 are strongly associated with GC development[83].
Gastric intestinal metaplasia: This lesion is characterized by the replacement of the gastric epithelium by two types of intestinal epithelium. This replacement is generally considered a condition that predisposes to malignancy and an increased risk for GC, especially type III (incomplete)[84,85]. Although the presence of this lesion is considered by many authors as a mild form of dysplasia[86], it is still controversial whether gastric intestinal metaplasia is really a precancerous lesion. After all, several studies have shown that gastric intestinal metaplasia is not always seen in patients who progress to GC[87]. However, further studies are needed to define this question.
Along with this questioning, it remains uncertain whether the eradication of H. pylori promotes the improvement of these precancerous lesions, as there are recent works that have not found any change[88]. However, most studies that assess patients for more than 5 years after the eradication of the bacteria, demonstrate improvements in these lesions. Some possible reasons for these discrepancies are ethnic variations, disease stage, follow-up period, medications used and resistance to the drugs used[89,90].
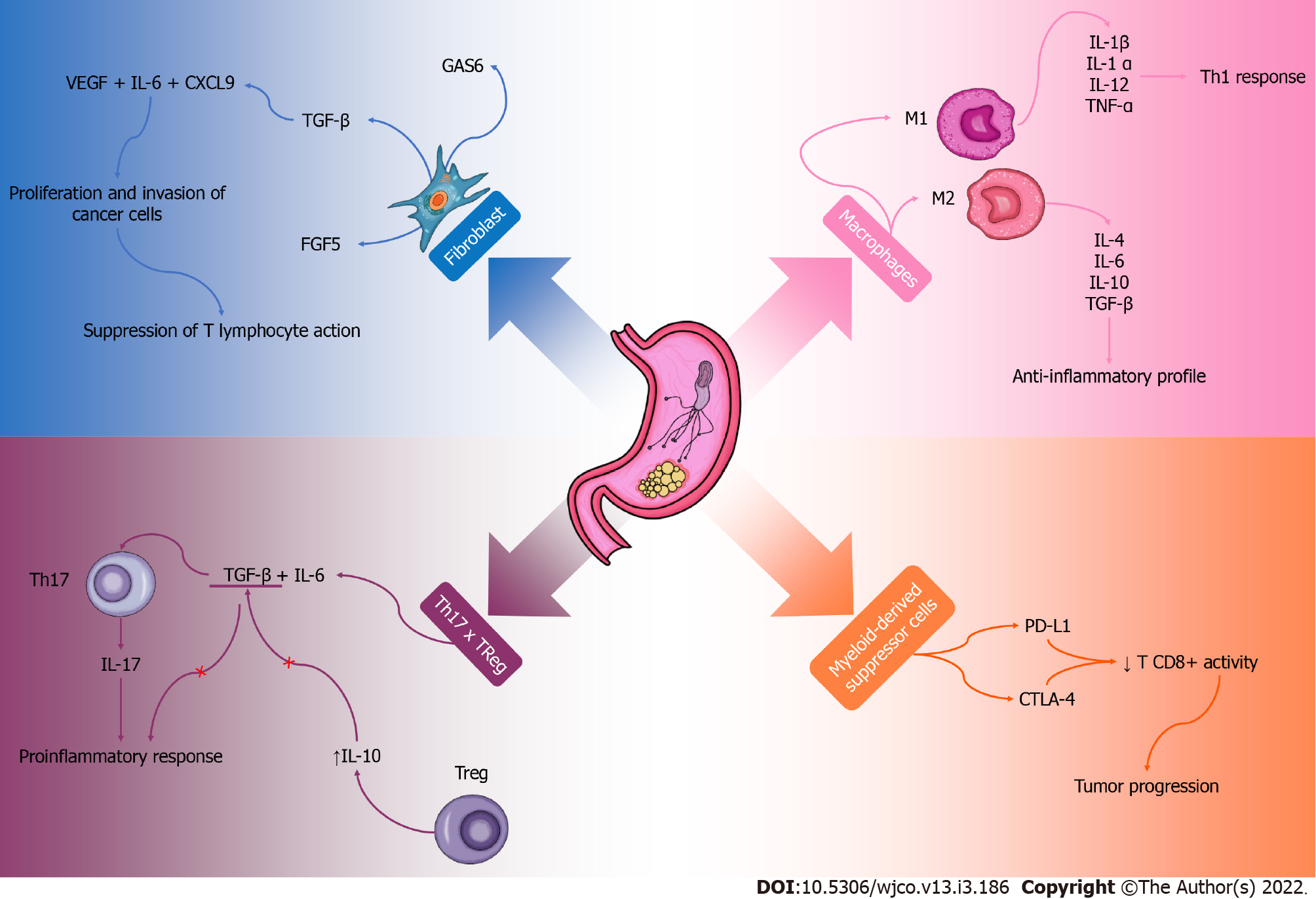
The GC microenvironment has a complex local immune response, and factors that promote the growth and expansion of cancer cells can be observed. Although the microenvironment is still poorly elucidated, some components have already been recognized. Inflammatory cells, fibroblasts and macrophages associated with cancer, endothelial cells and other infiltrating immune components play an important role in this process[91,92] (Figure 2).
Cancer-associated fibroblasts are involved in the synthesis and remodeling of the extracellular matrix and are directly related to angiogenesis, mechanical factors of the tumor and metastatic modulation[93]. The secretome of these cells produce TGF-β, FGF5 and specific growth arrest protein 6 that contribute to the proliferation and invasion of cancer cells[94]. In addition, the presence of vascular endothelial growth factor, IL-6 and chemokine ligand (CXCL9) can be observed, which together with TGF-β reduce the immune response of T lymphocytes[95]. Cancer-associated fibroblasts contribute to increase the stiffness of tumor tissue, compress blood vessels generating a hypoxemic process and contribute to a more aggressive cancer, providing immune evasion and less effective therapeutic response[96,97]. Interestingly, a study using mice found that these cells contribute to the progression of GC, but their functions are not fully understood[98]. This whole process contributes to an immunosuppression in the tumor microenvironment and creates an ideal environment for the development and progression of tumor cells in the affected tissue and possibly other tissues. These cells are probably important in the process of malignant or benign evolution of GC and should be better explored so that their knowledge is directed to therapeutic contexts.
Tumor-associated macrophages are also abundant components of the immune system that play an important role in the microenvironment of GC[99,100]. They can be M1 type and produce proinflammatory cytokines such as IL-1β, IL-1α, IL-12, tumor necrosis factor α, IL-12 and CXCL9, which polarize and recruit components of a Th1 response profile[93,101]. This process culminates in the inhibition of tumor growth[102]. In contrast, type M2 stimulates a Th2 profile secreting cytokines such as IL-4, IL-6, IL-10, IL-13, IL-33, TGF-β and IL-10, which have an anti-inflammatory profile and important tumor activity[103]. They act on tumor progression, metastasis and angiogenesis, which are important factors for the tumor formation microenvironment[104]. The polarization of M1/M2 macrophages is conducted according to the inflammatory profile of the tumor microenvironment or therapeutic intervention. More studies would be interesting in order to observe possible therapies in order to reverse the polarization of M2 macrophages in M1 so that with a more proinflammatory profile, the immune system is stimulated, and the fight against the tumor is more effective.
Myeloid-derived suppressor cells are part of the cell population involved in immune responses and are observed in the tumor microenvironment. They suppress the cytotoxic function of CD8+T cell activity and antitumor, as they have a high expression of programmed death ligand 1 and cytotoxic T-lymphocyte-associated protein 4[93,105]. An interesting study in mice observed that the expression of IL1-β is associated with a greater recruitment of these suppressor cells in the tumor microenvironment[106]. Baumann et al[107] observed that the neutralization of the activities of these cells, concomitant with the inhibition of the checkpoint, obtained a greater efficacy in cancer therapy.
Regarding the expression of cytokines in the microenvironment of GC, there is an important increase in Th17 and Treg cells causing an imbalance in the relationship between these cytokines. This phenomenon is observed gradually with the progression of cancer[108]. The increase in Th17 cells, stimulated by TGF-β and IL-6, promote tumor progression due to increased expression of IL-17 and consequently greater local inflammation[109]. However, Treg cells provide an immunosuppressed environment, stimulating a high production of IL-10 and inhibiting the production of TGF-β. Thus, these Treg cells stimulate the progression of the GC, decreasing the host’s immune surveillance in the tumor microenvironment[110]. Apparently, the balance of the Th17 and Treg relationship in the gastrointestinal tract reflects the integrity of the mucosal immune response and plays an important role in the mechanisms of tumor progression and metastasis.
Most studies are in vitro and murine models. Despite few studies on human models with GC, it is important to highlight the role of immunosuppression in the tumor microenvironment, which is essential for the development of cancer and for possible metastases. The immune response in the tumor microenvironment has a direct link to the host’s general immune response to cancer. These responses are dependent on factors such as genetics, polymorphisms, chronic diseases and the use of drug therapies. In addition, the lack of studies in different stages of human life is a problem since the immunological profile is different throughout life. More studies on the microenvironment of GC are needed, so it would be possible to better understand the mechanism of the immune response and possibly find even more effective therapies in the treatment of this cancer.
During the initial development of tumors, including GC, their cells use several mechanisms to resist innate immune response and prevail in the organism, and when the tumor achieves more advanced stages it evades from the action of T effector cells[111]. Of note, the tumor microenvironment is a protagonist in that context[112]. The immunosuppression promoted by the programmed cell death protein 1 is strongly related to the immune evasion and worse outcomes in GC[113]. In that context, the podoplanina is an immune checkpoint molecule that has been associated with the immune response evasion in various malignancies[114].
Liu et al[115] reported in their study that high levels of podoplanina-expressing cells infiltrating gastric tumors were associated with an increased recruitment of protumoral macrophages and T effector cell dysfunction. Moreover, the infiltrating podoplanina cells contributed to the reduction of INF-γ, granzyme B and perforin-1 levels as well as with an increased expression of programmed cell death protein 1, T cell immunoglobulin and immunoglobulin and mucin-domain containing-3. These findings suggest that the expression of those cells is a tumor evasion mechanism by gastric tumor cells[115].
Another study observed that the macrophage-derived chemokine CXCL8 plays a crucial role in tumor progression in GC patients by mediating immune response evasion and metastasis. CXCL8 acts by reducing the infiltration of Ki67 CD8+ T cells and inhibiting them through the expression of programmed death ligand 1 in macrophages[116]. Interestingly, a study evaluated a GC-derived extracellular compound and concluded that it presents immunosuppressive activities through selective inhibition of CD161CD3 natural killer cells, proliferative stimulus and reduction of the intracellular levels of IFN-γ, granzyme B and perforin[117]. Complementarily, Zhang et al[118] investigated the importance of IL-10-related tumor-associated macrophages in the GC immune response evasion and observed that a tumor microenvironment with high levels of IL-10 tumor-associated macrophages was characterized by the infiltration of Treg cells and dysfunction of CD8+ T cells.
Shi et al[119] demonstrated in a study that the density of Foxp3+ Treg cells and A2aR/CD8+ T cells were highly expressed in the tumor microenvironment and were able to avoid immune responses against GC. The mechanism used by the FoxP3+ Treg cells was the cell apoptosis induction through the ATP decomposition into adenosine as well as the inhibition of CD8+ T cells through the A2aR pathway, leading to an immunosuppressive effect[119]. Notably, a study showed that IL-10-producing regulatory B cells have the potential to avoid the immune surveillance in patients with GC and predict poorer outcomes since the population of CD19CD24+hiCD27 B cells included cells that are able to suppress CD4+ T cells and the production of IFN-γ by autologous CD4+ T cells[120].
Another protein, the costimulatory molecule B7-H4, is a member of the B7 inhibitors family expressed in tumor-related monocytes and macrophages. It is considered an important component of GC immune system evasion, being that it is correlated to invasion depth and to the presence of venous and lymphatic invasion as well as to the expression of HLA-DR[121]. Interestingly, circulating tumor cells undergo an epithelial-mesenchymal transition that allows them to survive in several metastatic environments. In this sense, an assay demonstrated that patients with GC had subtypes of epithelial-mesenchymal transition markers able to regulate ULBP1 (a major member of the natural killer group 2 member D ligand family) in the circulating tumor cells, which aid in immune response evasion[122].
Considering that the tumor purity consists of the proportion of cancer cells in the tumor and is intimately related to the tumor microenvironment characteristics, it is important to emphasize that the low tumor purity implies an unfavorable prognosis, accentuated infiltration of Treg, M1 and M2 macrophages, high expression of immune checkpoints and recruitment of immunosuppressor molecules[123]. A computational study used the interface mimicry technology in order to predict host-pathogen interactions in the context of H. pylori infection and their repercussions in GC. This study found that the H. pylori infection interferes with the apoptosis of host cells through proteins such as HP0231, which is able to impair the CASP6 homodimerization, a crucial step for apoptotic signaling.
The aforementioned interaction might explain the H. pylori-induced resistance to death of host cells, a well-known characteristic associated with carcinogenesis[124]. The studies mentioned here highlight the importance of understanding the mechanisms that lead to the immune system evasion of GC in order to better understand the pathophysiology of the disease as well as to improve prognosis assessments and therapeutic tools for affected individuals. Finally, it is evident that the H. pylori virulence factors are closely related to the gastric carcinogenesis by means of adaptative mechanisms that not only contribute to the infection persistence but also unleash premalignant changes in the gastric microenvironment. These variations progress and perpetuate along with tumor development, aiding in its evasion from the immune response.
Faced with such complex pathways to be understood, future work needs to detail the immune response to bacterial infection, especially to help with early intervention in patients who may develop CG. This may enhance the discovery of new pharmacological therapies that interfere with precancerous lesions and even in advanced stages of cancer. In addition, they should also help in the discovery of new, non-conventional, non-invasive, highly specific biomarkers capable of providing early detection of GC.
Infection by H. pylori, its relationship with the host’s immune system and oncogenesis as well as the tumor evasion mechanisms is crucial for the understanding of the mechanisms involved in the appearance and progression of GC. In view of the chronic inflammation process, a variety of factors can affect the patient’s prognosis, especially according to the individual’s age. The gastric microenvironment has not been well established, and some fundamental components for the progression of GC have been recognized to be related to the host’s immune response.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng H, Cho JH, Kosuga T, Liu XM, Melit LE, Modun D S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Wang AY, Peura DA. The prevalence and incidence of Helicobacter pylori-associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest Endosc Clin N Am. 2011;21:613-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Elzouki AN, Buhjab SI, Alkialani A, Habel S, Sasco AJ. Gastric cancer and Helicobacter pylori infection in the eastern Libya: a descriptive epidemiological study. Arab J Gastroenterol. 2012;13:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 569] [Article Influence: 51.7] [Reference Citation Analysis (1)] |
| 5. | Noto JM, Peek RM Jr. Helicobacter pylori: an overview. Methods Mol Biol. 2012;921:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Berger H, Marques MS, Zietlow R, Meyer TF, Machado JC, Figueiredo C. Gastric cancer pathogenesis. Helicobacter. 2016;21 Suppl 1:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 438] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 8. | Gu H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr Microbiol. 2017;74:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 9. | Lertsethtakarn P, Ottemann KM, Hendrixson DR. Motility and chemotaxis in Campylobacter and Helicobacter. Annu Rev Microbiol. 2011;65:389-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 10. | Mizote T, Yoshiyama H, Nakazawa T. Urease-independent chemotactic responses of Helicobacter pylori to urea, urease inhibitors, and sodium bicarbonate. Infect Immun. 1997;65:1519-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Worku ML, Karim QN, Spencer J, Sidebotham RL. Chemotactic response of Helicobacter pylori to human plasma and bile. J Med Microbiol. 2004;53:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (6)] |
| 12. | Johnson KS, Ottemann KM. Colonization, localization, and inflammation: the roles of H. pylori chemotaxis in vivo. Curr Opin Microbiol. 2018;41:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Ansari S, Yamaoka Y. Helicobacter pylori BabA in adaptation for gastric colonization. World J Gastroenterol. 2017;23:4158-4169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Yamaoka Y. Roles of Helicobacter pylori BabA in gastroduodenal pathogenesis. World J Gastroenterol. 2008;14:4265-4272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Björnham O, Bugaytsova J, Borén T, Schedin S. Dynamic force spectroscopy of the Helicobacter pylori BabA-Lewis b binding. Biophys Chem. 2009;143:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 16. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Borén T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 665] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 17. | Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Braga LLBC, Batista MHR, de Azevedo OGR, da Silva Costa KC, Gomes AD, Rocha GA, Queiroz DMM. oipA "on" status of Helicobacter pylori is associated with gastric cancer in North-Eastern Brazil. BMC Cancer. 2019;19:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, Trust TJ. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect Immun. 2000;68:4155-4168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 20. | Coppens F, Castaldo G, Debraekeleer A, Subedi S, Moonens K, Lo A, Remaut H. Hop-family Helicobacter outer membrane adhesins form a novel class of Type 5-like secretion proteins with an interrupted β-barrel domain. Mol Microbiol. 2018;110:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Rad R, Gerhard M, Lang R, Schöniger M, Rösch T, Schepp W, Becker I, Wagner H, Prinz C. The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. J Immunol. 2002;168:3033-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 22. | Sakamoto S, Watanabe T, Tokumaru T, Takagi H, Nakazato H, Lloyd KO. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 1989;49:745-752. [PubMed] |
| 23. | Walz A, Odenbreit S, Mahdavi J, Borén T, Ruhl S. Identification and characterization of binding properties of Helicobacter pylori by glycoconjugate arrays. Glycobiology. 2005;15:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Gur C, Maalouf N, Gerhard M, Singer BB, Emgård J, Temper V, Neuman T, Mandelboim O, Bachrach G. The Helicobacter pylori HopQ outermembrane protein inhibits immune cell activities. Oncoimmunology. 2019;8:e1553487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 25. | Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 552] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 26. | Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 334] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 27. | Schwartz JT, Allen LA. Role of urease in megasome formation and Helicobacter pylori survival in macrophages. J Leukoc Biol. 2006;79:1214-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Mahawar M, Tran V, Sharp JS, Maier RJ. Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase. J Biol Chem. 2011;286:19159-19169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Basu M, Czinn SJ, Blanchard TG. Absence of catalase reduces long-term survival of Helicobacter pylori in macrophage phagosomes. Helicobacter. 2004;9:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Richter C, Mukherjee O, Ermert D, Singh B, Su YC, Agarwal V, Blom AM, Riesbeck K. Moonlighting of Helicobacter pylori catalase protects against complement-mediated killing by utilising the host molecule vitronectin. Sci Rep. 2016;6:24391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Harris AG, Hazell SL. Localisation of Helicobacter pylori catalase in both the periplasm and cytoplasm, and its dependence on the twin-arginine target protein, KapA, for activity. FEMS Microbiol Lett. 2003;229:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Matos JI, de Sousa HA, Marcos-Pinto R, Dinis-Ribeiro M. Helicobacter pylori CagA and VacA genotypes and gastric phenotype: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 387] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 34. | Giri V, Jha AK, Tomar V, Banerjee I. Re: Novel Bladder Preservation Therapy with Osaka Medical College Regimen: H. Azuma, T. Inamoto, K. Takahara, H. Nomi, H. Hirano, N. Ibuki, H. Uehara, K. Komura, K. Minami, T. Uchimoto, K. Saito, T. Takai, N. Tanda, K. Yamamoto, Y. Narumi and S. Kiyama J Urol 2015;193:443-450. J Urol. 2015;194:263; author reply 264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Raghwan, Chowdhury R. Host cell contact induces fur-dependent expression of virulence factors CagA and VacA in Helicobacter pylori. Helicobacter. 2014;19:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Argent RH, Kidd M, Owen RJ, Thomas RJ, Limb MC, Atherton JC. Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology. 2004;127:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Raju D, Hussey S, Ang M, Terebiznik MR, Sibony M, Galindo-Mata E, Gupta V, Blanke SR, Delgado A, Romero-Gallo J, Ramjeet MS, Mascarenhas H, Peek RM, Correa P, Streutker C, Hold G, Kunstmann E, Yoshimori T, Silverberg MS, Girardin SE, Philpott DJ, El Omar E, Jones NL. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Cover TL, Tummuru MK, Cao P, Thompson SA, Blaser MJ. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566-10573. [PubMed] |
| 39. | Ferreira RM, Machado JC, Letley D, Atherton JC, Pardo ML, Gonzalez CA, Carneiro F, Figueiredo C. A novel method for genotyping the Helicobacter pylori vacA intermediate region directly in gastric biopsy specimens. J Clin Microbiol. 2012;50:3983-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | González CA, Figueiredo C, Lic CB, Ferreira RM, Pardo ML, Ruiz Liso JM, Alonso P, Sala N, Capella G, Sanz-Anquela JM. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Am J Gastroenterol. 2011;106:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Sgouras D, Tegtmeyer N, Wessler S. Activity and Functional Importance of Helicobacter pylori Virulence Factors. Adv Exp Med Biol. 2019;1149:35-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J Exp Med. 2000;191:587-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 906] [Cited by in RCA: 926] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 44. | Chung WC, Jung SH, Lee KM, Paik CN, Kawk JW, Jung JH, Lee MK, Lee YK. The detection of Helicobacter pylori cag pathogenicity islands (PAIs) and expression of matrix metalloproteinase-7 (MMP-7) in gastric epithelial dysplasia and intramucosal cancer. Gastric Cancer. 2010;13:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Ali M, Khan AA, Tiwari SK, Ahmed N, Rao LV, Habibullah CM. Association between cag-pathogenicity island in Helicobacter pylori isolates from peptic ulcer, gastric carcinoma, and non-ulcer dyspepsia subjects with histological changes. World J Gastroenterol. 2005;11:6815-6822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Hatakeyama M. The role of Helicobacter pylori CagA in gastric carcinogenesis. Int J Hematol. 2006;84:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Higashi H, Nakaya A, Tsutsumi R, Yokoyama K, Fujii Y, Ishikawa S, Higuchi M, Takahashi A, Kurashima Y, Teishikata Y, Tanaka S, Azuma T, Hatakeyama M. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004;279:17205-17216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 48. | Hatakeyama M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:196-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 49. | Moran AP. Lipopolysaccharide in bacterial chronic infection: insights from Helicobacter pylori lipopolysaccharide and lipid A. Int J Med Microbiol. 2007;297:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Yokota S, Okabayashi T, Rehli M, Fujii N, Amano K. Helicobacter pylori lipopolysaccharides upregulate toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect Immun. 2010;78:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 198] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (15)] |
| 52. | Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 53. | Razavi A, Bagheri N, Azadegan-Dehkordi F, Shirzad M, Rahimian G, Rafieian-Kopaei M, Shirzad H. Comparative Immune Response in Children and Adults with H. pylori Infection. J Immunol Res. 2015;2015:315957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 397] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 56. | Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995;48:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Harris PR, Mobley HL, Perez-Perez GI, Blaser MJ, Smith PD. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Santos MLC, de Brito BB, da Silva FAF, Sampaio MM, Marques HS, Oliveira E Silva N, de Magalhães Queiroz DM, de Melo FF. Helicobacter pylori infection: Beyond gastric manifestations. World J Gastroenterol. 2020;26:4076-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 59. | Chen J, Zhong Y, Liu Y, Tang C, Zhang Y, Wei B, Chen W, Liu M. Parenteral immunization with a cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) adjuvanted Helicobacter pylori vaccine induces protective immunity against H. pylori infection in mice. Hum Vaccin Immunother. 2020;16:2849-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Liu X, Xu D, Huang C, Guo Y, Wang S, Zhu C, Xu J, Zhang Z, Shen Y, Zhao W, Zhao G. Regulatory T cells and M2 macrophages present diverse prognostic value in gastric cancer patients with different clinicopathologic characteristics and chemotherapy strategies. J Transl Med. 2019;17:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 62. | Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 63. | Rocha GA, de Melo FF, Cabral MMDA, de Brito BB, da Silva FAF, Queiroz DMM. Interleukin-27 is abrogated in gastric cancer, but highly expressed in other Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2020;25:e12667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748-5756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 65. | Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Wang X, Wan F, Pan J, Yu GZ, Chen Y, Wang JJ. Tumor size: a non-neglectable independent prognostic factor for gastric cancer. J Surg Oncol. 2008;97:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Bordi C, Falchetti A, Buffa R, Azzoni C, D'Adda T, Caruana P, Rindi G, Brandi ML. Production of basic fibroblast growth factor by gastric carcinoid tumors and their putative cells of origin. Hum Pathol. 1994;25:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Miyahara R, Niwa Y, Matsuura T, Maeda O, Ando T, Ohmiya N, Itoh A, Hirooka Y, Goto H. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: data from a single institute over 30 years. J Gastroenterol Hepatol. 2007;22:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Zhai Z, Zhu ZY, Zhang Y, Yin X, Han BL, Gao JL, Lou SH, Fang TY, Wang YM, Li CF, Yu XF, Ma Y, Xue YW. Prognostic significance of Borrmann type combined with vessel invasion status in advanced gastric cancer. World J Gastrointest Oncol. 2020;12:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4314] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 71. | Waldum HL, Fossmark R. Types of Gastric Carcinomas. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 73. | Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1136] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 74. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2405] [Article Influence: 481.0] [Reference Citation Analysis (3)] |
| 75. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4828] [Article Influence: 438.9] [Reference Citation Analysis (2)] |
| 76. | Yamamoto H, Perez-Piteira J, Yoshida T, Terada M, Itoh F, Imai K, Perucho M. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 1999;116:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K, Suehiro Y, Yamasaki T, Sakaida I. Clinical Importance of Epstein⁻Barr Virus-Associated Gastric Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 78. | Keller G, Grimm V, Vogelsang H, Bischoff P, Mueller J, Siewert JR, Höfler H. Analysis for microsatellite instability and mutations of the DNA mismatch repair gene hMLH1 in familial gastric cancer. Int J Cancer. 1996;68:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 79. | Maleki SS, Röcken C. Chromosomal Instability in Gastric Cancer Biology. Neoplasia. 2017;19:412-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3545] [Article Influence: 122.2] [Reference Citation Analysis (3)] |
| 81. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3179] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 82. | Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 216] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fassan M, Basso D, Plebani M, Graham DY. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Filipe MI, Muñoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer. 1994;57:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 294] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 85. | Shiotani A, Haruma K, Uedo N, Iishi H, Ishihara R, Tatsuta M, Kumamoto M, Nakae Y, Ishiguro S, Graham DY. Histological risk markers for non-cardia early gastric cancer. Pattern of mucin expression and gastric cancer. Virchows Arch. 2006;449:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Tosi P, Filipe MI, Luzi P, Miracco C, Santopietro R, Lio R, Sforza V, Barbini P. Gastric intestinal metaplasia type III cases are classified as low-grade dysplasia on the basis of morphometry. J Pathol. 1993;169:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Sasaki I, Yao T, Nawata H, Tsuneyoshi M. Minute gastric carcinoma of differentiated type with special reference to the significance of intestinal metaplasia, proliferative zone, and p53 protein during tumor development. Cancer. 1999;85:1719-1729. [PubMed] |
| 88. | Watari J, Das KK, Amenta PS, Tanabe H, Tanaka A, Geng X, Lin JJ, Kohgo Y, Das KM. Effect of eradication of Helicobacter pylori on the histology and cellular phenotype of gastric intestinal metaplasia. Clin Gastroenterol Hepatol. 2008;6:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Vannella L, Lahner E, Bordi C, Pilozzi E, Di Giulio E, Corleto VD, Osborn J, Delle Fave G, Annibale B. Reversal of atrophic body gastritis after H. pylori eradication at long-term follow-up. Dig Liver Dis. 2011;43:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, Shiota S, Nakagawa Y, Mizukami K, Fujioka T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 92. | Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 410] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 93. | Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1940] [Cited by in RCA: 2424] [Article Influence: 484.8] [Reference Citation Analysis (0)] |
| 94. | Wu Y, Grabsch H, Ivanova T, Tan IB, Murray J, Ooi CH, Wright AI, West NP, Hutchins GG, Wu J, Lee M, Lee J, Koo JH, Yeoh KG, van Grieken N, Ylstra B, Rha SY, Ajani JA, Cheong JH, Noh SH, Lim KH, Boussioutas A, Lee JS, Tan P. Comprehensive genomic meta-analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut. 2013;62:1100-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 95. | Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol Lett. 2017;14:2611-2620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 96. | Mohammadi H, Sahai E. Mechanisms and impact of altered tumour mechanics. Nat Cell Biol. 2018;20:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 97. | Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 671] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 98. | Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 865] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 99. | Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, Chen L, Zhang P, Chen H, Liu Y, Dong P, Xie G, Ma Y, Jiang L, Yuan X, Shen L. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 100. | Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 1212] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 101. | Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló S, Roda D, Huerta M, Cervantes A, Fleitas T. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020;86:102015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 102. | Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6:1670-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1188] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 103. | Räihä MR, Puolakkainen PA. Tumor-associated macrophages (TAMs) as biomarkers for gastric cancer: A review. Chronic Dis Transl Med. 2018;4:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 104. | Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 105. | Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, Greenberg PD, Hingorani SR. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63:1769-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 106. | Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 679] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 107. | Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, Laketa V, Donakonda S, Ahting U, Lorenz-Depiereux B, Heil JE, Schredelseker J, Simeoni L, Fecher C, Körber N, Bauer T, Hüser N, Hartmann D, Laschinger M, Eyerich K, Eyerich S, Anton M, Streeter M, Wang T, Schraven B, Spiegel D, Assaad F, Misgeld T, Zischka H, Murray PJ, Heine A, Heikenwälder M, Korn T, Dawid C, Hofmann T, Knolle PA, Höchst B. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol. 2020;21:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 108. | Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B, Ai J, Zhang Z, Song J, Shan B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep. 2013;30:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Rezalotfi A, Ahmadian E, Aazami H, Solgi G, Ebrahimi M. Gastric Cancer Stem Cells Effect on Th17/Treg Balance; A Bench to Beside Perspective. Front Oncol. 2019;9:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 110. | Kindlund B, Sjöling Å, Yakkala C, Adamsson J, Janzon A, Hansson LE, Hermansson M, Janson P, Winqvist O, Lundin SB. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer. 2017;20:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 111. | Campoli M, Ferrone S, Zea AH, Rodriguez PC, Ochoa AC. Mechanisms of tumor evasion. Cancer Treat Res. 2005;123:61-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2809] [Article Influence: 561.8] [Reference Citation Analysis (5)] |
| 113. | Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J. 2019;17:661-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 114. | Ugorski M, Dziegiel P, Suchanski J. Podoplanin - a small glycoprotein with many faces. Am J Cancer Res. 2016;6:370-386. [PubMed] |
| 115. | Liu X, Cao Y, Lv K, Gu Y, Jin K, He X, Fang H, Fei Y, Shi M, Lin C, Liu H, Li H, He H, Xu J, Li R, Zhang H. Tumor-infiltrating podoplanin+ cells in gastric cancer: clinical outcomes and association with immune contexture. Oncoimmunology. 2020;9:1845038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 116. | Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, Li H, Zhang W, Sun Y, Xu J. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68:1764-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 117. | Adithan A, John Peter JS, Mohammad AH, Kim B, Kang CW, Kim NS, Hwang KC, Kim JH. A gastric cancer cell derived extracellular compounds suppresses CD161+CD3- lymphocytes and aggravates tumor formation in a syngeneic mouse model. Mol Immunol. 2020;120:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X, Lv K, He X, Fang H, Jin K, Fei Y, Chen Y, Wang J, Liu H, Li H, Zhang H, He H, Zhang W. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann Surg. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 119. | Shi L, Feng M, Du S, Wei X, Song H, Yixin X, Song J, Wenxian G. Adenosine Generated by Regulatory T Cells Induces CD8+ T Cell Exhaustion in Gastric Cancer through A2aR Pathway. Biomed Res Int. 2019;2019:4093214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 120. | Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Ashida K, Sakabe T, Nakayama Y, Fujiwara Y. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep. 2019;9:13083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 121. | Matsunaga T, Saito H, Ikeguchi M. Increased B7-H1 and B7-H4 Expressions on Circulating Monocytes and Tumor-Associated Macrophages are Involved in Immune Evasion in Patients with Gastric Cancer. Yonago Acta Med. 2011;54:1-10. [PubMed] |
| 122. | Hu B, Tian X, Li Y, Liu Y, Yang T, Han Z, An J, Kong L. Epithelial-mesenchymal transition may be involved in the immune evasion of circulating gastric tumor cells via downregulation of ULBP1. Cancer Med. 2020;9:2686-2697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. | Gong Z, Zhang J, Guo W. Tumor purity as a prognosis and immunotherapy relevant feature in gastric cancer. Cancer Med. 2020;9:9052-9063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 124. | Guven-Maiorov E, Tsai CJ, Ma B, Nussinov R. Prediction of Host-Pathogen Interactions for Helicobacter pylori by Interface Mimicry and Implications to Gastric Cancer. J Mol Biol. 2017;429:3925-3941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (1)] |