Published online Jan 24, 2022. doi: 10.5306/wjco.v13.i1.62
Peer-review started: April 2, 2021
First decision: August 18, 2021
Revised: September 22, 2021
Accepted: December 28, 2021
Article in press: December 28, 2021
Published online: January 24, 2022
Processing time: 294 Days and 7.2 Hours
Late relapses of early-stage germ cell tumors are rare. Most patients (-85%) with stage I seminoma are cured by radical orchiectomy. The detection of late relapse is challenging given the relative rarity of this phenomenon, and the fact that patients who have completed surveillance are usually not undergoing regular oncologic workup nor imaging. While many treatment options do exist for a patient with late relapse of seminoma, surgery is typically the mainstay as these tumors are generally thought to be more chemo-resistant.
In this article, we describe the management of a patient with an early-stage pure seminoma who was subsequently identified to have a recurrence two decades later. We provide a review of the literature not only focused on clinical factors and biology, but also the management of late recurrences specifically in pure semi
There is a paucity of data and treatment recommendations for this clinical entity, and a multidisciplinary approach emphasizing subspecialty expert consultation and patient education is imperative.
Core Tip: This case report describes of a patient with early-stage pure seminoma subsequently identified to have a recurrence within the prostate over two decades later. We provide a robust review of the literature focused on clinical factors, biology, and management of late recurrences specifically in pure seminoma and in prostate gland. There remains a lack of consensus data on treatment recommendations for this clinical disease. As such, a multidisciplinary approach emphasizing subspecialty expert consultation and patient education is imperative.
- Citation: Baweja A, Mar N, Rezazadeh Kalebasty A. Late recurrence of localized pure seminoma in prostate gland: A case report. World J Clin Oncol 2022; 13(1): 62-70
- URL: https://www.wjgnet.com/2218-4333/full/v13/i1/62.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i1.62
Late relapses of early-stage germ cell tumors (GCTs) [both seminomatous and non-seminomatous GCTs (NSGCTs)] are rare and are typically defined as recurrence at least two years after completion of successful primary treatment[1]. Notably, most patients (-85%) with stage I seminoma are cured by radical orchiectomy[2]. As such, the detection of late relapse is challenging given the relative rarity of this phe
The purpose of this report is to describe the management of a patient with an early-stage pure seminoma who was subsequently identified to have a recurrence two decades later. A majority of the reports studying late relapse combine a mixed patient population of seminoma and NSGCTs, and focus more on clinical characteristics that may be associated with the late relapse. As such, we aim to provide a brief review of the literature not only focused on clinical factors and biology, but also the mana
The patient complained of urinary frequency, nocturia and intermittent urinary stream 20 years after his initial diagnosis of stage I pure seminoma.
The patient is a 60 years old man who originally presented in 1998 to a different facility and underwent a right radical orchiectomy for a testicular mass. Pathology showed a stage I (pT1 N0 M0) pure seminoma. He received adjuvant radiation therapy to the periaortic fields and subsequently did well with no evidence of disease recurrence.
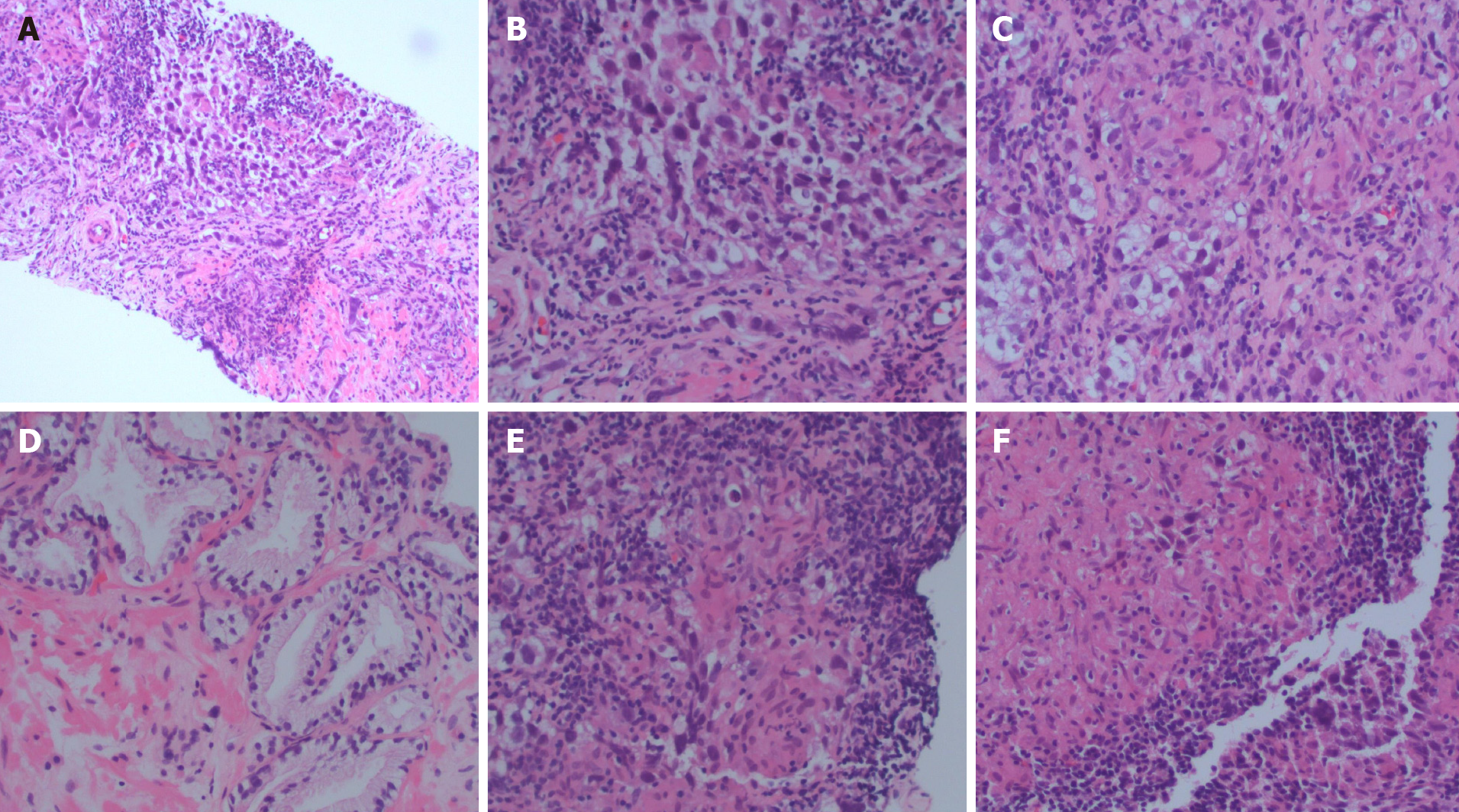
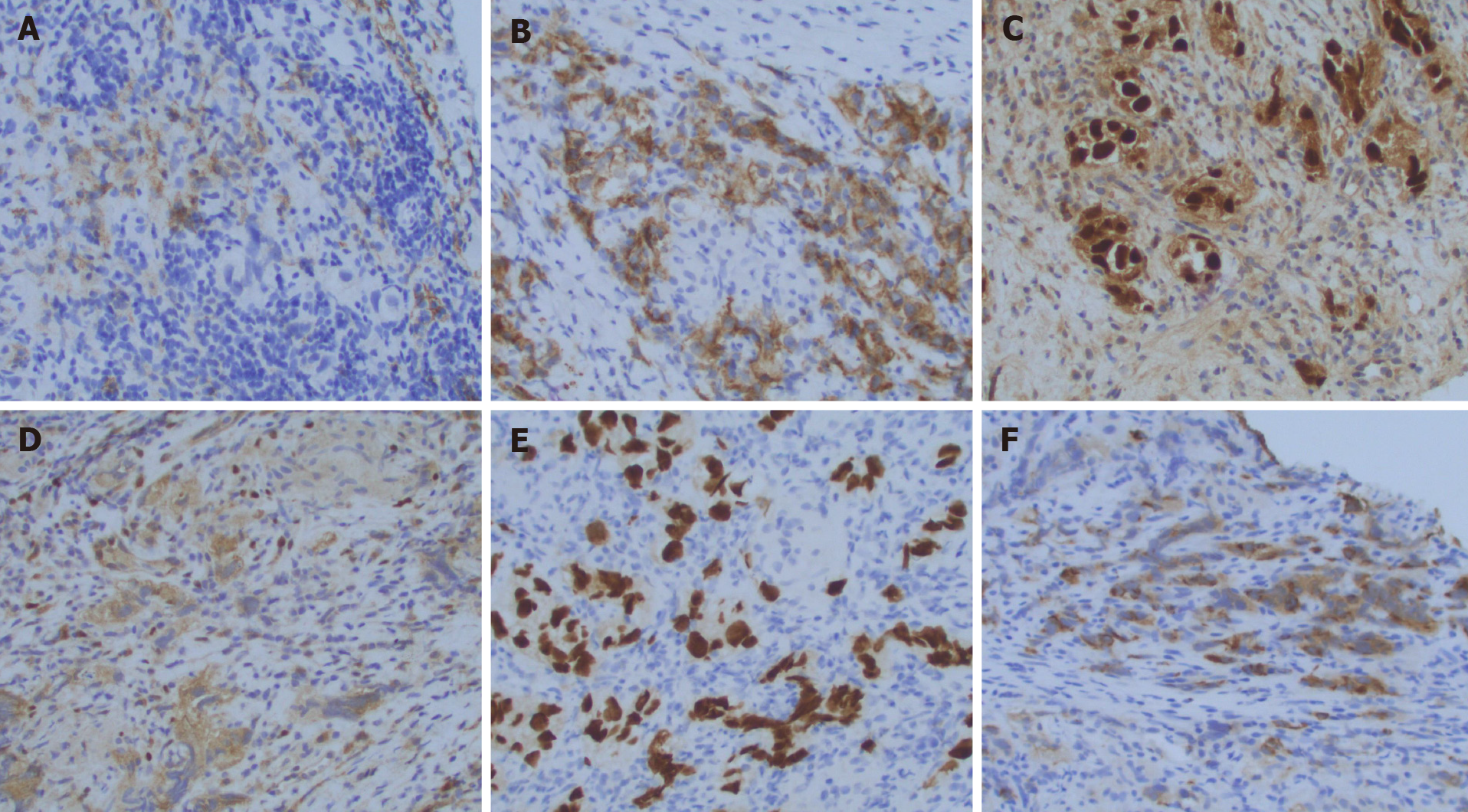
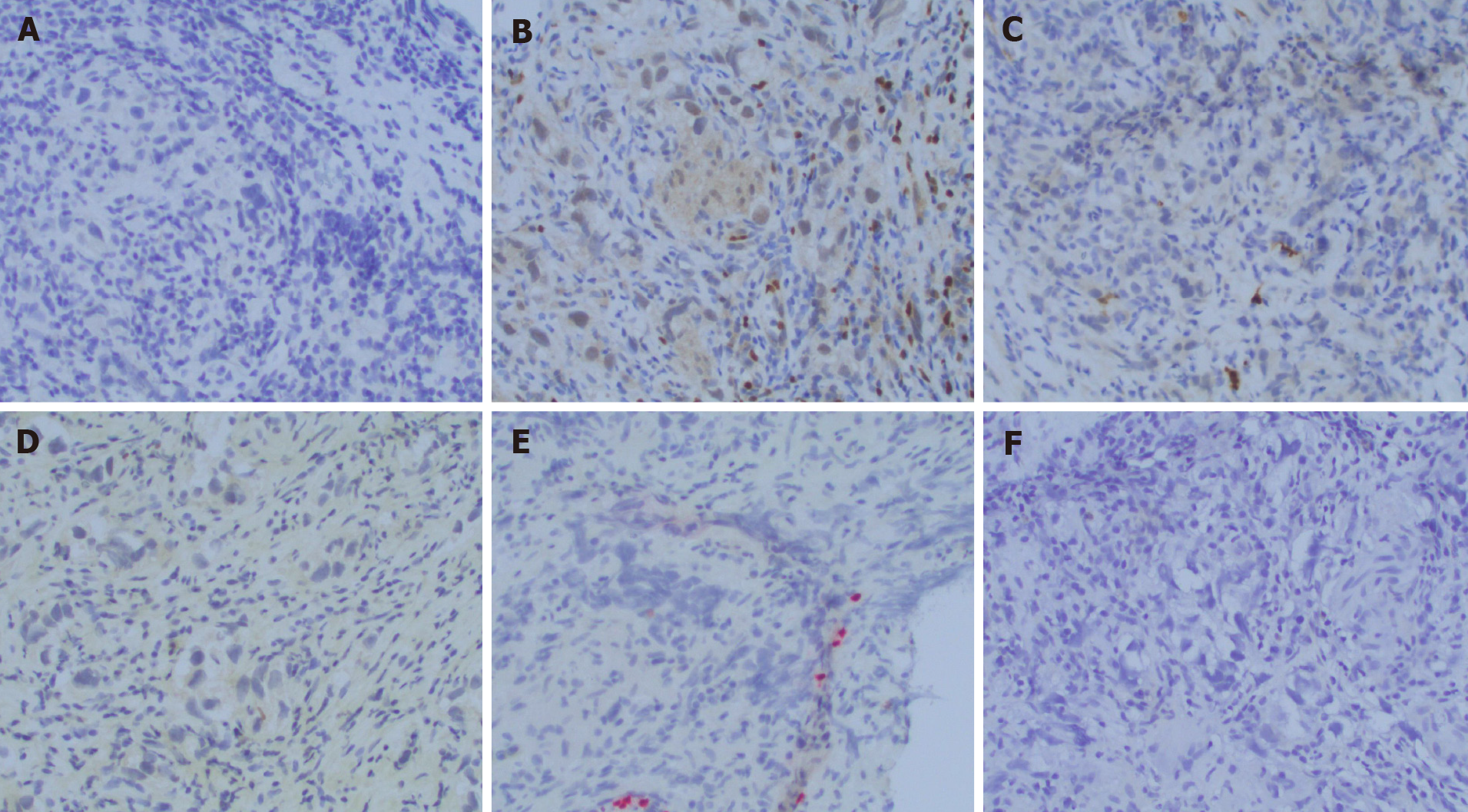
Twenty years later, he developed frequency of urination, nocturia, and intermittent urinary stream. He was found to have an abnormal digital rectal exam and underwent a trans-rectal ultrasound which showed an irregular prostate gland. He ultimately underwent a prostate biopsy which revealed pure seminoma in 10 out of 12 cores bilaterally (Figures 1-3).
Tumor markers after diagnosis of recurrence were as follows: Alpha fetoprotein (AFP) 3.4 and human chorionic gonadotropin (HCG) < 2. A left testicular ultrasound showed a spermatocele, but no obvious masses. Staging computed tomography (CT) scans of the chest, abdomen and pelvis demonstrated an enlarged prostate with extracapsular extension, seminal vesicle invasion, possible tumor invasion into the rectal wall, an enhancing 1 cm periprostatic lymph node, and a 6 mm right pelvic sidewall lymph node. He was then referred to our tertiary care center for further management. His case was presented at a multidisciplinary genitourinary tumor board where further imaging was recommended.
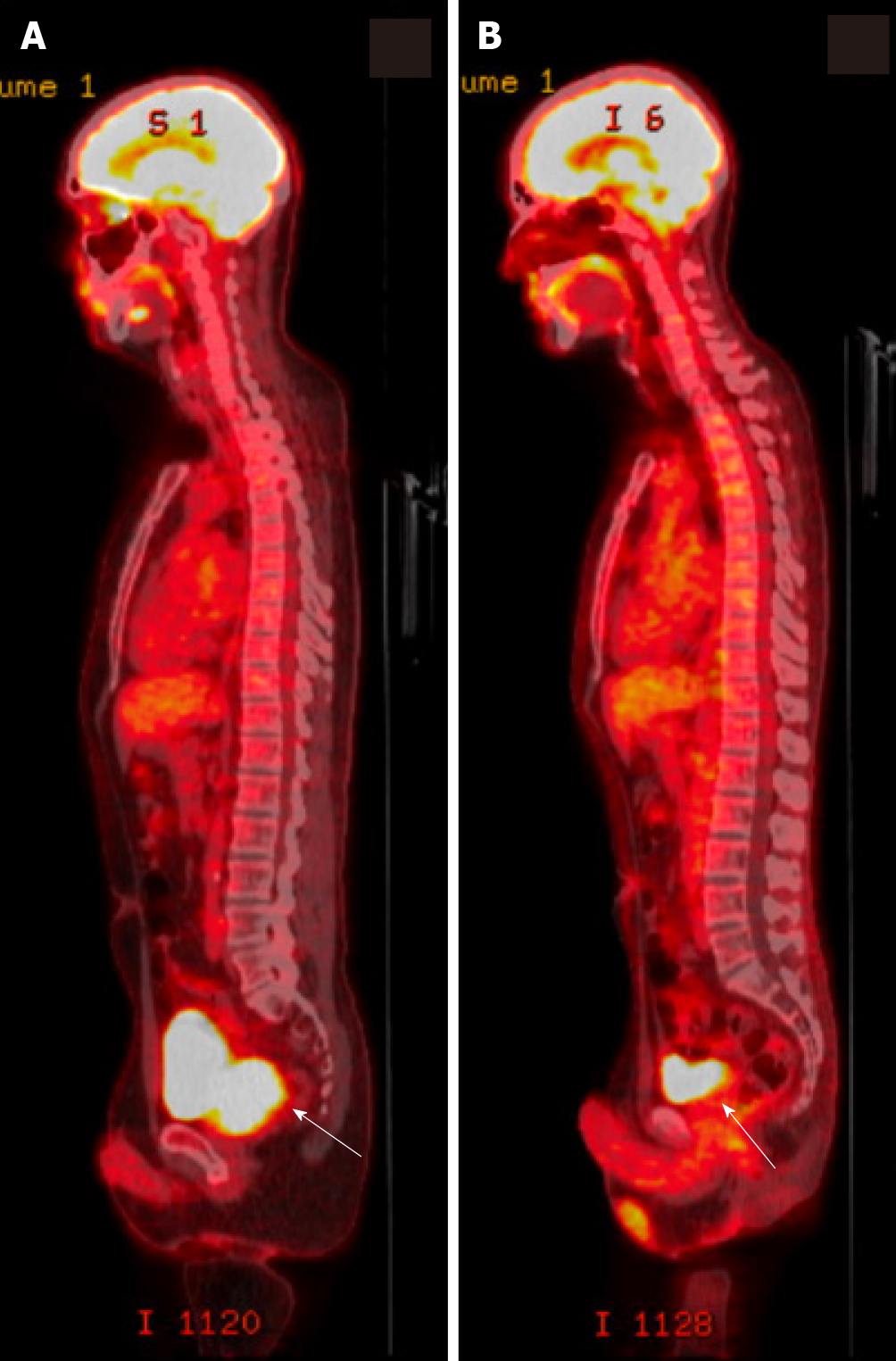
He underwent a positron emission tomography (PET) scan which showed an fluorodeoxyglucose (FDG)- avid mass replacing the prostate gland with invasion into the right seminal vesicle, loss of the normal fat planes between the prostate, bladder and rectum concerning for extracapsular invasion, and an FDG-avid prominent right mesenteric lymph node consistent with nodal metastasis (Figure 4A).
Past medical history: Gastroesophageal reflux disease; post-nasal drip/recurrent sinusitis; hyperlipidemia - diet controlled; low testosterone; B12 deficiency.
Past surgical history: Left inguinal hernia repair - 2014; right orchiectomy - 1998; tonsillectomy - age 3.
Allergies: No known drug allergies.
Family history: Mother - lymphoma at age 94; maternal uncle - prostate cancer in his 80 s.
Social history: Patient denied history of smoking, alcohol or drug use. He currently lives with his wife. He has 3 children. He is a pilot.
Electrocorticography performance status: 0; pain score: 0/10; general: Alert and oriented to place, self, time, in no acute distress well-appearing; head, ears, eyes, nose, and throat: Normocephalic, atraumatic, anicteric sclera, moist mucous membranes, no oral lesions or thrush; chest: Chemotherapy port site clean; cardiovascular: Normal S1, S2, regular rate and rhythm, no murmurs/rubs/gallops; pulmonary: Clear to auscultation bilaterally, no crackles/wheezing/rales; abdomen: Soft, non-distended, non-tender, normal bowel sounds, no rebound/guarding, no hepatosplenomegaly; back: No costovertebral angle tenderness. No tenderness along the spine. Extremities: Warm, well perfused, no joint deformities. No cyanosis, clubbing, or edema.
Tumor markers after diagnosis of recurrence were as follows: AFP 3.4 and HCG < 2.
Staging CT scans of the chest, abdomen and pelvis demonstrated an enlarged prostate with extracapsular extension, seminal vesicle invasion, possible tumor invasion into the rectal wall, an enhancing 1 cm periprostatic lymph node, and a 6 mm right pelvic sidewall lymph node.
Literature review: Late recurrences of early stage GCTs remains a rare clinical entity. This applies to both pure seminoma and NSGCTs. Studies detailed below have shed light on additional clinical factors associated with late recurrences aside from the clinical stage. In particular, there is an observation that NSGCTs have a higher propensity to recur late than do pure seminomatous tumors[3]. While the treatment of a germ cell tumor recurrence can generally involve surgery or chemotherapy[2,3], a thorough assessment of tumor locality, patient functional status and prior treatments must be taken into account in detailing a personalized approach especially for curative intent.
A study of 1263 patients with late recurrence of GCTs has demonstrated that positive tumor markers at initial presentation and the presence of differentiated teratoma in post-chemotherapy surgical specimens are predictors of late recurrence. Additionally, late recurrences more than 5 years from initial therapy occurred mainly in patients with metastatic NSGCTs, whereas late recurrence was only seen in one case of metastatic seminoma and in one case of stage I NSGCT managed by surveillance. In patients with stage I seminoma treated with adjuvant radiation, the latest recurrence in this study was seen at 21 mo[3]. Interestingly, very late recurrences after 5 years in stage I and II seminoma treated with post- operative radiotherapy have been reported and were more common in bulky stage II disease[4].
The possibility of a new extra-gonadal primary tumor rather than a recurrence of the initial primary tumor for these patients must be seriously considered. Although ionizing radiation can have late effects mediating new tumorigenesis, the recurrence we have described has occurred outside of the radiated field, or “landing zone”. As such, it is important to note that there have been very few cases reported of primary seminoma of the prostate. To our knowledge, this entity has only been described in at least 5 circumstances. Hashimoto et al[12] recently reported the case of a 54-year old man with difficulty urinating who was found to have an enlarged and irregular prostate, as in our patient. Core biopsy demonstrated cells positive for placental alkaline phosphatase, CD117, periodic acid-Schiff, and negative for cytokeratin 7, leukocyte common antigen, vimentin, S100 protein, CD30, and prostate-specific antigen. The pathological exam was therefore consistent with seminoma. Notably, this patient had completely normal testes on ultrasound and physical exam with no other distant disease on imaging by chest and abdominal CT scan. He was treated with three cycles of bleomycin, etoposide, and cisplatin (BEP) chemotherapy and has responded well with complete remission of disease. Hence, although rarely described, the notion of an extra-gonadal germ cell tumor is certainly plausible and data does point towards platinum-sensitivity of these particular tumors[12,13].
A population based study by Oldenburg et al[5] evaluated 1123 patients with seminoma and 826 patients with non-seminoma, identifying twenty-five patients who developed a late relapse. Of note, four of ten initial seminoma patients relapsed with non-seminomatous pathology, one non-seminoma patient relapsed as a seminoma, and three relapses were noted to have undifferentiated carcinomas. This observation implies the significance of biological heterogeneity within these relapses, which necessitates a comprehensive multidisciplinary approach to their management. All ten of the seminoma patients in this study and seven of the eight non-seminoma patients received salvage chemotherapy regimens. The authors caution regarding the use of salvage chemotherapy in patients considered to have a late germ cell tumor relapse. Indeed, the role for surgical resection can lead to cure, as it did for eight of their non- seminoma patients. A suggested treatment strategy includes cisplatin-based chemotherapy followed by a complete resection of residual masses, if needed.
A descriptive analysis of 122 cases of malignant GCTs assessed the characteristics of late relapse in 50 patients with pure seminoma and 72 patients with non-seminoma. The time course to late relapse was 42 mo (range 25 to 276 mo) in seminoma and 64.5 mo (range 28 to 216 mo) in non-seminoma[6]. The wide ranges seen for both disease states are intriguing and implies that specific biological factors are at play to mediate relapses such as the one presented above. Another intriguing observation from the authors was that for late recurring seminoma, 80% of cases developed from stage 1 disease while for non-seminoma, 75% of late relapses developed from primary systemic disease.
Hence, in regards to pure seminoma relapse we postulate that cellular and molecular mechanisms governing self-renewal, senescence and even extracellular contacts could play a viable role. Factors that may be at play in the evolution of a late relapse include a derangement in genes regulating these processes, thereby resulting in a persistence of seminoma cancer stem cells. Gene expression studies have clearly documented that the human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in seminoma, supporting the role for such a mechanistic evolution of late relapse[7]. Whether these cancer progenitor cells can maintain a long-term quiescence followed by transformation to a more mitogenic phenotype is a matter of debate, but is certainly possible given rare but notable reports of pure semi
Importantly, in comparing the pattern of relapses in pure seminoma, it is noted that both early (< 2 years) and late (> 2 years) relapses harbor similar anatomical distributions with few distinctions. In one retrospective review of 1060 stage I seminoma patients, it was observed that a single site of relapse with isolated para-aortic or pelvic nodes were the most predominant distributions. However, all late relapses after adjuvant radiotherapy occurred in the mediastinum, whereas early relapse sites were inguinal, supraclavicular and lung. There were no significant differences between the times to late relapse between patients who were managed with adjuvant radiation vs active surveillance[1]. This supports, to some degree, a similar biology in both early and late seminoma patients. While studies may be limited into understanding these mechanistic factors due to the small number of relapses, treatment decisions for late relapse can be made in similar fashion to early relapse. Guidance is provided in the National Comprehensive Cancer Network guidelines, with surgical excision preferred for solitary localized relapses as well as for early (< 2 years) relapses and systemic chemotherapy preferred for late (> 2 years) relapses where surgery is not feasible.
It is not well established what the ideal modality of therapy is for relapse after adjuvant radiotherapy, but as these tumors may be chemo-sensitive it is reasonable to consider systemic and localized approaches with a preference for surgery where possible. In the study previously mentioned, among 294 stage I pure seminoma patients treated with adjuvant radiotherapy after orchiectomy, 14 (5%) had a relapse after a median time of 15 mo (range 5-72 mo). Specifically, later relapses were noted after adjuvant radiotherapy in three patients, representing 1% of the entire adjuvant radiotherapy group (3/294) and 21% of the relapsed patients after adjuvant radiation (3/14). To date, our case is the only known report of relapsed stage I pure seminoma occurring approximately 20 years after adjuvant radiation.
Nine of the patients in the review by Hosni et al[1] who relapsed after adjuvant radiation went on to receive salvage chemotherapy with platinum based regimens such as etoposide and cisplatin (EP). One patient with late relapse in the mediastinum received both chemotherapy and salvage radiation, whereas four patients with isolated inguinal early relapse either received salvage radiation (3 patients) or inguinal lymph node dissection (1 patient). Importantly, none of the patients in the adjuvant radiotherapy group developed a second relapse. Similarly, among patients with relapse of seminoma managed with active surveillance, none of the patients in the late relapse group developed a second relapse after either salvage radiotherapy or chemotherapy. Although not conclusive, this suggests a similar chemo-sensitivity profile for relapsed seminoma originally managed with active surveillance or adjuvant radiation.
While treatment of these relapses has curative potential, the outcomes for seminoma tend to be better than for non-seminoma. In the retrospective study by Dieckmann et al[6], thirty-seven out of 72 (51.3%) patients with non-seminoma failed to be cured in contrast to only 6 out of 48 (12.5%) patients with seminoma who failed to be cured. The use of surgery increased the chance of cure for these patients. This again supports a distinct and perhaps more chemo-resistant tumor biology for late relapses, more-so for patients who have previously received chemotherapy. Hence, while seminomas and other chemotherapy-naive cases may respond to chemotherapy, inclusion of experienced urological surgeons in care of these patients is crucial to determine the appropriate intervention for best possible outcomes.
Pure seminoma localized to prostate gland.
The patient mentioned in this review subsequently was consented for treatment with EP and completed 4 cycles of therapy.
Response assessment with CT chest, abdomen and pelvis scans showed reduction in the size of the dominant prostate mass and resolution of the pelvic adenopathy. A restaging PET scan showed no FDG-avid lesions (Figure 4B). His case was again reviewed at the multidisciplinary tumor board, where review of imaging confirmed response to chemotherapy.
Subsequent scans continued to show complete response to therapy. At this point, the patient is over two years from completion of cancer-directed treatment for his recurrence of pure seminoma. Clinically, he is doing well without major functional limitations.
Our case represents an anomaly of late relapsed stage I pure seminoma due to its discovery after approximately 20 years since completion of adjuvant postoperative radiation therapy. He has maintained remission successfully after receipt of platinum-based chemotherapy. This observation sparks an intriguing discussion into the clinical factors, biological mechanisms, and treatment modalities surrounding this rare clinical entity. While a lack of concrete evidence for management is marred by the small number of late relapsed cases in pure seminoma, a few retrospective studies have shed light on patient outcomes after varied approaches inclusive of surgery, platinum-based chemotherapy and radiation.
The clinical vignette presented in our paper is also unique in that he has achieved a complete response to treatment with a less intensive chemotherapy regimen. Although the standard of therapy for non- pulmonary metastasis from testicular cancer generally includes 4 cycles of BEP or etoposide, ifosfamide and cisplatin, this patient has received less than that and has been cured. Factors that may help explain this include the fact that his disease was managed with adjuvant radiation alone after orchiectomy, implying chemo-sensitive disease at relapse. Nonetheless, it remains unknown whe
Studies suggested that serum levels of microRNA (miR)-371a-3p (the so-called M371 test) have a much higher sensitivity and specificity than the classic markers of testicular GCTs and are applicable toward both seminoma and non-seminoma. The M371 test outperforms the classic markers of GCT with both a sensitivity and a specificity greater than 90%. All histologic subgroups, except teratoma, express this marker. If validated, M371 can be used for detection of late recurrence without concern for excess radiation exposure over time[14]. Furthermore, in a case like ours, M371 can help to detect residual disease after completion of chemotherapy and offer early salvage surgery.
Whether his recurrence was a manifestation of true relapse from his original seminoma or a completely new primary is also unclear and not likely to be proven with the present scope of our knowledge. We have presented a literature review that details findings by other groups in regards to primary seminoma of the prostate. This clinical entity has demonstrated chemosensitivity to platinum-containing regimens and remains a plausible diagnosis to consider. Whether pure seminoma can manifest with a late relapse into the prostate is not known, but the theory of an extra-gonadal second primary tumor should be considered in this regard. Given our patient’s pure seminomatous pathology and the fact that he has responded well to chemotherapy, the biology of his initial and relapsed disease states may share a common evolutionary tumorigenesis.
In summary, further research will be required to identify the ideal follow-up strategy to detect late relapses in pure seminoma and prognostic factors including more specific biological characteristics. The identification of patients at risk for late relapse is limited by its rarity and a lack of large-scale data[1,2]. The surveillance schedule for stage I seminoma patients has generated some controversy. While some experts suggest lifelong follow-up in contrast to 5-10 years of follow-up[8-10], a more personalized approach taking into account patient characteristics with risk-adapted projections can be considered.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bi LK S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Hosni A, Warde P, Jewett M, Bedard P, Hamilton R, Moore M, Nayan M, Huang R, Atenafu EG, O'Malley M, Sweet J, Chung P. Clinical Characteristics and Outcomes of Late Relapse in Stage I Testicular Seminoma. Clin Oncol (R Coll Radiol). 2016;28:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Boujelbene N, Cosinschi A, Boujelbene N, Khanfir K, Bhagwati S, Herrmann E, Mirimanoff RO, Ozsahin M, Zouhair A. Pure seminoma: a review and update. Radiat Oncol. 2011;6:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Shahidi M, Norman AR, Dearnaley DP, Nicholls J, Horwich A, Huddart RA. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer. 2002;95:520-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Bauman GS, Venkatesan VM, Ago CT, Radwan JS, Dar AR, Winquist EW. Postoperative radiotherapy for Stage I/II seminoma: results for 212 patients. Int J Radiat Oncol Biol Phys. 1998;42:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Oldenburg J, Alfsen GC, Waehre H, Fosså SD. Late recurrences of germ cell malignancies: a population-based experience over three decades. Br J Cancer. 2006;94:820-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Dieckmann KP, Albers P, Classen J, De Wit M, Pichlmeier U, Rick O, Müllerleile U, Kuczyk M. Late relapse of testicular germ cell neoplasms: a descriptive analysis of 122 cases. J Urol. 2005;173:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Sato S, Tanaka T, Takahashi A, Sasai M, Kitamura H, Masumori N, Tsukamoto T. Late recurrence and second primary malignancy among 139 patients with germ cell tumors: long-term outcome of the disease in a single-center experience. Jpn J Clin Oncol. 2010;40:157-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Oldenburg J, Martin JM, Fosså SD. Late relapses of germ cell malignancies: incidence, management, and prognosis. J Clin Oncol. 2006;24:5503-5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Mead GM, Fossa SD, Oliver RT, Joffe JK, Huddart RA, Roberts JT, Pollock P, Gabe R, Stenning SP; MRC/EORTC seminoma trial collaborators. Randomized trials in 2466 patients with stage I seminoma: patterns of relapse and follow-up. J Natl Cancer Inst. 2011;103:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Baniel J, Foster RS, Gonin R, Messemer JE, Donohue JP, Einhorn LH. Late relapse of testicular cancer. J Clin Oncol. 1995;13:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 235] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Hashimoto T, Ohori M, Sakamoto N, Matsubayashi J, Izumi M, Tachibana M. Primary seminoma of the prostate. Int J Urol. 2009;16:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Israel A, Bosl GJ, Golbey RB, Whitmore W Jr, Martini N. The results of chemotherapy for extragonadal germ-cell tumors in the cisplatin era: the Memorial Sloan-Kettering Cancer Center experience (1975 to 1982). J Clin Oncol. 1985;3:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Dieckmann KP, Radtke A, Geczi L, Matthies C, Anheuser P, Eckardt U, Sommer J, Zengerling F, Trenti E, Pichler R, Belz H, Zastrow S, Winter A, Melchior S, Hammel J, Kranz J, Bolten M, Krege S, Haben B, Loidl W, Ruf CG, Heinzelbecker J, Heidenreich A, Cremers JF, Oing C, Hermanns T, Fankhauser CD, Gillessen S, Reichegger H, Cathomas R, Pichler M, Hentrich M, Eredics K, Lorch A, Wülfing C, Peine S, Wosniok W, Bokemeyer C, Belge G. Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J Clin Oncol. 2019;37:1412-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |