Published online Oct 24, 2022. doi: 10.5306/wjco.v13.i10.822
Peer-review started: April 26, 2022
First decision: June 22, 2022
Revised: July 8, 2022
Accepted: October 11, 2022
Article in press: October 11, 2022
Published online: October 24, 2022
Processing time: 176 Days and 12.5 Hours
The inflammatory response to tumor has been proven to be closely related to the prognosis of colorectal cancer. Neutrophil to lymphocyte ratio (NLR) is a widely available inflammatory biomarker that may have prognostic value for patients with colorectal liver metastasis (CRLM).
To assess the role of NLR as a prognostic factor of survival and tumor recurrence in patients with CRLM.
A systematic literature search of PubMed, Cochrane Library and clinicaltrials.gov was conducted by two independent researchers in order to minimize potential errors and bias. Conflicts were discussed and settled between three researchers. Studies including patients undergoing different types of medical interventions for the treatment of CRLM and evaluating the correlation between pretreatment NLR and disease-free survival (DFS) and overall survival (OS) were included in the review. Nineteen studies, involving 3283 patients matched our inclusion criteria.
In the studies included, NLR was measured before the intervention and the NLR thresholds ranged between 1.9 and 7.26. Most studies used 5 as the cut-off value. Liver metastases were treated with hepatectomy with or without chemotherapy regimens in 13 studies and with radiofrequency ablation, radioembolization, chemoembolization or solely with chemotherapy in 6 studies. High NLR was associated with decreased OS and DFS after liver resection or other medical intervention. Moreover, high NLR was associated with poor chemosensitivity. On the contrary, CRLM patients with low pretreatment NLR demonstrated improved OS and DFS. NLR could potentially be used as a predictive factor of survival and tumor recurrence in patients with CRLM treated with interventions of any modality, including surgery, chemotherapy and ablative techniques.
NLR is an inflammatory biomarker that demonstrates considerable prognostic value. Elevated pretreatment NLR is associated with poor OS and DFS in patients with CRLM who are submitted to different treatments.
Core Tip: Colorectal cancer is the third most common cancer globally and liver is the most common site of metastasis. Even though surgery and chemotherapy are the main treatment options, prognostic markers are also essential for the progress and future management of the disease. Neutrophil-to-lymphocyte ratio (NLR) is a promising biomarker that has been recently proposed as an indicator for the survival and recurrence of various malignancies. In our review we assess the role of NLR in the overall survival of patients with colorectal liver metastases.
- Citation: Papakonstantinou M, Fiflis S, Christodoulidis G, Giglio MC, Louri E, Mavromatidis S, Giakoustidis D, Papadopoulos VN, Giakoustidis A. Neutrophil-to-lymphocyte ratio as a prognostic factor for survival in patients with colorectal liver metastases: A systematic review. World J Clin Oncol 2022; 13(10): 822-834
- URL: https://www.wjgnet.com/2218-4333/full/v13/i10/822.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i10.822
According to GLOBOCAN 2020 Data, colorectal cancer (CRC) is the third most frequent cancer in both men and women with an estimated 1931500 new cases and 935173 deaths worldwide in 2020. The liver is the most common site of metastasis in patients with CRC as almost 50% of these patients will develop liver metastases (LM) during the course of their disease of whom 15%-25% have LM at initial diagnosis. The remaining 18%-25% will have metachronous LM in the next 5 years[1,2]. The management of patients with colorectal liver metastases (CRLM) consists of different treatment options such as tumor resection, radiofrequency ablation (RFA), which can destroy the tumor by the use of high-frequency electromagnetic current and can be applied in unresectable CRLM, or microwave ablation. Other treatment options include systemic therapy, such as Irinotecan-loaded drug-eluting beads and radioembolization (RE), that administer high doses of chemotherapy and radiation, respectively, and chemotherapy. The intra-arterial techniques aim specifically at the tumor’s vasculature, thus minimizing systemic toxicity, and may be an option in patients not eligible for surgery or ablation[3]. Different treatment methods are selected depending on the patient’s clinical and radiological data[4]. Advancements in treatment for patients with CRLM have resulted in improved 5-year survival rates as high as 46%; however, survival remains low in patients where all sites of disease are not surgically resectable[5]. The low 5-year overall survival (OS) and the fact that recurrences occur in more than half of CRLM patients, highlights the need for more prognostic factors that could be easily applied to predict OS as well as disease-free survival (DFS)[6].
Many studies have examined the prognostic role of neutrophil to lymphocyte ratio (NLR) in CRLM patients. NLR is a widely available, low-cost prognostic index that is calculated by dividing the number of neutrophils by the number of lymphocytes and reflects the inflammatory response of the patient against the tumor, which is correlated with tumor development and poor outcomes[7,8]. Neutrophils play a role in cancer development and metastases, while lymphocytes mediate an immune response against the malignancy, consequently an elevated NLR value could indicate a protumorigenic status.
In this systematic review we investigated the association between NLR and the prognosis of CRLM in patients treated with interventions of any modality including surgery, chemotherapy and ablative techniques[9,10]. High NLR was associated with poor survival in CRLM patients in the systematic review and meta-analysis by Tang et al[11], which included 8 studies and in the systematic review by Haram et al[12] which also included 8 studies. Our systematic review includes 19 studies thus making the analysis results more robust. It consists of 12 studies including 2442 patients treated surgically, 6 studies including 641 patients treated with RFA or RE or solely chemotherapy and 1 study (Kishi et al[15]) including 200 patients treated surgically and 90 different patients treated with RFA. We studied the different impact of pretreatment NLR as a prognostic factor depending on the medical intervention and we present the analysis results in two categories. The first category included 2642 patients who were treated surgically and the second category included 731 patients who were treated with ablative techniques or solely chemotherapy. All the studies included demonstrated that CRLM patients with low pretreatment NLR had better survival and DFS in comparison to high pretreatment NLR patients regardless of the medical intervention received.
A systematic literature search of PubMed and the Cochrane Library was performed using the following search terms: “Neutrophil to Lymphocyte Ratio and liver metastas* and survival”, “NLR and liver metastas* and survival”, “NLR and liver metastasis and prognostic factor”, “NLR and liver metastas*” and “NLR”. The same search strategy was used for the trial registry “ClinicalTrials.gov'' in order to minimize publication bias by identifying unpublished studies.
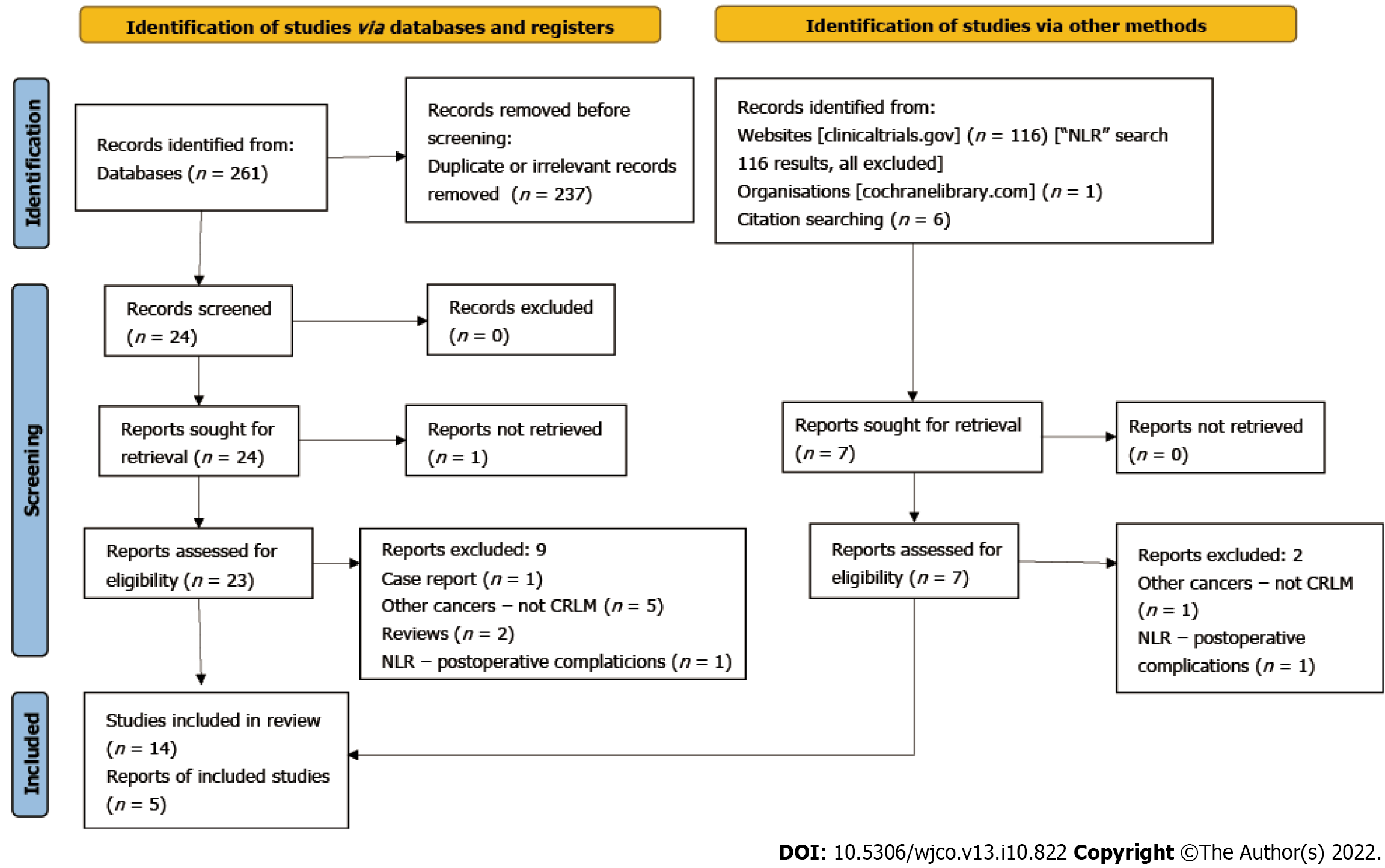
The titles of the articles were screened and relevant abstracts were assessed for eligibility. After excluding duplicates, eligible articles were further evaluated and then the references of those studies were also checked. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart is shown in Figure 1.
In order to minimize possible errors and bias, two independent researchers blindly reviewed the literature and extracted data using the method of completely independent data extraction. After that, any potential differences were cleared up through discussion between them and a third reviewer. The following data were extracted from each study: (1) Patients’ clinicopathological characteristics; (2) The treatment modalities used to treat CRLM; (3) The median survival, 3-year and 5-year OS, 3-year and 5-year DFS; and (4) The univariate and multivariate analysis outcomes.
In order to be included in the analysis, the studies must meet all of the following criteria: (1) Include patients older than 18 years of age diagnosed with CRLM; (2) Define NLR as the absolute number of neutrophils divided by the absolute number of lymphocytes in the peripheral blood; (3) Clearly stated pretreatment NLR values and NLR thresholds; and (4) Analyzing the correlation between pretreatment NLR value and OS outcome and/or DFS. The following exclusion criteria were applied: (1) Not specifically reported colorectal metastasis to the liver; (2) Patients with liver metastases originating from outside the colorectum; (3) Pre-clinical studies; and (4) Studies published in a language other than English.
NLR was defined as the absolute number of neutrophils divided by the absolute number of lymphocytes in the peripheral blood. OS was defined as the time between treatment (hepatectomy, RFA, RE, chemotherapy) and death or last follow-up. DFS was defined as the time between the treatment and the first detection of disease recurrence, including local tumor recurrence, intrahepatic recurrence and extrahepatic metastases. Progression-free survival (PFS) was defined as the duration between primary tumor resection and disease progression.
NLR is a predictor of survival after hepatectomy with neoadjuvant or adjuvant chemotherapy. Eleven studies assessed the prognostic significance of NLR for patients undergoing hepatectomy for CRLM after neoadjuvant chemotherapy. Details on patient demographics and the different NLR thresholds are shown in Tables 1 and 2. Five studies that included 902 patients in total, used 5 as the cut-off value for the NLR. Elevated NLR was significantly associated with worse OS[13-17]. Peng et al[18] used 4.63 as the NLR threshold in 59 patients who received neoadjuvant chemotherapy yielded the same results. Elevated NLR was also significantly correlated with poor OS when the threshold was 1.9, 2.3, 2.4, 2.5, 2.6 or 7.26[19-23].
| Ref. | Number of patients | Sex | Mean age (years) | NLR threshold | Follow up (mo), mean (range) | Sample acquisition |
| Erstad et al[13] | 151 | 84 M, 67 F | 58 | 5 | 41.3 ± 36.7 (2-186) | Within 6 mo prior to surgery and prior to chemotherapy |
| Halazun et al[14] | 440 | 289 M, 151 F | 64 (32-88) | 5 | 24 (11-97) | 1 d prior to surgery |
| Kishi et al[15] | 290 | Resection group 132 M, 68 F; non resection group 61 M, 29 F | Resection, 57; non resection, 56 | 5 | 28 (2-102) | Resection group: Before chemotherapy and before resection |
| Neal et al[16] | 302 | 192 M, 110 F | 64.8 (26-85) | 5 | 29.5 (4-96) | Prior to surgery |
| Hand et al[17] | 322 | 205 M, 117 F | 252 p < 70 yr; 70 p > 70 yr | 5 | 41 | On admission; the night prior to or on the morning of surgery |
| Peng et al[18] | 150 | 97 M, 53 F | 58 (20-82) | 4.63 | 36 (2-126) | Within 7 d prior to surgery |
| Kim et al[19] | 83 | 62 M, 21 F | 59.5 | 1.94 | NS | Within 1 wk prior to surgery |
| Mao et al[20] | 183 | 123 M, 60 F | 67 p > 60 yr | 2.3 | 36.3 | Within 10 d before chemotherapy and surgery |
| Neofytou et al[21] | 140 | 88 M, 52 F | 78% < 70 yr | 2.4 | 33 (1-103) | Within 10 d prior to surgery |
| Giakoustidis et al[22] | 169 | 104 Μ, 65 F | 135 p < 70 yr, 34 p > 40 yr | 2.5 | 34.6 | 10 d prior to surgery - after preoperative chemotherapy |
| Dupré et al[23] | 343 | 236 M, 107 F | 65.8 ± 10.9 | 2.5, 2.6 and 7.261 | 49 | Within 1 wk prior to surgery |
| Hamada et al[24] | 29 | 20 M, 9 F | 63 ± 11.6 (41-83) | 4.1 | 51 (2-97) | NS |
| Zeman et al[25] | 130 | 70 M, 60 F | 60 (33-82) | 5 | 39.3 | NS |
| Ref. | Median Survival | 5-year OS | 5-year DFS | Extrahepatic Disease | Primary Tumor | Chemotherapy |
| Erstad et al[13] | 3.1 yr, NLR > 5; 6.3 yr NLR < 5 | 28.7%, NLR > 5; 59.6%, NLR < 5 | NS | Νο | Previous resection of rectum or colon | Neoadjuvant |
| Halazun et al[14] | NS | 22%, NLR > 5; 43%, NLR < 5 | 12%, NLR > 5; 42%, NLR < 5 | No disseminated or unresectable EHD | Previously resected | Neoadjuvant |
| Kishi et al[15] | 34 mo, NLR > 5; 45 mo, NLR < 5 | 26%, NLR > 5; 36%, NLR < 5 | NS | No | Previously resected | Neoadjuvant, n = 200; Without resection, n = 90 |
| Neal et al[16] | 27.8 mo, NLR > 5; 39.8 mo, NLR < 5 | 18.5% NLR > 5; 30.6% NLR < 5 | 22.3%, NLR > 5; 35.2%, NLR < 52 | NS | Rectum n = 149, Colon n = 153 | Adjuvant, n = 126 |
| Hand et al[17] | 59 mo | Chemotherapy group, 50.8%; No chemotherapy group, 42.5% | NS | NS | No | Neoadjuvant, n = 202 |
| Peng et al[18] | NS | 18.8%, NLR > 4.63; 46.7%, NLR < 4.63 | NS | No | 58% colon, 42% rectum | Neoadjuvant, n = 59 |
| Kim et al[19] | NS | NS | NS | No | NS | Neoadjuvant, n = 24 |
| Mao et al[20] | 31.1 mo NLR > 2.3 43.1 mo NLR < 2.3 | NS | NS | No | Colon n = 104 | Neoadjuvant, n = 183 |
| Neofytou et al[21] | 55 mo, NLR > 2.4; Not reached, NLR < 2.4 | 42%, NLR > 2.4; 69%, NLR < 2.4 | Total 27%. 14%, NLR > 2.4; 40%, NLR < 2.4 | Νο | Resection prior to hepatectomy in 81% | Neoadjuvant |
| Giakoustidis et al[22] | 75 mo | 51%, NLR > 2.5; 74% NLR < 2.5 | NS | No | Synchronous resection, n = 26; ‘liver first’, n = 7 | Neoadjuvant, n = 169 |
| Dupré et al[23] | 50.3, NLR < 2.5; 38.4, NLR > 2.5; 44.8, NLR < 7.26; 25.4, NLR > 7.26 | NS | 11.6, NLR < 2.5; 9.7, NLR > 2.5; 10.3, NLR < 7.26; 6.3, NLR > 7.261 | Resectable EHD in 36 patients | Synchronous, n = 169; Right colon, n = 73; Left colon, n = 126; Rectum, n = 142; Multiple, n = 2 | Neoadjuvant, n = 198 |
| Hamada et al[24] | NS | NS | NS | Yes, n = 5 | NS | Adjuvant, n = 15 |
| Zeman et al[25] | Resection group, 56 mo | 46.6%, resection group; 9.5%, RFA group | 30.5%, resection group, 21 mo median | No | Rectum n = 60, colon n = 70 | Adjuvant, n = 25 |
Ninety-eight patients in 3 studies received only adjuvant chemotherapy after metastasectomy. Elevated NLR was associated with significantly worse DFS[19,24,25]. The OS was also significantly shorter with elevated preoperative NLR in two of the studies[19,24]. However, the NLR cut-off value was different in each cohort (4.1, 1.94 and 5)[19,24,25]. Further information on the OS and DFS, the tumor characteristics as well as the results of univariate and multivariate analyses for the studies mentioned above are shown in Tables 3 and 4.
| Ref. | Univariate analysis | Multivariate analysis |
| Erstad et al[13] | NLR > 5 was significantly associated with reduced OS (P = 0.001) | NLR > 5 associated with reduced OS (P = 0.032) |
| Halazun et al[14] | NLR > 5 was associated with reduced OS (P < 0.0001); NLR > 5 was the sole positive predictor of recurrence (P < 0.0001) | NLR > 5 was associated with reduced OS (P < 0.0001) |
| Kishi et al[15] | NLR > 5 predicted worse survival (P = 0.011) | NLR > 5 predicted worse survival using variables available before surgery (P = 0.016) and after surgery (P = 0.048) |
| Neal et al[16] | NLR > 5 was significantly associated with worse OS (P = 0.001) and CSS (P < 0.001) following metastasectomy | NLR > 3 was associated with shorter survival (P < 0.001); NLR > 3 was associated with adverse outcomes regarding CSS (P < 0.001) |
| Hand et al[17] | Following index hepatectomy, patients with NLR > 5 had a median survival of 55 mo vs 70 mo when NLR < 5 (P = 0.027); Following neoadjuvant chemotherapy, no association between NLR and survival was demonstrated (P = 0.93); NLR > 5 had no impact on prognosis following repeat hepatectomy | There is an independent association between elevated preoperative neutrophil count and shortened overall survival (P = 0.001); no such association was found for NLR |
| Peng et al[18] | Patients with NLR > 4.63 were more likely to present multiple hepatic metastases than those with low NLR (68.8% vs 40.3%, P = 0.030); 5-year RFS and OS for high NLR were significantly inferior to those for low NLR (RFS: 12.5% vs 38.5%, P = 0.015; OS: 18.8% vs 46.7%, P = 0.004) | NLR was not identified as an independent inflammatory factor for better RFS |
| Kim et al[19] | NLR > 1.94 was a prognostic factor for poor OS (P = 0.035) and DFS; High NLR was associated with recurrence (P = 0.031) | NLR > 1.94 was an independent prognostic factor for OS (P = 0.01) and prognostic factor for poor DFS; High NLR was associated with recurrence (P = 0.006) |
| Mao et al[20] | Pre- and post-chemotherapy NLR > 2.3 was associated with decreased OS (P = 0.012) | NLR > 2.3 was a significant predictor both for worse OS (P < 0.001) and for RFS (P = 0.017) |
| Neofytou et al[21] | NLR > 2.4 was associated with decreased DFS (P = 0.033) and OS (P = 0.007) | No significant correlation was found between NLR and OS/DFS |
| Giakoustidis et al[22] | NLR > 2.5 was associated with decreased OS (P = 0.009) and decreased DFS (P = 0.09) | High NLR remained a significant prognostic factor for poor OS (P = 0.012) |
| Dupré et al[23] | NLR of 2.5 was an independent prognostic factor for OS and PFS | High NLR was significantly associated with decreased OS (P < 0.002) |
| Hamada et al[24] | NLR > 4.1 was significantly correlated with better CSS (P = 0.026) | Only univariate analysis was performed |
| Zeman et al[25] | NLR > 5 was significantly associated with DFS (P = 0.044); OS was significantly affected by the preoperative number of leukocytes (P = 0.0014) and neutrophils (P = 0.0036) but not by the NLR. | NLR > 5 was significantly associated with DFS (P = 0.03); Leukocyte number was significantly associated with OS (P = 0.0014); no effect of NLR was demonstrated on OS |
| Ref. | Number of patients and procedure | Sex | Mean age (yr) | NLR threshold | Follow up (mo) | Sample acquisition |
| Tohme et al[29] | 104 RE | 69 M, 35 F | 60.8 ± 12.2, NLR > 5, 66.4 ± 12.2, NLR < 5 | 5 | 100 patients died during follow up | Same day before RE |
| Chang et al[26] | 98 RFA | 56 M, 42 F | 62 (28-92) | 2.5 | 35.2 ± 21.89 | 1 d before RFA - 1 mo after RFA |
| Zhang et al[27] | 92 RFA | 51 M, 41 F | 59 (43-78) | 5 | 27.1 ± 9.8 (range 5-62) | Preoperatively as part of the routine workup. 1-3 d before RFA |
| Weiner et al[28] | 131 RE | 84 M, 47 F | 59 | 5 | 117 deaths during follow up | NS |
| Kishi et al[15] | 90 chemotherapy | 61 M, 29 F | 56 | 5 | 28 (2-102) | |
| Wu et al[31] | 55 chemotherapy | 35 M, 20 F | 59 | 4 | Complete clinical records (no exact mention) | Preoperatively and before 2nd cycle of chemotherapy |
| Philips et al[30] | 71 | - | - | 5 | - | - |
Five studies included 494 patients who underwent RFA or RE or intraarterial therapy and they investigated the correlation between NLR and OS or DFS.
Chang et al[26] and Zhang et al[27] included 190 patients with CRLM without concomitant metastases outside of the liver. Patients were treated with RFA and both studies showed that preoperative high NLR (> 2.5) was associated with worse OS and DFS. Weiner et al[28] and Tohme et al[29] enrolled 235 patients, 100 of whom had extrahepatic metastases and an unspecified number of patients had unresected primary CRC both of which affect NLR and its correlation to OS. All of the patients underwent RE and high NLR was significantly associated with reduced OS. The fifth study investigated the correlation between NLR and OS in CRLM patients with unresectable CRC who were treated with Irinotecan drug-eluting beads against a control group and high NLR was significantly associated with reduced OS[30].
Two studies included 145 patients with unresectable or potentially resectable liver-only metastases from CRC and all of them were treated with primary tumor resection followed by chemotherapy. Both studies revealed that high NLR was significantly associated with worse survival and that prolonged survival was anticipated when NLR was normalized after chemotherapy. Wu et al[31] demonstrated that the normalization of high NLR was significantly associated with better PFS[15,31]. More details of patient demographics, medical treatments provided to the patients and survival outcomes are shown in Tables 5 and 6.
| Ref. | Median Survival | 5-year OS | 5-year DFS | Extrahepatic disease | Primary tumor | Chemotherapy |
| Tohme et al[29] | 5.6 m high NLR 10.6 m low NLR | - | - | 40 (less than 10% of tumor burden) | Some patients had undergone CRC resection (number not mentioned) | All patients |
| Chang et al[26] | - | - | (Preoperative NLR) 11.1% high NLR 22.6% low NLR (NLR increase after RFA 8.6%) (No postoperative NLR increase 22.2%) | No | All patients had undergone CRC resection | No mention |
| Zhang et al[27] | - | 18,4% high NLR 41.7% low NLR | 9.5% high NLR 26.7% low NLR | No | All patients had undergone CRC resection | If necessary after primary tumor resection (number not mentioned) |
| Weiner et al[28] | 7.9 m high NLR 13 low NLR | - | - | 59 | 79% had undergone primary CRC resection | All patients |
| Kishi et al[15] | 11 m high NLR 21 m low NLR | 0% high NLR 14% low NLR | - | No | All patients had undergone CRC resection | All patients |
| Wu et al[31] | 24 m high NLR 56 m low NLR | - | - | No | All patients had undergone CRC resection | All patients |
| Philips et al[30] | 14.7 m high NLR 31.9 m low NLR | - | - | Liver dominant disease | Not mentioned | All patients |
| Ref. | Univariate analysis | Multivariate analysis |
| Tohme et al[29] | High NLR associated with decreased OS P < 0.001 | High NLR associated with decreased OS (HR = 1.927, 95%CI: 1.202-3.089, P = 0.006) |
| Chang et al[26] | Preoperative high NLR and postoperative increase in NLR were associated with decreased DFS (P = 0.044 and P = 0.022, respectively) | Only postoperative NLR increase was associated with decreased DFS (P = 0.029) |
| Zhang et al[27] | High NLR associated with decreased OS (P = 0.007) and DFS (P = 0.007) | High NLR associated with decreased OS (P = 0.039, HR = 3.59, 95%CI: 1.54-9.67) and DFS (P = 0.022, HR = 3.19, 95%CI: 1.87-8.24). |
| Weiner et al[28] | High NLR associated with decreased OS (HR = 2.18, 95%CI: 1.45-3.28, P = 0.0002) | High NLR associated with decreased OS (HR = 2.22, 95%CI: 1.46-3.38, P = 0.0002) |
| Kishi et al[15] | High NLR associated with decreased OS (HR = 3.1, 95%CI: 1.7-5.9, P < 0.001) | High NLR associated with decreased OS (HR = 2.9, 95%CI: 1.5-5.5, P = 0.001). |
| Wu et al[31] | High NLR (HR = 3.182, 95%CI: 1.277-7.933, P = 0.013) associated with decreased OS and DFS (HR = 2.284, 95%CI: 1.156-4.498, P = 0.017). Patients with normalization of NLR had better DFS than those with high NLR that did not decrease (P = 0.002). | No association between NLR and survival |
| Philips et al[30] | High NLR associated with decreased OS P = 0.067 (statistically significant) | No association between NLR and survival |
Many studies have shown the significance of elevated NLR as a prognostic marker in various cancers and the role of NLR in predicting survival remains unanimous across diverse studies that included different cancer types, disease stages and sites[32]. In the studies we analyzed, the NLR cut-off values were determined either by using receiver operating characteristic (ROC) curve analysis that assigned patients in high and low NLR groups or arbitrarily by the authors based on previous studies or the decision-making for the threshold was not mentioned. There is currently no perfect cut-off value that could be used for all CRLM patients as the NLR is greatly affected by chemotherapy regimens, other inflammatory conditions and the tumor burden of each patient. The most frequently used cut-off values in CRLM patients are 2.5 and 5 but further research is needed in order to establish the ideal NLR threshold.
NLR is an inexpensive and easily calculated marker by dividing the total count of neutrophils by the total count of lymphocytes in peripheral blood as measured in a complete blood count (CBC) examination[11,12]. NLR is also immediately available as CBC is part of the routine examinations in patients with cancer.
The association between high NLR and worse prognosis in CRLM patients is complicated and is being rigorously studied. Inflammation plays a significant role in tumor initiation, promotion and progression through pro-inflammatory cytokines, growth factors, chemokines and pro-angiogenic factors. Neutrophils promote tumorigenesis through several mechanisms. They induce DNA damage and mutations by producing toxic substances such as reactive nitrogen species, they promote neoangiogenesis and tumor progression by releasing matrix metalloproteinase-9 (MMP-9) followed by the release of vascular endothelial growth factor. Moreover, neutrophils release a granule protein, called Arg-1, which restricts CD3-cell mediated T cell activation, thus establishing an immunosuppressive microenvironment that contributes to cancer growth. Therefore, a high neutrophil count could indicate worse prognosis in patients with cancer[33,34]. On the contrary, a low lymphocyte count is associated with poorer tumor infiltration and insufficient cell immunity and therefore with worse prognosis in patients with cancer[32]. Consequently, high NLR as a result of increased neutrophils and/or decreased lymphocytes could be an indicator of poor host against tumor response and poor prognosis.
It is plausible that NLR could have an impact in clinical practice as it represents a readily-available biomarker which could potentially assist in the decision-making with prognostic significance. In the studies included in our literature review, patients were treated with different interventions such as surgery with or without adjuvant or neo-adjuvant chemotherapy and other patients were treated with RFA or RE or solely chemotherapy. High NLR was associated with worse OS and DFS in all of the studies.
Patients with CRLM have a poor prognosis if not treated appropriately. The current surgical approach when applicable is to resect the primary tumor followed by liver metastases resection 2-3 mo later with or without chemotherapy, but in certain cases there can be synchronous resection of the primary colon cancer and the hepatic metastases or the ‘liver first approach’[35]. The role of systemic chemotherapy before or after a surgical procedure is well-established both for resectable and non-resectable disease, as it can improve OS[36].
This systematic review points out the role of the NLR as a prognostic factor for the OS and DFS of patients with CRLM. Patients with low preoperative NLR value had better outcomes with longer OS. Similar results were presented by another systematic review which concluded that preoperative NLR calculation could contribute to the identification of patients who could benefit from adjuvant therapies[12]. Measurement of the NLR is inexpensive and easily applied with its value possibly being able to add to the management strategy for patients.
It would be of interest if we could clarify whether different types of chemotherapy affect the NLR or vice versa. In two of the studies included, some patients received adjuvant and others neoadjuvant chemotherapy. The results showed that an elevated NLR is indeed significantly associated with worse survival, but the patients who received neoadjuvant chemotherapy were not separated from the adjuvant group[14,16]. However, Kishi et al[15] showed that preoperative chemotherapy normalized the NLR in 68% of patients with elevated pretreatment NLR, who eventually had similar survival to those with normal pretreatment NLR. Of note, Hand et al[17] showed that OS was significantly shorter for chemotherapy-naive patients with elevated NLR, but not for those who received neoadjuvant chemotherapy. For the latter, the OS resembled that of the patients with normal NLR. Hand et al[17] did not measure the NLR after chemotherapy, but their results are consistent with the fact that neoadjuvant chemotherapy could normalize NLR. Finally, the role of chemotherapy was also investigated in the study by Mao et al[20]. They separated patients into four groups depending on pretreatment and presurgical NLR values. Simultaneous pretreatment and presurgical NLR > 2.3 was significantly associated with worse OS, and the authors hypothesized that high NLR may also indicate poor chemosensitivity[20]. Wu et al[31] included patients with synchronous CRLM who were treated with chemotherapy after primary tumor resection. They showed that patients with normalization of the NLR after one cycle of chemotherapy had better PFS than patients in whom the NLR remained high after chemotherapy and CRC resection. Consequently, the reduction of NLR could imply better chemosensitivity.
To this day, hepatectomy is the “gold standard” treatment in patients with CRLM offering the longest OS, but as a matter of fact only 25% of those patients are eligible for surgery mainly because their clinical condition does not allow them to be surgical candidates or sometimes they refuse surgical treatment[37].
In two studies where patients were treated with RE, the median OS ranged between 5.6 to 7.9 mo in the high NLR group and between 10.6 to 13 mo in the low NLR group. Zhang et al[27] and Chang et al[26] included 190 patients with liver-only CRC metastases. They showed that high NLR patients had worse 5-year DFS ranging between 9.5 to 11% whereas low NLR patients had better 5-year DFS ranging between 22.6 to 26.7%. Zhang et al[27] also showed that high NLR is associated with worse 5-year OS (18.4%) after RFA in comparison to 41.7% in low NLR patients.
It is plausible that the studies investigating the correlation between NLR and OS or DFS in patients with unresectable tumors will demonstrate worse OS or/and DFS compared to studies in surgically treated patients, since as mentioned before non-surgical candidates present a worse clinical condition in general which affects their course of disease.
Even though increased preoperative NLR is correlated with shorter OS and DFS in general, the NLR cut-off values varied between individual studies. The most commonly used threshold was 5. However, the NLR threshold ranged from 1.94 to 7.26[19,23]. That wide distribution could be attributed to the NLR depending on many pro- and anti-inflammatory parameters and the extent of the body’s inflammatory response to cancer, in other words the cancer’s biology being unpredictable[7]. In a study where eight different cut-off values were used (2.2, 2.5, 2.6, 3, 3.5, 4, 5 and 7.26), elevated NLR was consistently associated with decreased OS, even though the results were not statistically significant for every cut-off value[23]. The optimal threshold is based on molecular data analyzed by computer applications, such as Cutoff Finder[38]. The cut-off value is calculated with various models, such as ROC curve analysis or Kaplan-Meier curves and proportional hazards regression[39].
NLR is an easily calculated tool with a possible prognostic significance. High NLR could inform the clinicians about the worse OS and DFS that would be expected. Since worse DFS would be expected, patients with high NLR could be submitted to earlier and more frequent diagnostic imaging examinations, in order to diagnose disease recurrence. Moreover, better prognosis would be anticipated in patients with low NLR since they present better OS and DFS.
Moreover, some patients are initially diagnosed with unresectable or potentially resectable CRLM. Many studies have shown that inoperable CRLM can be down-staged to resectable CRLM after chemotherapy, but this happens in less than 35% of patients with inoperable CRLM[40]. Therefore, more than 65% of patients with unresectable CRLM will not benefit from chemotherapy and it would be important to identify biomarkers that could identify chemosensitive patients. Later, those patients would be submitted to secondary CRLM curative resection. Mao et al[20] and Wu et al[31] demonstrated in their studies that the normalization of NLR after chemotherapy is correlated to chemosensitivity. Consequently, NLR could be used as an assisting tool in stratifying patients as chemosensitive or chemoresistant. Chemoresistant patients would possibly benefit more from interventions such as RFA or RE rather than chemotherapy. More studies are needed to assess whether NLR can be used as an indicator of chemosensitivity or if NLR could be combined with other biomarkers to increase accuracy.
Our results clearly demonstrate that elevated NLR is associated with adverse OS and DFS. These results are significant and they are the same across heterogeneous studies that included different populations, age groups, cancer stages, chemotherapy regimens and medical interventions, which shows that NLR could be an important prognostic factor that can be used in CRLM patients. High pretreatment NLR is associated with worse outcomes independently of the treatment received by the patients.
Prospective studies are needed in order to examine whether NLR could be used as part of an algorithm for the treatment of CRLM. It could also be potentially used in combination with other biomarkers or parameters such as CEA, CA19-9, primary tumor location and primary tumor TNM stage, which have been used in other studies in order to create a novel scoring system that would improve the predictive accuracy of recurrence and survival[19,41].
One limitation of our study is that the cut-off values were different among the studies. That prevents the utilization of NLR as a tool for the management of patients in clinical practice. The timing of blood sampling was also not consistent among the studies. Regarding neoadjuvant chemotherapy, even though it appears to improve outcomes, there is a need for larger studies that distinguish different chemotherapy types and regimens to reach a certain conclusion. Finally, the heterogeneity of patient demographics and clinicopathological characteristics (e.g., primary tumor location and treatment, size or extent of the metastases) prevented the conduction of a meta-analysis.
It is obvious that more research is needed in order to enhance the role of NLR as an inexpensive, independent, crucial prognostic marker. More prospective randomized trials should be designed and executed as all the articles that were available to us were retrospective except one. In upcoming studies the authors should clearly state the clinicopathological details of every patient, the dates of blood sampling, the primary tumor and liver metastasis characteristics and how they were treated. Ideally, all patients should have their primary colorectal tumor resected and not have extrahepatic metastasis as these raise the tumor burden of patients with CRLM and therefore affect NLR. Moreover, all of these patients should be treated with similar chemotherapy sessions and with interventions by surgeons with similar levels of experience and training.
Neutrophil to lymphocyte ratio calculation could potentially be an assisting tool in identifying patients with CRLM who have a higher probability of poor prognosis after treatment, so that the periprocedural management could be adjusted to benefit the patient. Overall, high pretreatment NLR was significantly associated with worse OS and DFS. Larger studies could help identify a standard, widely accepted cut-off value and therefore make the NLR’s prognostic significance applicable in clinical practice.
Patients with CRLM can be treated surgically or non-surgically, but regardless of the medical intervention they have low overall survival and disease-free survival.
It is important to develop prognostic biomarkers that could predict survival, tumor recurrence and response to treatment in order for patients to benefit most from medical interventions and receive personalized treatment.
To identify all possible articles related to our topic and examine the use of NLR as a prognostic factor in CRLM patients in clinical practice. We aimed to demonstrate that NLR is a possible significant biomarker that could assist in the management of CRLM patients by predicting survival, tumor recurrence or response to treatment.
We performed an extensive search of PubMed, the Cochrane Library and also searched for unpublished articles in “clinicaltrials.gov”. We used combinations of the words “Neutrophil to Lymphocyte ratio”, “NLR”, “survival”, “prognostic factor”, “metastasis”, “metastases”, “liver metastasis”, “liver metastases”. The results were screened by two independent researchers and any potential differences were resolved between them and a third researcher through discussion. The aim was to identify studies that investigated the correlation between NLR and survival or tumor recurrence in CRLM patients.
We included 19 studies that included CRLM patients who were treated with different medical approaches, surgically or non-surgically. All the studies demonstrated that high NLR was associated with poor survival, disease-free survival and response to chemotherapy.
The NLR could potentially be used as a predictor of survival, tumor recurrence and chemosensitivity in CRLM patients.
Prospective, well-structured studies are needed in order to examine the role of the neutrophil to lymphocyte ratio (NLR) as a prognostic factor and establish it as part of the decision-making tools of clinicians in the management of colorectal liver metastasis (CRLM) patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Li T, China; Morozov S, Russia S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Chow FC, Chok KS. Colorectal liver metastases: An update on multidisciplinary approach. World J Hepatol. 2019;11:150-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (3)] |
| 2. | Kow AWC. Hepatic metastasis from colorectal cancer. J Gastrointest Oncol. 2019;10:1274-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Boas FE, Bodei L, Sofocleous CT. Radioembolization of Colorectal Liver Metastases: Indications, Technique, and Outcomes. J Nucl Med. 2017;58:104S-111S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Martin J, Petrillo A, Smyth EC, Shaida N, Khwaja S, Cheow HK, Duckworth A, Heister P, Praseedom R, Jah A, Balakrishnan A, Harper S, Liau S, Kosmoliaptsis V, Huguet E. Colorectal liver metastases: Current management and future perspectives. World J Clin Oncol. 2020;11:761-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (6)] |
| 5. | Lemke J, Cammerer G, Ganser J, Scheele J, Xu P, Sander S, Henne-Bruns D, Kornmann M. Survival and Prognostic Factors of Colorectal Liver Metastases After Surgical and Nonsurgical Treatment. Clin Colorectal Cancer. 2016;15:e183-e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 7. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11279] [Article Influence: 490.4] [Reference Citation Analysis (2)] |
| 8. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5765] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 9. | Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34:735-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment. Front Immunol. 2017;8:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 11. | Tang H, Li B, Zhang A, Lu W, Xiang C, Dong J. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Colorectal Liver Metastasis: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0159447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 2017;115:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Erstad DJ, Taylor MS, Qadan M, Axtell AL, Fuchs BC, Berger DL, Clancy TE, Tanabe KK, Chang DC, Ferrone CR. Platelet and neutrophil to lymphocyte ratios predict survival in patients with resectable colorectal liver metastases. Am J Surg. 2020;220:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 15. | Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 269] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Neal CP, Cairns V, Jones MJ, Masood MM, Nana GR, Mann CD, Garcea G, Dennison AR. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol. 2015;32:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Hand F, Ryan EJ, Harrington C, Durand M, Maguire D, O'Farrelly C, Hoti E, Geoghegan JG. Chemotherapy and repeat resection abrogate the prognostic value of neutrophil lymphocyte ratio in colorectal liver metastases. HPB (Oxford). 2020;22:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Peng J, Li H, Ou Q, Lin J, Wu X, Lu Z, Yuan Y, Wan D, Fang Y, Pan Z. Preoperative lymphocyte-to-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival. Onco Targets Ther. 2017;10:3789-3799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kim H, Jung HI, Kwon SH, Bae SH, Kim HC, Baek MJ, Lee MS. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann Surg Treat Res. 2019;96:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Mao R, Zhao JJ, Bi XY, Zhang YF, Li ZY, Huang Z, Zhou JG, Zhao H, Cai JQ. A Low Neutrophil to Lymphocyte Ratio Before Preoperative Chemotherapy Predicts Good Outcomes After the Resection of Colorectal Liver Metastases. J Gastrointest Surg. 2019;23:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol. 2014;31:239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Giakoustidis A, Neofytou K, Khan AZ, Mudan S. Neutrophil to lymphocyte ratio predicts pattern of recurrence in patients undergoing liver resection for colorectal liver metastasis and thus the overall survival. J Surg Oncol. 2015;111:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Dupré A, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ, Malik HZ. Preoperative Leucocyte-Based Inflammatory Scores in Patients with Colorectal Liver Metastases: Can We Count on Them? World J Surg. 2019;43:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Hamada T, Ishizaki H, Haruyama Y, Hamada R, Yano K, Kondo K, Kataoka H, Nanashima A. Neutrophil-to-Lymphocyte Ratio and Intratumoral CD45RO-Positive T Cells as Predictive Factors for Longer Survival of Patients with Colorectal Liver Metastasis after Hepatectomy. Tohoku J Exp Med. 2020;251:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Zeman M, Maciejewski A, Półtorak S, Kryj M. Evaluation of outcomes and treatment safety of patients with metastatic colorectal cancer to the liver with estimation of prognostic factors. Pol Przegl Chir. 2013;85:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Chang Z, Zheng J, Ma Y, Zhao J, Wang C, Liu Z. The neutrophil-to-lymphocyte ratio as a predictor for recurrence of colorectal liver metastases following radiofrequency ablation. Med Oncol. 2014;31:855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Peng Z, Chen M, Liu F, Huang J, Xu L, Zhang Y. Elevated neutrophil to lymphocyte ratio might predict poor prognosis for colorectal liver metastasis after percutaneous radiofrequency ablation. Int J Hyperthermia. 2012;28:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Weiner AA, Gui B, Newman NB, Nosher JL, Yousseff F, Lu SE, Foltz GM, Carpizo D, Lowenthal J, Zuckerman DA, Benson B, Olsen JR, Jabbour SK, Parikh PJ. Predictors of Survival after Yttrium-90 Radioembolization for Colorectal Cancer Liver Metastases. J Vasc Interv Radiol. 2018;29:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Tohme S, Sukato D, Chalhoub D, McDonald KA, Zajko A, Amesur N, Orons P, Marsh JW, Geller DA, Tsung A. Neutrophil-lymphocyte ratio is a simple and novel biomarker for prediction of survival after radioembolization for metastatic colorectal cancer. Ann Surg Oncol. 2015;22:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Friday, March 13, 2015, 4:30pm-6:30pm Long Oral E - Liver Oncology. HPB. 2015;17:16-20. [DOI] [Full Text] |
| 31. | Wu Y, Li C, Zhao J, Yang L, Liu F, Zheng H, Wang Z, Xu Y. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol. 2016;14:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2232] [Article Influence: 202.9] [Reference Citation Analysis (0)] |
| 33. | Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 425] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 34. | Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 321] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 35. | Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Tabernero J, Teh C, Van Cutsem E; Jean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 413] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 36. | Chakedis J, Squires MH, Beal EW, Hughes T, Lewis H, Paredes A, Al-Mansour M, Sun S, Cloyd JM, Pawlik TM. Update on current problems in colorectal liver metastasis. Curr Probl Surg. 2017;54:554-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Lang H. Liver resection is beneficial for patients with colorectal liver metastases and extrahepatic disease. Ann Transl Med. 2020;8:1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 783] [Cited by in RCA: 1016] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 39. | Mazzara S, Rossi RL, Grifantini R, Donizetti S, Abrignani S, Bombaci M. CombiROC: an interactive web tool for selecting accurate marker combinations of omics data. Sci Rep. 2017;7:45477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 40. | Guo M, Jin N, Pawlik T, Cloyd JM. Neoadjuvant chemotherapy for colorectal liver metastases: A contemporary review of the literature. World J Gastrointest Oncol. 2021;13:1043-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 41. | Kim WJ, Lim TW, Kang SH, Park PJ, Choi SB, Lee SI, Min BW, Kim WB. Development and validation of novel scoring system for the prediction of disease recurrence following resection of colorectal liver metastasis. Asian J Surg. 2020;43:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |