Published online Apr 24, 2021. doi: 10.5306/wjco.v12.i4.195
Peer-review started: December 24, 2020
First decision: January 11, 2021
Revised: January 23, 2021
Accepted: March 9, 2021
Article in press: March 9, 2021
Published online: April 24, 2021
Processing time: 116 Days and 18.8 Hours
Thymic epithelial tumours (TET) are rare, heterogeneous neoplasms that range from resectable indolent tumours to aggressive thymic carcinomas with a strong tendency to metastasize. The pathological diagnosis is complex, in part due to the existence of several different classification systems. The evidence base for the management of TETs is scant and mainly based on non-randomised studies and retrospective series. Consequently, the clinical management of TETs tends to be highly heterogenous, which makes it difficult to improve the evidence level. The role of technological advances in the field of radiotherapy and new systemic therapies in the treatment of TETs has received little attention to date. In the present clinical guidelines, developed by the GOECP/SEOR, we review recent developments in the diagnosis and classification of TETs. We also present a consensus-based therapeutic strategy for each disease stage that takes into consideration the best available evidence. These guidelines focus primarily on the role of radiotherapy, including recent advances, in the management of TETs. The main aim of this document is to promote the standardisation of clinical practice and lay the foundations for future studies to clarify the main unresolved questions related to the optimal management of TET.
Core Tip: Thymic epithelial tumours (TET) are rare, heterogeneous tumours. The clinical management of these tumours is largely based on studies with a low level of evidence. For this reason, it is especially important to develop guidelines to help guide the optimal approach to managing this disease. The present clinical guidelines review the main recommendations for the diagnosis and classification of TET, providing recommendations for the therapeutic approach based on the tumour stage and pathological anatomy. An important focus of these guidelines is the role of radiotherapy and novel therapies.
- Citation: Rico M, Flamarique S, Casares C, García T, López M, Martínez M, Serrano J, Blanco M, Hernanz R, de Ingunza-Barón L, Marcos FJ, Couñago F. GOECP/SEOR radiotherapy guidelines for thymic epithelial tumours. World J Clin Oncol 2021; 12(4): 195-216
- URL: https://www.wjgnet.com/2218-4333/full/v12/i4/195.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i4.195
Thymic epithelial tumours (TET) are a group of rare, heterogeneous thoracic tumours originating in the thymus gland. They account for 20% of mediastinal tumours and up to 30% of all anterior mediastinal masses in adults. The thymus gland is composed of two identical lobes involved in the process and maturation of T lymphocytes. At birth, the thymus gland weighs approximately 15 g, reaching its maximum size during puberty (40-45 g). Later, the gland undergoes a physiological involution and atrophies in adulthood, where the gland consists mainly of adipose tissue with scattered lymphocytes, weighing only 5 g in older people. Macroscopic or microscopic thymic tissue remains have been described in other locations between the hyoid bone and the diaphragm. This could be due to variations in the migration of the embryonic endodermal epithelium from the third pharyngeal pouches[1].
The World Health Organization (WHO) classification divides TETs into three histological types: Thymomas, thymic carcinomas, and thymic neuroendocrine tumours[2]. Together, these three types of TETs represent less than 1% of all neoplasms. Thymomas are the most common type of TETs, with an annual incidence ranging from 0.13 to 0.32 cases per 100000 people. By contrast, thymic carcinomas, with an annual incidence of 0.02-0.05 per 100000 people, account for only 10%-15% of TETs[3-5].
Thymomas are usually slow-growing, indolent tumours. The most common presentation is an incidental finding on imaging tests. They tend to be locally invasive. The most common route of dissemination is the pleura, pericardium, and diaphragm; extrathoracic metastases are very rare[6].
Thymic carcinomas are aggressive tumours with a worse prognosis than other TETs. Their behavior pattern is similar to other tumours that share the same histology. Most thymic carcinomas are diagnosed in advanced stages, presenting lymphatic and/or hematogenous metastases in 10% to 30% of cases[7]. Thymic neuroendocrine tumours should be considered a distinct clinical entity and its management is not covered in the present guidelines.
The incidence rate for TETs is similar in men and women. The peak incidence rate occurs in the 6th decade of life, although the age of presentation may be earlier in patients with myasthenia gravis (MG)[4,8]. Genetic risk factors, such as multiple endocrine neoplasia 1, could influence the development of thymomas and thymic carcinomas[9]. Environmental or infectious factors have not been shown to play a role in the pathogenesis of TETs. However, there does seem to be an association between thymomas and systemic diseases such as MG (the most commonly associated illness), pure red-cell aplasia, hypogammaglobulinemia, and systemic lupus erythematosus. Some hematopoietic cancers (such as leukaemia or diffuse large B-cell lymphoma), as well as other solid tumours (colon, stomach, pancreas, and thyroid) occur more frequently in patients diagnosed with thymoma[10].
These guidelines have been prepared by radiation oncologists with extensive expertise in the management of thoracic tumours. All the physicians in this expert group are members of the GOECP of the SEOR.
These guidelines are based on a review of the most relevant studies published in high-impact, peer-reviewed, indexed journals, together with a review of the clinical guidelines and recommendations produced by the major scientific societies.
The Medline database was searched for relevant systematic reviews, clinical guidelines, and primary studies up to September 2020. Search terms included ‘thymoma’, ‘thymic carcinoma’, ‘TET’, ‘thymic neoplasms’, ‘thymectomy’, ‘guidelines’, ‘International Thymic Malignancy Interest Group (ITMIG)’, ‘staging’, ‘postoperative radiotherapy (PORT)’, ‘radiation therapy’, ‘chemotherapy’, ‘proton’, ‘recurrent’, ‘reirradiation’, ‘targeted therapies’, ‘immunotherapy’.
To define the levels of evidence and degrees of recommendation, we used the classification of the Standard Operating Procedures for authors and templates for the European Society of Medical Oncology (ESMO) Clinical Practice Guidelines and ESMO-Magnitude of Clinical Benefit Scale Scores, adapted from the system of the American Society of Infectious Diseases[11,12].
The levels of evidence and grades of recommendation are defined in Table 1.
| Levels of evidence | |
| I | Evidence from at least one large RCT of good methodological quality (low potential for bias) or meta-analyses of well-conducted randomised trials without heterogeneity |
| II | Small or large RCTs with suspicion of bias (lower methodological quality) or meta-analyses of such trials with demonstrated heterogeneity |
| III | Prospective cohort studies |
| IV | Retrospective cohort studies or case-control studies |
| V | Studies without control group, case reports, expert opinions |
| Grades of recommendation | |
| A | Strong evidence for efficacy with a substantial benefit, strongly recommended |
| B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs, etc.), optional |
| D | Moderate evidence against efficacy or for adverse outcome, generally not recommended |
| E | Strong evidence against efficacy or for adverse outcome, never recommended |
The diagnostic process requires an appropriate differential diagnosis to rule out other types of anterior mediastinal masses, including lymphomas, teratomas, seminomas and non-seminomatous germ cells tumours[13]. A complete, meticulous medical history should be performed, including a comprehensive physical examination (especially neurological). The appropriate complementary examinations (imaging studies and blood tests) should also be performed.
TETs can have a variable form of presentation. Approximately one-third of patients are asymptomatic at diagnosis[10]. The most common clinical manifestations include compressive thoracic symptoms (chest pain, cough, dyspnea, dysphagia, superior vena cava syndrome) and/or symptoms derived from related autoimmune disorders, mainly MG[4]. MG is more common in type AB, B1 and B2 thymomas and much less common in thymic carcinomas[14,15]. Approximately 25%-45% of patients diagnosed with thymoma have MG, although only 15%-20% of patients with MG develop thymoma[14,16,17].
The imaging test of choice is chest computed tomography (CT) with intravenous contrast, which enables a complete evaluation of the mediastinum and pleura from the apex to the costodiaphragmatic recesses (IVA)[18]. CT imaging is comparable or superior to magnetic resonance imaging (MRI) for the diagnosis of masses in the anterior mediastinum, except for cystic lesions (IVB)[13,19].
In terms of laboratory tests, a complete blood analysis is advisable, including hemogram and biochemistry, as well as the assessment of biomarkers (beta-human chorionic gonadotropin and alpha-fetoprotein) for the differential diagnosis with germ cell tumours[13].
The use of 18F-fluorodeoxyglucose (FDG) positron-emission tomography-computed tomography (PET/CT) is not recommended for use in routine diagnostic imaging. However, this imaging modality may be useful to complete the imaging study to assess distant disease if dissemination is suspected. 18F-FDG PET-CT may be especially valuable during follow-up if there are doubts about the persistence or recurrence of the disease. A recent meta-analysis highlighted an association between types B3 and C (thymic carcinoma) and increased uptake on 18F-FDG PET-CT, although it should be noted that thymic hyperplasia can also present elevated FDG uptake[20].
Table 2 Lists the most common clinical manifestations of TET, as well as the most characteristic clinical criteria and signs to establish the differential diagnosis with other anterior mediastinal masses[4,10,13,14,16-18,20].
| Speed of development | Associated clinical manifestations | Characteristic signs | |
| Thymoma | Long course or indolent | Compressive symptoms: Chest pain, dyspnea, dysphagia, vena cava syndrome | Laboratory abnormalities: Anti-acetylcholine receptor antibodies (common if associated with MG), hypogammaglobulinemia, erythropenia, pancytopenia |
| Neurological autoimmune disorders: MG, myotonic dystrophy, limbic encephalitis, peripheral neuropathy | |||
| Hematologic autoimmune disorders: Red-cell aplasia, pernicious anemia, erythrocytosis, pancytopenia | |||
| Thymic carcinoma | Rapid growth | Collagen autoimmune disorders: Systemic lupus erythematosus, rheumatoid arthritis, Sjogren's syndrome, scleroderma | PET-CT: Thymoma types A, AB, B1-2: Low uptake. |
| Endocrine disorders: Multiple endocrine neoplasia, Cushing's syndrome, thyroiditis | PET-CT: Type B3 thymoma and thymic carcinoma: High uptake, with loss of bilobar structure of the thymus | ||
| Autoimmune deficiencies: Hypogammaglobulinemia, T-cell deficiency | |||
| Lymphoma | Fulminant onset | Dermatological disorders: Pemphigus, Lichen planus | Elevated LDH |
| “B” symptoms: Fever, weight loss, sweating | |||
| Teratoma | Slow development | Lymphadenopathy. Asymptomatic or compressive symptoms of long duration | CT: Heterogeneous mass, with cystic component and fat/calcifications |
| Germ cell tumors | Rapid development | Testicular mass | Seminoma: Elevated beta-HCG |
| No seminoma: Elevated beta-HCG and AFP | |||
| Thymic hyperplasia | Indolent | Asymptomatic | PET-CT: Elevated uptake in mass that maintains the thymic bilobar structure |
If the diagnostic tests and imaging studies suggest a high probability of a clinical diagnosis of thymoma, and if the lesion is resectable (no break in continuity with the thyroid gland), then no preoperative biopsy is necessary (IVE). In all other cases, a histological sample must be obtained, preferably by CT-guided core needle biopsy or surgical incisional biopsy by mediastinotomy or thoracoscopy (IVD). Whenever possible, transpleural access should be avoided due to the risk of pleural dissemination[21].
Thymomas are the most common TET subtype. Pathologically, they are classified into types A, A/B, B1, B2, and B3, in addition to several newer, less frequent subtypes. Use of the term “benign thymoma” is discouraged, as aggressive behaviour can never be completely ruled out. In some cases, it can be highly challenging to accurately determine the pathological type. In the 4th edition of the WHO classification system, updated in 2015, the histological criteria were refined, and parameters based on immunohistochemical characteristics introduced. These changes led to a more reproducible and robust classification, thus facilitating diagnosis of thymomas with an uncertain histology (Table 3)[2,5,22].
| Thymoma subtype | Obligatory criteria | Optional criteria |
| Type A | Occurrence of bland, spindle-shaped epithelial cells (at least focally); paucity1 or absence of immature (TdT+) T cells throughout the tumor | Polygonal epithelial cells; CD20+ epithelial cells |
| Atypical type A variant | Criteria of type A thymoma; in addition, comedo-type tumor necrosis; increased mitotic count (> 4/2 mm2); nuclear crowding | Polygonal epithelial cells; CD20+ epithelial cells |
| Type AB | Occurrence of bland, spindle-shaped epithelial cells (at least focally); abundance of immature (TdT+) T cells focally or throughout tumor | Polygonal epithelial cells; CD20+ epithelial cells |
| Type B1 | Thymus-like architecture and cytology: Abundance of immature T cells, areas of medullary differentiation (medullary islands); paucity of polygonal or dendritic epithelia cells without clustering (i.e., < 3 contiguous epithelial cells) | Hassall’s corpuscles; perivascular spaces |
| Type B2 | Increased numbers of single or clustered polygonal or dendritic epithelial cells intermingled with abundant immature T cells | Medullary islands; Hassall’s corpuscles; perivascular spaces |
| Type B3 | Sheets of polygonal slightly to moderately atypical epithelial cells; absent or rare intercellular bridges; paucity or absence of intermingled TdT+ T cells | Hassall’s corpuscles; perivascular spaces |
| MNT | Nodules of bland spindle or oval epithelial cells surrounded by an epithelial cell-free lymphoid stroma lymphoid stroma | Lymphoid follicles; monoclonal B cells and/or plasma cells (rare) |
| Metaplasticthymoma | Biphasic tumor composed of solid areas of epithelial cells in a background of bland-looking spindle cells; absence of immature T cells | Pleomorphism of epithelial cells; actin, keratin, or EMA-positive spindle cells |
| Rare others2 |
Thymic carcinomas have several histological subtypes, as follows: Squamous carcinoma (the most common), basaloid carcinoma, mucoepidermoid carcinoma, lymphoepithelioma-like carcinoma, clear cell carcinoma, sarcomatoid carcinoma, adenocarcinoma, “NUT” carcinoma, and undifferentiated carcinoma[2].
Neuroendocrine tumours include small-cell neuroendocrine carcinomas, large-cell neuroendocrine carcinomas, and carcinoids with neuroendocrine differentiation. Neuroendocrine markers include: Chromogranin-A, synaptophysin, CD-56, and neuronal-specific enolase[2].
The classification of TETs by clinical or tumour stage is based on the pathological and surgical criteria first proposed in 1978 and published by Masaoka et al[23] in 1981 and modified by Koga in 1994 (Table 4) (IIIA). The Masaoka-Koga classification system is the most widely used as it correlates pathologic and surgical findings with overall survival (OS)[23,24].
| Stage | Diagnostic criteria | 10-yr survival |
| I | Tumours encapsulated macroscopically and microscopically (without capsular invasion) | 84% (81%-86%) |
| II | A: Microscopic transcapsular invasion | 83% (79%-87%) |
| B: Macroscopic invasion of fatty tissue or pleural and/or pericardial adhesion | ||
| III | Macroscopic involvement of adjacent structures (pericardium, great vessels, or lung). A: With invasion of great vessels | 70% (64%-75%) |
| B: Without invasion of great vessels | ||
| IV | A: Pleural or pericardial spread | 42% (26%-58%) |
| B: Hematogenous or lymphogenic metastasis | 53% (32%-73%) |
The International Association for the Study of Lung Cancer (IASLC), together with the ITMIG, jointly developed a tumor-node-metastasis (TNM) classification system based on an international retrospective analysis of more than 10000 cases (Table 5)[25]. This classification system emphasises the degree of invasion of adjacent structures to determine levels of involvement. This system is highly useful to assess tumour resectability. Levels T1-3 indicate invasion of structures susceptible to surgical resection, while level T4 indicates the involvement of unresectable structures. In addition, this classification system deemphasises features not considered clinically relevant, such as whether the tumour is encapsulated or not[26].
| Stage | Description | |
| Primary tumor (T) | ||
| T1 | T1a | Encapsulated or unencapsulated, with or without extension into mediastinal fat |
| T1b | Extension into mediastinal pleura | |
| T2 | Direct invasion of the pericardium (partial or full thickness) | |
| T3 | Direct invasion of the lung, brachiocephalic vein, superior vena cava, chest wall, phrenic nerveand/or hilar (extrapericardial) pulmonary vessels | |
| T4 | Direct invasion of the aorta, main pulmonary artery, myocardium, trachea, or esophagus | |
| Lymph nodes (N) | ||
| N0 | No nodal involvement | |
| N1 | Anterior (perithymic) lymph nodes (IASLC levels 1, 3a, 6 and/or supradiaphragmatic/inferior phrenic/pericardial) | |
| N2 | Deep cervical or intrathoracic nodes (IASLC levels 2, 4, 5, 7, 10 and/or internal mammary nodes) | |
| Distant metastasis (M) | ||
| M0 | No pleural, pericardial, or distant metastases | |
| M1 | M1a | Pleural or pericardial nodules |
| M1b | Pulmonary intraparenchymal nodule or distant organ metastasis | |
| TNM stage | MASOKA-KOGA stage | |
| I | T1N0M0 | I, IIA, IIB, III |
| II | T2N0M0 | III |
| IIIA | T3N0M0 | III |
| IIIB | T4N0M0 | III |
| IVA | Any T, N0-1, M0-1a | IVA, IVB |
| IVB | Any T, N0-2, M0-1b | IVB |
The IASLC-ITMIG system has been incorporated into the 8th edition of the American Joint Committee on Cancer-TNM classification as the official classification system for TETs, offering a universal, standardised system with a consistent nomenclature. As this system becomes more widely used, it should further improve our understanding of this disease[26-30].
The management of TETs is complex and not without controversy, mainly due to the rarity and heterogeneous nature of these tumours. In addition, the recent introduction of the new staging system (the aforementioned TNM developed by the IASLC/ITMIG) may generate confusion when comparing studies that use different staging criteria[29,30]. The Masaoka-Koga stage classification system remains the most widely used system and it has been employed for this guidelines[23,24,26].
Numerous clinical guidelines and recommendations from expert working groups are available. However, the recommendations in these documents are based mainly on data from non-randomised trials and small, retrospective case series[18,31-33]. Due to the lack of high-level scientific evidence, many recommendations are based exclusively on expert opinion. In this context, all cases should be referred to a multidisciplinary tumour board for clinical decision-making.
In early-stage disease, complete surgical resection is achieved in nearly all cases, with negligible mortality rates. Consequently, surgery is the treatment of choice for stages I, IIa, and IIb (IVA).
The aim of surgical treatment should be complete resection of the tumour, the residual thymus, and the perithymic fat (IVC). In the case of thymomas, unlike thymic carcinomas, thymectomy should not include systematic lymphadenectomy due to the minimal risk of nodal metastasis. Nodal sampling could be considered in more locally-advanced stages or less favourable histologies, although there is a lack of clear evidence to support this decision.
The standard surgical approach is median sternotomy (IVA). Minimally-invasive techniques, such as video-assisted thoracoscopy or robotic surgery, are not usually recommended due to the lack of mature data in TETs[18,31]. However, in the hands of an expert surgeon, these techniques could be considered in stages I and II provided that complete oncological resection can be assured[34-36].
In stage III disease, the use of minimally-invasive surgery is discouraged due to the lack of long-term data and the difficulty of achieving complete resection in cases that require resection of the pericardium, phrenic nerve, pleura, lung parenchyma, and even vascular structures. During thymectomy, the pleural surface must be carefully checked for the presence of metastatic implants, which, if present, should be resected if possible[18,31].
In the case of extensive tumours with a high risk of close surgical margins or residual tumour, the placement of surgical clips to guide PORT is highly recommended.
Adjuvant PORT is not indicated in completely resected early-stage tumours (IIE). A key trial randomised patients with stage I disease (n = 29) to surgery or surgery plus adjuvant PORT, with significantly better 10-year survival rates in the group treated with surgery alone (92% vs 88%)[37].
An analysis by the Surveillance, Epidemiology, and End Results group evaluated the role of adjuvant radiotherapy in 901 patients with thymomas treated with surgical resection between the years 1973 and 2005. The results of that analysis showed that PORT did not provide any clinical benefit in stage I disease: The cause-specific survival rate at 5 years was 98% with surgery alone vs 91% in the group that received adjuvant PORT (P = 0.03)[38].
In stage II TETs, there is some controversy between clinical guidelines and the recommendations of expert working groups. The ESMO guidelines recommend considering radiotherapy in patients with unfavourable histologies (e.g., B3), but that recommendation is not included in other guidelines such as the Italian collaborative group for thymic malignancies (TYME) or the recommendations of a Canadian group by Falkson et al[33], who consider that there is insufficient evidence to recommend PORT in stage II disease due to the toxicity risk. Falkson and colleagues recommend discussing the advantages and disadvantages of this option with the patient. However, some groups still recommend considering PORT after complete resection in cases with unfavourable histologies (IVC)[18,32,33].
Several studies have shown that adjuvant radiotherapy provides some survival benefits in stage IIB and III disease, increasing 5-year survival rates from 66% to 76% (P = 0.01)[38]. The value of adjuvant radiotherapy in patients with higher tumour stages may be that higher stages have lower complete resection (R0) rates, with R0 rates of 47% in stage III disease and 26% in stage IV disease[39-41].
Adjuvant radiotherapy is indicated in most patients who undergo resection for stage IIB, III, and IV thymoma. However, this indication is not absolute as the evidence to support this approach is limited and the reported results are sometimes contradictory[38,42-47].
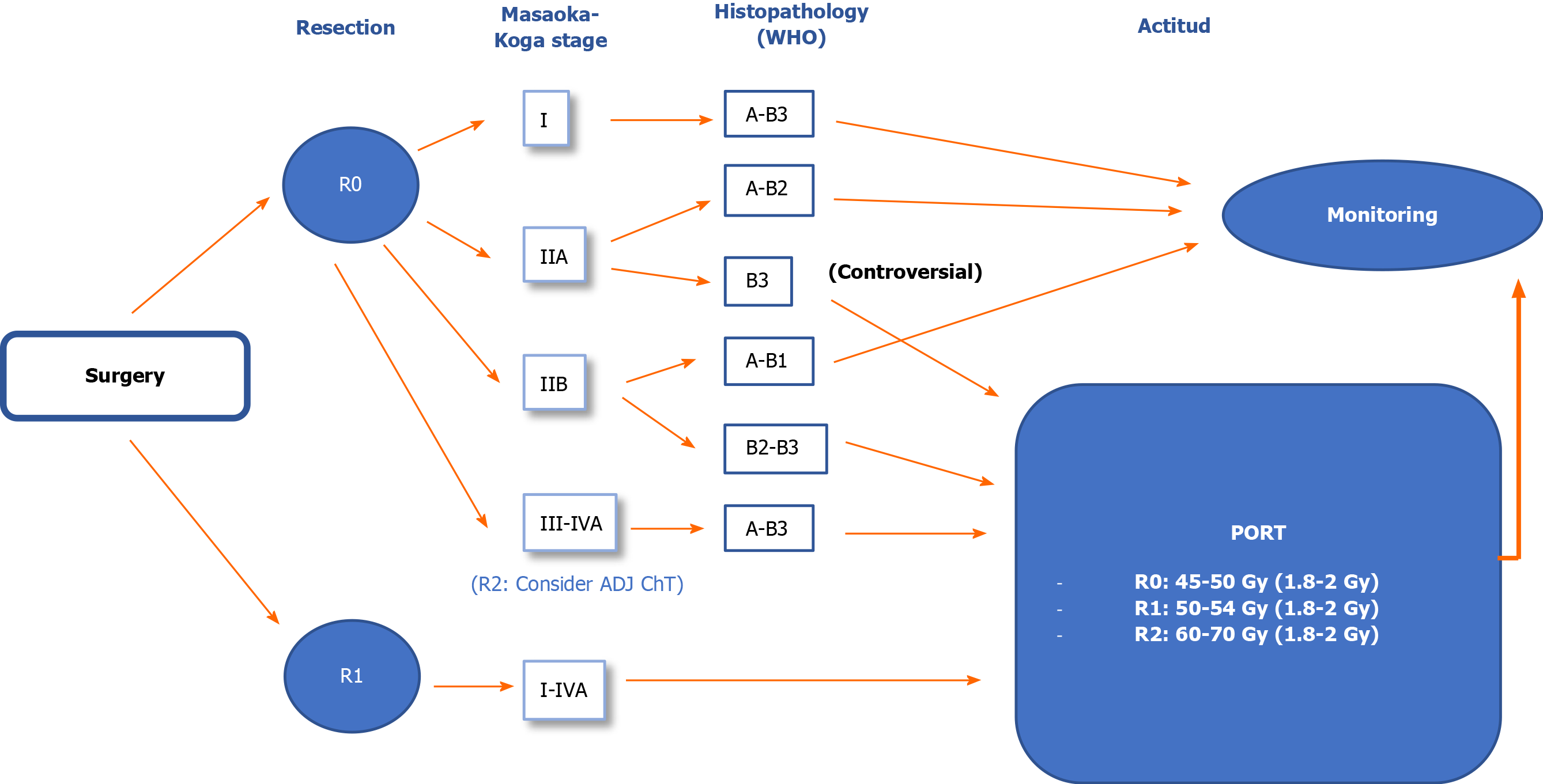
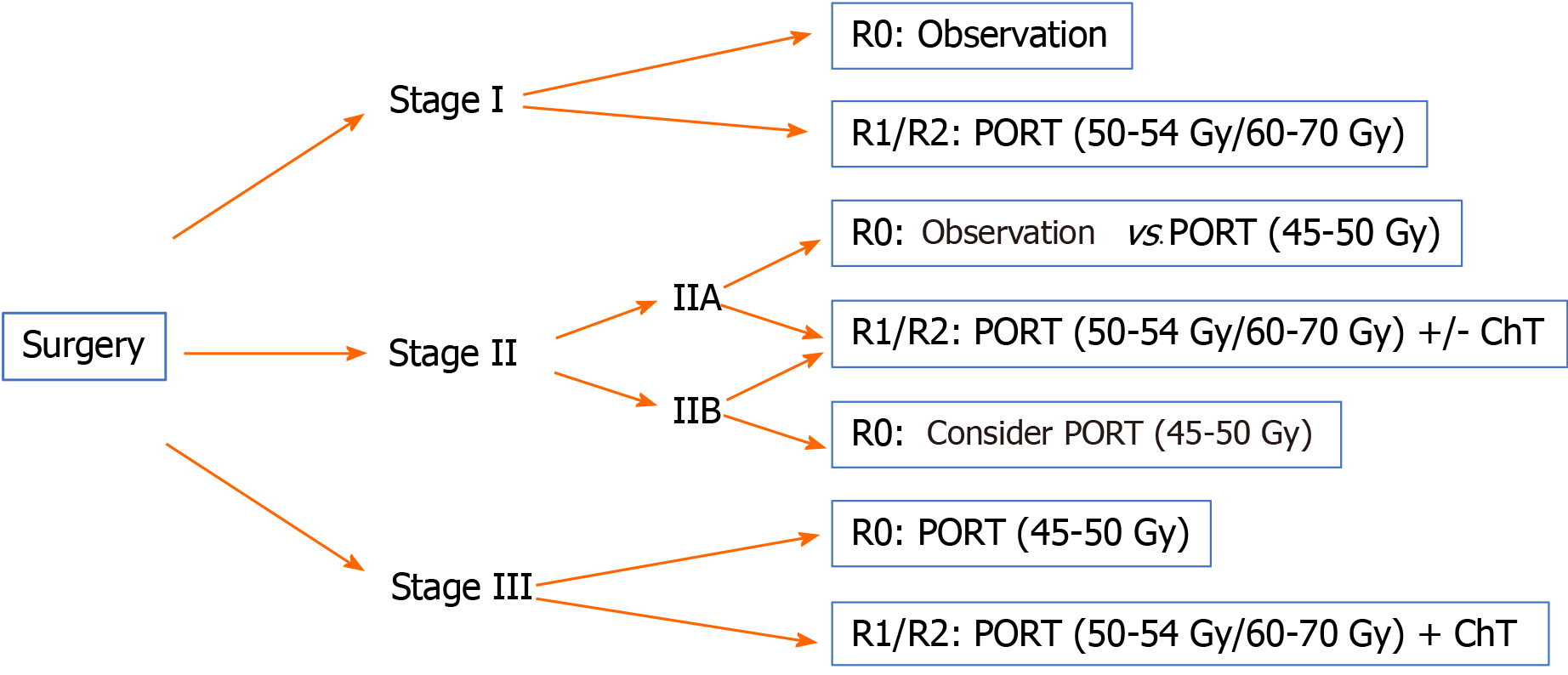
Below we provide treatment recommendations for thymomas according to disease stage using the Masaoka-Koga classification shown in Figures 1 and 2.
Stage I: Biopsy not indicated (IVE).
The treatment of choice is surgical resection (IVA).
Complete resection (R0): No adjuvant treatment (IIE)[42,43].
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage IIA: Biopsy not indicated (IVE).
The treatment of choice is surgical resection (IVA).
Complete resection (R0): A-B2: No adjuvant treatment (IVC); B3: Consider PORT (45-50 Gy) (IVC).
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage IIB: Biopsy not indicated (IVE).
The treatment of choice is surgical resection (IVA).
Complete resection (R0): A-B1: No adjuvant treatment (IVC); B2-B3/pericardium+: Consider PORT (45-50 Gy) (IVC).
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
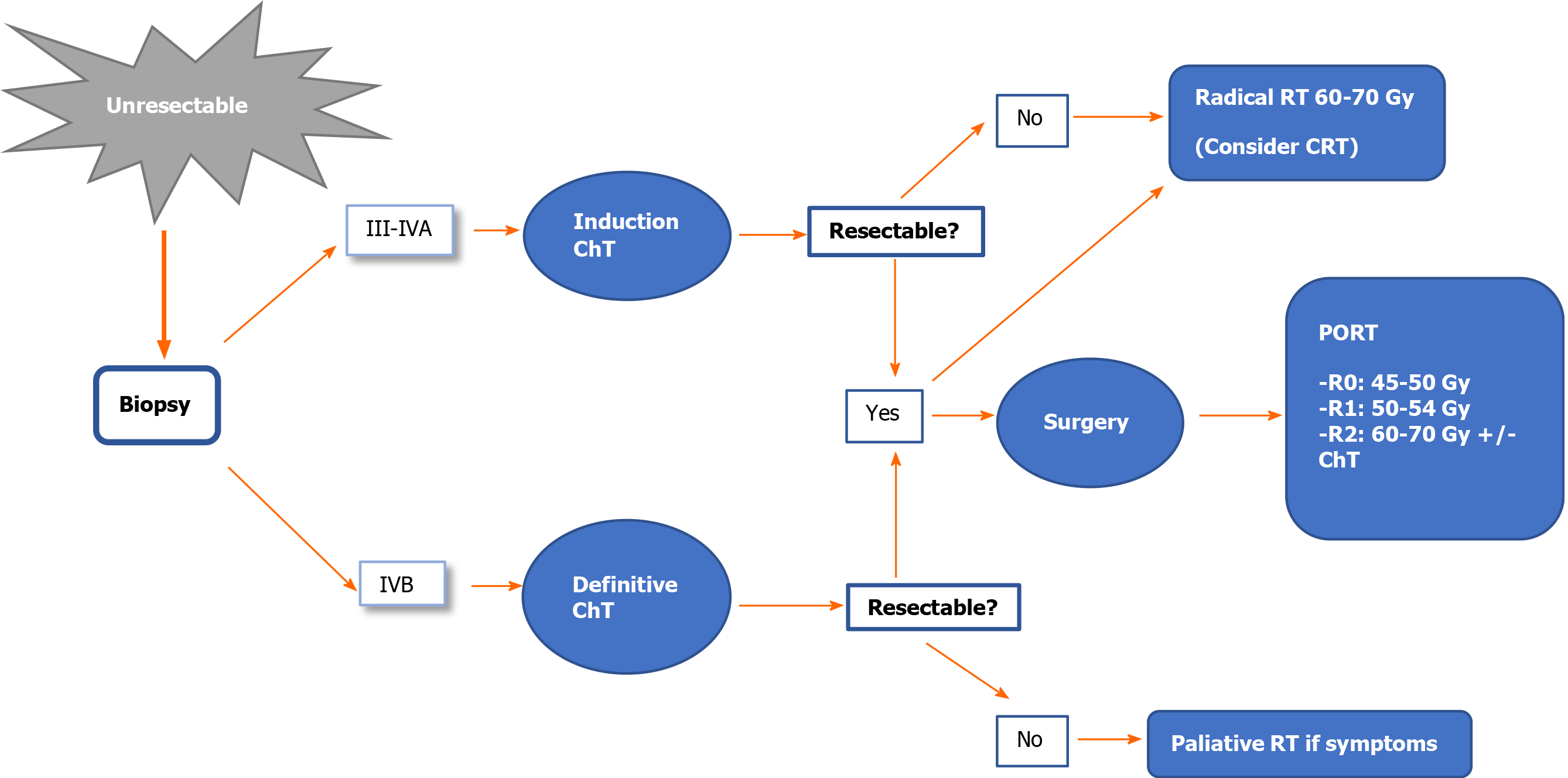
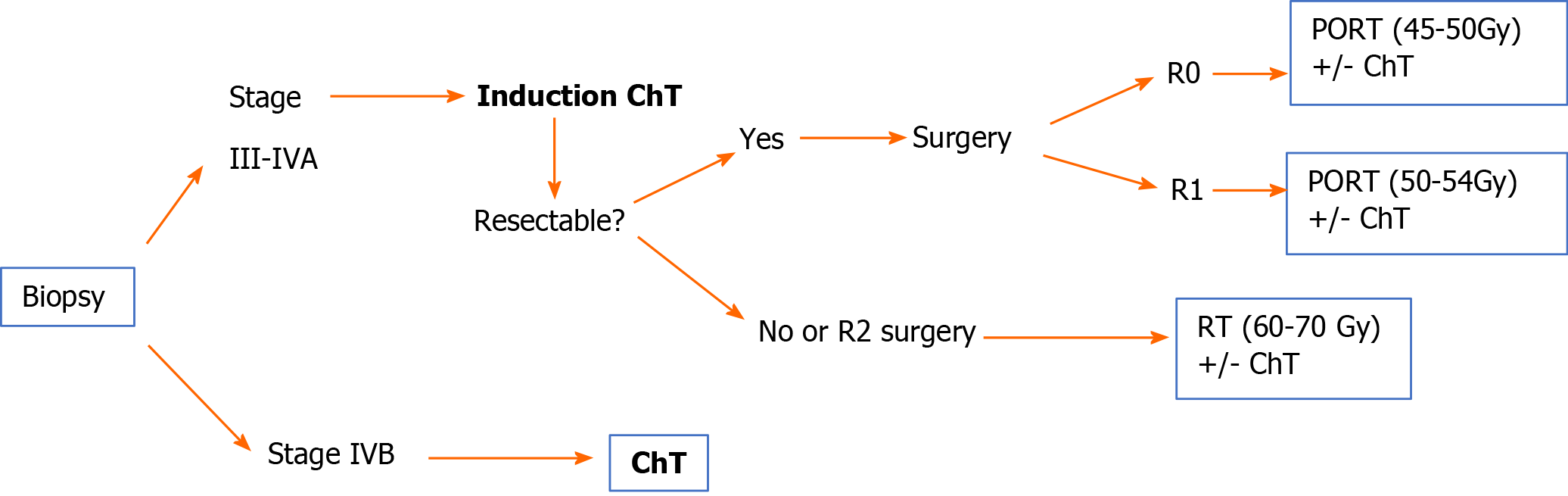
Stage III-IVA: Resectable tumour. The treatment of choice is surgical resection (IVA). After surgery, administer PORT (IVB). The recommended dose is 45-50 Gy for R0, with a boost to 54 Gy for R1 or 60-70 Gy for R2[38,48-51].
Unresectable tumour. A biopsy should be performed to confirm the diagnosis of thymoma and to rule out thymic carcinoma. Initiate anthracycline/cisplatin-based induction chemotherapy (ChT). After ChT, re-evaluate tumour resectability with imaging tests[48-51].
If the tumour is resectable after ChT: Surgical resection (IIIA) + PORT (IVB). The recommended dose is 45-50 Gy for R0; 54 Gy for R1; 60-70 Gy for R2. In cases of R2, assess concomitant platinum/etoposide-based chemoradiotherapy (CRT) (IVB).
If the tumour is unresectable after ChT: Radical radiotherapy (60-70 Gy) with or without concomitant platinum/etoposide-based ChT (IVB)[18].
Stage IVB: The treatment of choice is chemotherapy (IIIA).
If the patient is in good general condition [performance status (PS), 0-1], the use of anthracycline-based ChT regimens is recommended.
The most common regimen is capecitabine (cyclophosphamide + doxorubicin + cisplatin) for a maximum of 6 cycles (IIIB).
Imaging re-evaluation (using the same imaging tests used for diagnosis) is recommended mid-treatment (VA)[52].
In allergic patients or patients with poor PS, carboplatin-paclitaxel ChT could be considered[53].
If the tumour is resectable after ChT, assess surgery and PORT, or radical radiotherapy (IVC).
Debulking surgery is not recommended[32].
In advanced stages, palliative radiotherapy for pain relief or decompressive radiotherapy could be considered in cases with severe vascular involvement.
Thymic carcinoma is a very rare but aggressive tumour commonly diagnosed in advanced stages. These tumours have the capacity to metastasize to the lymph nodes and even to extrathoracic regions[54,55]. Thymic carcinoma has a worse prognosis than thymoma, mainly related to tumour resectability and stage. The 5-year survival rates are 90% in stage I-II disease and 28% in stages III-IV[56].
Thymic carcinoma differs from thymoma in terms of the histologic, immunohistochemical and genetic characteristics. The new WHO classification clearly identifies thymic carcinoma as a distinct entity. Determination of the immunohistochemical parameters is often required for the differential diagnosis[5]. It is important to differentiate thymic carcinomas from primary squamous cell lung cancers that have metastasized to the thymus, since these tumours sometimes have histological characteristics that are similar to those of thymic carcinoma[57].
As with thymomas, clinical staging of thymic carcinoma is essential to determine the appropriate treatment[33]. However, given the rarity of this tumour and the lack of data from prospective, randomised studies to define the optimal strategy, there is no consensus regarding the ideal therapeutic approach. Given the aggressiveness of these tumours, multimodal treatment involving multidisciplinary teams is common.
Complete surgical resection is an independent prognostic factor and a key treatment recommendation (IVA)[58]. ITMIG/IASLC guidelines define complete resection as complete resection of the tumour, residual thymus, the surrounding fat, and lymphadenectomy[59,60].
Below we provide treatment recommendations for thymic carcinoma according to disease stage using the Masaoka-Koga classification (Figures 3 and 4).
Stage I: Surgical resection is the treatment of choice (IVA).
Complete resection (R0): No additional treatment required.
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage II: Surgical resection is the treatment of choice (IVA).
Complete resection (R0): Consider PORT (45-50 Gy), especially for stage IIB (IVB).
Incomplete resection (R1): PORT (50-54 Gy) (IVB). Postoperative ChT should be considered.
Stage III-IVA: Resectable tumour. Surgical resection (IVA) followed by PORT (IB).
The recommended doses are 45-50 Gy, 54 Gy, and 60-70 Gy for R0, R1 and R2 resections, respectively.
In R0 resections, postoperative ChT is optional; in R1-R2 cases, postoperative ChT is recommended[61].
Unresectable tumour. The diagnosis of thymic carcinoma should be confirmed by biopsy. 2-4 cycles of induction ChT followed by imaging studies to reassess tumour resectability are recommended[48-51]. Various ChT schemes are available based on cisplatin (IIIA) combined with other agents such as etoposide (IIIB), doxorubicin, or cyclophosphamide[18,34].
If the tumour is resectable after ChT: Surgery (IIIA) followed by PORT (in R0, 45-50 Gy; in R1, 54 Gy) (IVB). Consider postoperative ChT.
If the tumour is unresectable after ChT or the resection is classified as R2, the treatment indication is radiotherapy (60-70 Gy) with or without concomitant ChT (IVB)[18].
Stage IVB: The treatment of choice is cisplatin-based ChT (IIIA). Various ChT regimens are available[18]. Table 6 shows the most common schemes.
| Scheme | Drugs |
| ADOC | Doxorubicin + Cisplatin + Vincristine + Cyclophosphamide |
| CAP | Cisplatin + Doxorubicin + Cyclophosphamide |
| PE | Cisplatin + Etoposide |
| VIP | Etoposide + Ifosfamide + Cisplatin |
| CODE | Cisplatin + Vincristine + Doxorubicin + Etoposide |
| Carbo-Px | Carboplatin + Paclitaxel |
| CAP-GEM | Capecitabine + Gemcitabine |
Due to advances in the molecular characterisation of TETs in recent years, our understanding of the tumour response to targeted agents has improved substantially, especially in thymic carcinomas. Several signalling pathways have been evaluated as potential targets, including KIT, vascular endothelial growth factor receptor (VEGFR), and mammalian target of rapamycin (mTOR).
In chemotherapy-refractory thymic carcinoma, the targeted agent imatinib may be a treatment option, although this drug is not recommended in patients without a KIT mutation (IVB)[62]. Sunitinib may also be an alternative in refractory tumours, regardless of KIT status (IIIA)[63]. Everolimus can also be considered in treatment-refractory cases (IIIB)[64].
Standardisation of the treatment approach is important to enable the comparison of results among different centres, which is why it is important to reach a consensus with regard to target volumes, techniques, and treatment schemes. In turn, this will allow us to develop clinical-therapeutic guidelines for TETs.
In terms of the radiotherapeutic treatment of TETs, various different approaches are used, ranging from preoperative radiotherapy alone, or radiotherapy combined with ChT, and PORT (the most common approach), in which the surgical margin status (R0, R1 or R2) is a crucial factor to select the radiation dose or the need for radical treatment, in which radiotherapy will be administered alone or concomitantly with ChT as the only treatment for the thymic tumour.
The patients should preferably be immobilized in the supine position with the neck extended and the arms above the head. A contrast-enhanced CT should be performed to help identify neighbouring structures and to assess postsurgical changes (CT slice thickness: 3-5 mm).
If four-dimensional (4D)-CT is available, acquisition of the full respiratory cycle will improve volume delimitation by determining tumour motion with respiration (internal margin), thus allowing for smaller margins than conventional CT[65]. PET/CT fusion (18F-FDG PET/CT) may be useful in certain circumstances to improve volume definition[66].
Gross tumour volume (GTV): The GTV, if present, is the identifiable macroscopic disease.
Clinical target volume (CTV): Defined as the GTV plus microscopic disease. The incorporation of preoperative imaging tests, as well as pathological findings for the surgical margins, will help define these limits.
Internal target volume (ITV): This is obtained by adding the internal motion of the lesion (internal margin) to the CTV.
Planning target volume (PTV): Defined as the ITV plus systematic uncertainties for each treatment unit (set-up margin).
Depending on the planning technique, daily verification, patient imaging control (IGRT), tumour location, and differences in routine clinical practice, the margins may vary, as follows[67]:
GTV to CTV: 0.5 to 1 cm.
CTV to PTV (conventional CT): 1 to 1.5 cm.
ITV to PTV (4D-CT without IGRT): 0.5 to 1 cm.
ITV to PTV (4D-CT and IGRT): 0.5 cm.
Given the thoracic localization of TETs, it is essential to pay careful attention to the radiation doses to the surrounding organs at risks (OARs), particularly the lungs, esophagus, heart, spinal cord, trachea, and great vessels. The proposed dose limits for the main OARs are shown in Table 7[68].
| Organ | Constraints | ||
| Spinal cord | Dmax < 45 Gy | ||
| Dmax < 40 Gy if 3 Gy/fraction | |||
| Lung (total lung minus GTV; solely total lung for postoperative cases without GTV) | Mean dose < 20 Gy | ||
| Mean dose < 8 Gy if post-pneumonectomy | |||
| RT alone | RT with chemotherapy | Neoadjuvant treatment before surgery | |
| V20 ≤ 40% | V20 ≤ 35% | V20 ≤ 30% | |
| V10 ≤ 45% | V10 ≤ 40% | ||
| V5 ≤ 65% | V5 ≤ 55% | ||
| V20 < 10% and V5 < 60% if post-pneumonectomy | |||
| Heart | Mean dose < 26 Gy | ||
| V30 ≤ 45% | |||
| Esophagus | Mean dose < 34 Gy | ||
| Dmax ≤ 80 Gy | |||
| V70 < 20% | |||
| V50 < 50% | |||
| Kidney | 20 Gy < 32% bilateral kidney | ||
| Liver | Mean dose < 30 Gy | ||
| V30 ≤ 40% | |||
When evaluating the role of radiotherapy in TETs, it is important to consider that most of the available evidence is based on large retrospective series and multicentre databases. A common limitation of these studies is high variability in diagnostic and treatment standards — including radiotherapy — which makes comparative analyses difficult. In addition, rapid technological progress in the field of radiotherapy has significantly improved treatment precision while simultaneously reducing the associated toxicity. However, the clinical impact of these advances in the treatment of TETs has received scant attention to date[69]. Nevertheless, given the mediastinal location of these tumours, experience and lessons learned from treating other thoracic cancers, such as lung cancer or malignant pleural mesothelioma, can likely be extrapolated to TETs.
Intensity-modulated radiation therapy (IMRT) is the standard radiotherapy modality today due to improved dose conformity to the target volume and lower doses to healthy tissues. If IMRT is unavailable, 3D conformal radiotherapy (3D-CRT) is an acceptable alternative[70]. A retrospective study compared IMRT/3D-CRT to conventional RT found that better survival outcomes in patients treated with IMRT/3D-CRT due to the smaller radiation field, increased precision, and lower doses to healthy organs[71].
The combination of IMRT and 4D-CT simulation could reduce radiation-induced toxicity, with better local control and OS[72,73]. Given the location of these tumours in the thorax, coordinated breathing control techniques could be beneficial in selected patients[65].
Due to the proximity of critical organs (spinal cord, heart, lungs, and esophagus), the risk of toxicity secondary to treatment — especially cardiovascular toxicity — must be carefully considered. One study assessed a consecutive cohort of 74 patients with thymoma treated with surgery plus adjuvant radiotherapy, finding that cardiovascular disease (CVD) was the primary cause of non-cancer mortality, accounting for 50% of deaths. The median time from treatment to CVD diagnosis was 101 mo. In that study, the mean heart dose was an independent risk factor for CVD[74].
It is important to underscore the increased risk of second malignant tumours (particularly esophageal and lung cancer, non-Hodgkin's lymphoma, and soft tissue sarcomas) in patients with thymoma. These second tumours may be related to the combination of immune dysregulation, and genetic or environmental factors[75-77]. This association has important implications, as irradiation of healthy tissues can promote the development of second tumours due to genetic damage; in addition, if chest reirradiation is required, there is an increased risk of toxicity due to overlapping fields.
IMRT improves dose conformity and distribution in the target volume; however, due to the physical properties of photons, it may not be possible to adequately reduce the dose to the OARs. This has led some authors to propose proton therapy as an alternative to limit the radiation dose to healthy tissues; predictive models suggest that this approach would reduce the risk of acute and late toxicity and second neoplasms[78-81].
A study published in 2018 performed a dosimetric comparison for advanced radiotherapy techniques in 10 surgically-treated patients with thymoma. Dosimetric plans were prepared for 3D-CRT, volumetric modulated arc therapy (VMAT), tomotherapy, proton therapy, and carbon ion therapy. The results showed that heavy particle radiotherapy significantly reduced radiation doses to the OARs compared to photon radiotherapy. In terms of target coverage, the best results were achieved with tomotherapy, although this also resulted in higher low doses to the OARs. The dosimetric parameters for VMAT were superior to those obtained with 3D-CRT[82]. Another study, published in 2019, compared the dosimetric characteristics of VMAT to proton therapy in 20 cases. While the two techniques yielded similar results in terms of coverage, doses to healthy organs were lower with proton therapy[81].
Despite the potential advantages of proton radiotherapy, the clinical evidence to support this approach in TETs is scant. However, one prospective series of 27 patients treated with proton therapy reported a local control rate of 100% and a 3-year OS rate of 94% at a median follow-up of 2 years. Toxicity was mild to moderate with no grade 3 or higher events[83]. Therefore, although proton beam radiation therapy has a clear dosimetric benefit, and theoretical models support a secondary clinical benefit, long-term prospective studies are still needed to compare this radiotherapy modality to other advanced radiotherapy techniques, such as tomotherapy and VMAT.
Disease recurrence is not uncommon in TETs, ranging from 7.5% to 36% of cases. However, in cases with complete surgical resection (R0), the recurrence rate is less than 15%[45,84]. In stage I and II disease, the relapse rate is only 5%-10%; by contrast, in more advanced stages, the recurrence rate is approximately 30% and some cases series have reported relapse rates > 50%[45,85].
Histology is a prognostic factor for recurrence, which can be as high as 28.6% in type B3. Thymic carcinoma is more aggressive than thymoma, with relapse rates greater than 50%[61]. Tumour size is another possible prognostic factor[86] . The mean time to recurrence is 5.5 years (range, 2 to 16)[84].
Locoregional recurrence is the most common pattern of recurrence after complete surgical resection, although thymic carcinomas tend to progress distantly[84,85,87]. There is some evidence suggesting that some thymomas can become more aggressive subtypes when they recur[87].
The management of recurrent disease in TETs should be individualized based on the resectability of the recurrent lesion(s), prior treatments received, and time interval since treatment. These patients should be included in clinical trials whenever possible.
Recurrences should be managed according to the same strategy used to treat the primary tumour (IVA). Thus, surgical salvage with complete resection is the treatment of choice for both local and regional recurrences (isolated pleural metastases)[33,88]. The available data indicate that surgery is superior to non-surgical treatment in resectable recurrences, with 10-year survival rates of around 65% after a second resection[85,87]. In patients with multiple pleural metastases, neoadjuvant ChT followed by surgical resection is worth considering when feasible[33].
In patients not previously treated with radiotherapy, the indication is the same as in newly-diagnosed tumours: PORT in patients with subtotal resections or stage III/IVA disease with complete resection (consider in stage II). Radiotherapy can be administered as definitive treatment for unresectable tumours or inoperable cases, potentially with concomitant ChT (IIIA)[18].
Historically, thoracic reirradiation has been limited to palliative doses due to the potential for toxicity related to the proximity of OARs. Cardiac toxicity merits special attention given the tumour localization, patient age, and the frequent use of cardiotoxic chemotherapy agents. However, reirradiation is increasingly being considered due to continuous technological advances in radiotherapy, which allows for increasingly conformal radiation dose delivery.
When considering reirradiation, it is essential to evaluate the patient’s clinical status, prior radiotherapy treatments (total dose, fractionation, treatment volumes, technique used, time elapsed since the initial radiation therapy) and other therapeutic alternatives. The risks and benefits of reirradiation are often equally balanced due to the potential toxicity of the dose and treatment volumes.
The available scientific evidence on reirradiation in thymic cancer is scant, although some studies have reported acceptable tolerance[89,90]. Most of the studies involving chest reirradiation have been performed in patients with lung cancer treated with 3D-CRT, IMRT, proton therapy, or stereotactic body radiation therapy. In general, radical doses achieve better disease control than palliative doses, and the best results are obtained in patients with good PS, small reirradiation volumes, recurrence outside the original radiotherapy field, and a longer interval between the two radiotherapeutic treatments[91-95]. By extrapolating these experiences to thymic tumours (in the absence of specific studies in TETs), it appears that reirradiation is safe and feasible in well-selected patients, as long as the appropriate technology is available, and the teams are experienced (IVB).
ChT is typically used to treat recurrent disease that is not suitable for surgical salvage, or to treat advanced metastatic disease. Generally, anthracycline/cisplatin-based chemotherapy is the regimen of choice. However, other first-line regimens include vincristine or cyclophosphamide. If the first-line treatment is effective, it can be re-administered in patients who develop disease progression (IVB), although patients should be closely monitored for cardiac toxicity due to the accumulated dose of anthracyclines[96]. In thymic carcinomas, carboplatin-paclitaxel ChT is the most widely-accepted first-line chemotherapy approach[31].
The use of successive lines of treatment in patients with progressive disease can prolong progression-free survival. Multiple ChT regimens are accepted, although the most common are carboplatin-paclitaxel, platinum-etoposide, capecitabine-gemcitabine, pemetrexed-etoposide, and 5-FU-leucovorin.
Patients not considered candidates for ChT but who have a positive octreoscan may benefit from treatment with octreotide, with or without prednisone (IIB)[18].
In recent years, targeted therapies have been incorporated into the therapeutic arsenal (IIIB)[97]. The identification of molecular alterations in various pathways—KIT signalling, VEGFR, and mTOR—has opened opportunities for the off-label use of targeted therapies in treatment-refractory thymic tumours.
The c-KIT receptor is overexpressed in 80% of thymic carcinomas, although it is mutated in only 9%. The efficacy of tyrosine inhibitors (TKIs) such as imatinib, sunitinib, and sorafenib has been evaluated in numerous studies. Some phase II trials involving imatinib in patients with non-mutated B3 thymoma and thymic carcinoma have found no response (IIIE)[62]. However, other studies have shown that treatment with TKIs in patients with mutated c-KIT can achieve disease control[98].
Sunitinib and sorafenib both act on the VEGFR receptor, with activity in tumour angiogenesis, and both have demonstrated efficacy in the treatment of TETs, regardless of the c-KIT receptor status[99]. Sunitinib has been evaluated in patients with TETs in two phase II trials. In one trial, which included patients with thymic carcinoma or thymoma, response rates were 26% and 6%, respectively[63]. The second trial (KOSMIC) included only patients with thymic carcinoma, achieving response rates of 22%, although disease stabilization was achieved in 70% (IIIA)[100].
Lenvatinib is a second-line TKI with activity on the VEGFR, fibroblast growth factor and platelet-derived growth factors receptors. In the phase II REMORA trial, lenvatinib significantly improved the response rate (up to 38%). At present, lenvatinib is only administered within clinical trials[101]. Other antiangiogenic agents (anti-VEGFR) such as bevacizumab or aflibercept have demonstrated efficacy in thymic tumours[18].
Zucali et al[64] conducted a phase II trial in 51 patients to evaluate everolimus, an mTOR inhibitor, reporting a disease control rate of 88%, although this was mainly disease stabilization rather than objective response. However, everolimus has been associated with the emergence of potentially severe pneumonitis. Consequently, more studies are needed to better define the indication for everolimus (IIIB)[64].
Several targeted therapies have shown promising results in patients with thymic tumours, but more studies are needed to better determine the most appropriate treatment type and sequence according to individual pathological and molecular characteristics[18]. Targeted therapies are currently used in clinical trials or as off-label drugs, mainly sunitinib or other TKI/anti-VEGFR agents and everolimus (IIB).
Several studies have evaluated the efficacy of anti-PD-1 antibodies, such as pembrolizumab. Giaccone et al[102] conducted a phase II trial involving 41 patients with thymic carcinoma, reporting a response rate of 23%, mainly disease stabilization (53%). However, these treatments induce a strong immune system response and were associated with non-negligible toxicity and potentially serious adverse effects (e.g., myocarditis, polymyositis, hepatitis, among others) in up to 15% of patients[102].
A study conducted in Korea in patients diagnosed with thymic carcinoma or thymoma reported results that were similar to those described by Giaccone and colleagues. Disease stabilization was achieved in 72% and 54% of patients with thymoma and thymic carcinoma, respectively; however, a high percentage of patients experienced autoimmune side effects[103].
The efficacy of nivolumab was assessed in phase II trial in Japan in 15 patients with thymic carcinoma. Of these, 11 achieved disease stabilization. However, none of the patients showed an objective response, which led to the premature termination of study recruitment[104].
At present, there are no prospective data on which to establish recommendations regarding the optimal post-treatment follow-up protocols for thymic tumours. European guidelines—based on the expert consensus statements and published data on the cumulative incidence of recurrences and the pattern of recurrence—recommend chest CT as the imaging technique of choice for follow-up purposes. The first CT image should be obtained 3-4 mo after surgery (VC)[18].
In patients with complete resection of a stage I/II thymoma, an annual CT scan is recommended during the first five years of follow-up and then every two years thereafter. In stage III/IV thymomas, thymic carcinoma, or after an incomplete (R1-2) resection, CT scans should be performed every 4-6 mo during the first two years and annually thereafter. Follow-up CT scans should be performed for 10-15 years after treatment.
The most recent version of the American guidelines recommend follow-up with contrast-enhanced CT every 6-12 mo for the first 2 years followed by annual CT scans for 5 (thymic carcinoma) or 10 years (thymoma). However, these guidelines do not indicate the recommended duration of follow-up. In some cases, the guidelines suggest that MRI may be a useful alternative to CT scans[33].
The emergence of second tumours and autoimmune diseases has been described during follow-up. Patients with clinical MG (or only anti-acetylcholine receptor antibody positivity) have a higher risk of myasthenic crisis in stressful situations or when certain drugs are administered (VA)[18].
TET are rare, heterogeneous tumours. The evidence base to support the diagnosis and therapeutic management of this disease is limited. Some recommendations are based mainly on data from retrospective or non-randomised studies, or even expert opinions (due to the lack of evidence). As a result, disease management is often highly heterogeneous. In recent years, several scientific societies and working groups have sought to standardise the management of TETs. The recent development of a universal, standardised classification system with a consistent nomenclature (IASLC-ITMIG) is expected to reduce variability in the management of these tumours. In addition, new guidelines have been published to provide a clear, schematic approach to treatment. Although these guidelines differ on certain points due to the lack of definitive evidence (e.g., the role of radiotherapy in stage II disease), the overall trend is towards the convergence of recommendations, which should facilitate the performance of multicentre studies and, in turn, raise the level of evidence for the management of TETs.
The present document was developed by the GOECP/SEOR to help unify the management of TET in Spain and to lay the foundations for the creation of a stable working group. We believe that it is crucial to prioritise closer collaboration between centres and the relevant scientific societies in order to carry out prospective studies and randomised clinical trials, particularly to resolve the most controversial aspects of this disease management.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawabata H S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Zdrojewicz Z, Pachura E, Pachura P. The Thymus: A Forgotten, But Very Important Organ. Adv Clin Exp Med. 2016;25:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, Jaffe ES, Kurrer MO, Marom EM, Moreira AL, Mukai K, Orazi A, Ströbel P. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol. 2015;10:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 417] [Article Influence: 46.3] [Reference Citation Analysis (1)] |
| 3. | Kalhor N, Moran CA. Thymoma: current concepts. Oncology (Williston Park). 2012;26:975-981. [PubMed] |
| 4. | Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5:S260-S265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 5. | Marx A, Ströbel P, Badve SS, Chalabreysse L, Chan JK, Chen G, de Leval L, Detterbeck F, Girard N, Huang J, Kurrer MO, Lauriola L, Marino M, Matsuno Y, Molina TJ, Mukai K, Nicholson AG, Nonaka D, Rieker R, Rosai J, Ruffini E, Travis WD. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9:596-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 6. | Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, Levasseur P. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 305] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Ruffini E, Detterbeck F, Van Raemdonck D, Rocco G, Thomas P, Weder W, Brunelli A, Guerrera F, Keshavjee S, Altorki N, Schützner J, Arame A, Spaggiari L, Lim E, Toker A, Venuta F; European Society of Thoracic Surgeons Thymic Working Group. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol. 2014;9:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Hsu CH, Chan JK, Yin CH, Lee CC, Chern CU, Liao CI. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS One. 2019;14:e0227197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Kojima Y, Ito H, Hasegawa S, Sasaki T, Inui K. Resected invasive thymoma with multiple endocrine neoplasia type 1. Jpn J Thorac Cardiovasc Surg. 2006;54:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 348] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | ESMO Guidelines Committee. Standard Operating Procedures (SOPs) for Authors and templates for ESMO Clinical Practice Guidelines (CPGs) and ESMO-MCBS Scores. 2020; 11. Available from: https://www.esmo.org/content/download/77789/1426712/file/ESMO-Clinical-Practice-Guidelines-Standard-Operating-Procedures.pdf. |
| 12. | Dykewicz CA; Centers for Disease Control and Prevention (U. S.); Infectious Diseases Society of America; American Society of Blood and Marrow Transplantation. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2001;33:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 467] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol. 2014;9:S102-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol. 2014;9:S143-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Chalabreysse L, Etienne-Mastroianni B, Adeleine P, Cordier JF, Greenland T, Thivolet-Bejui F. Thymic carcinoma: a clinicopathological and immunohistological study of 19 cases. Histopathology. 2004;44:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Romi F. Thymoma in myasthenia gravis: from diagnosis to treatment. Autoimmune Dis. 2011;2011:474512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol. 2011;8:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S; ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v40-v55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 19. | Seki S, Koyama H, Ohno Y, Nishio M, Takenaka D, Maniwa Y, Itoh T, Nishimura Y, Sugimura K. Diffusion-weighted MR imaging vs. multi-detector row CT: Direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol. 2014;83:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Treglia G, Sadeghi R, Giovanella L, Cafarotti S, Filosso P, Lococo F. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? Lung Cancer. 2014;86:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | De Montpréville VT. [Thymomas and thymic carcinomas]. Rev Mal Respir. 2010;27:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Wu J, Fang W, Chen G. The enlightenments from ITMIG Consensus on WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Dis. 2016;8:738-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, Shimosato Y. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 340] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6:S1698-S1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 26. | Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, Filosso PL, Frazier AA, Giaccone G, Huang J, Kim J, Kondo K, Lucchi M, Marino M, Marom EM, Nicholson AG, Okumura M, Ruffini E, Van Schil P; Staging and Prognostic Factors Committee; Members of the Advisory Boards; Participating Institutions of the Thymic Domain. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S65-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 27. | Detterbeck FC. Clinical implication of the new TNM classification of thymic malignancies. J Thorac Dis. 2018;10:S2692-S2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ried M, Eicher MM, Neu R, Sziklavari Z, Hofmann HS. Evaluation of the new TNM-staging system for thymic malignancies: impact on indication and survival. World J Surg Oncol. 2017;15:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Nicholson AG, Detterbeck FC, Marino M, Kim J, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, Filosso PL, Giaccone G, Huang J, Kondo K, Lucchi M, Marom EM, Okumura M, Ruffini E, Van Schil P; Staging and Prognostic Factors Committee; Members of the Advisory Boards; Participating Institutions of the Thymic Domain. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S73-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Kondo K, Van Schil P, Detterbeck FC, Okumura M, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, Filosso PL, Giaccone G, Huang J, Kim J, Lucchi M, Marino M, Marom EM, Nicholson AG, Ruffini E; Staging and Prognostic Factors Committee; Members of the Advisory Boards; Participating Institutions of the Thymic Domain. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9:S81-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | NCCN Clinical Practice Guidelines in Oncology. Thymic malignancies. 2020; 1. Available from: https://www.nccn.org. |
| 32. | Imbimbo M, Ottaviano M, Vitali M, Fabbri A, Leuzzi G, Fiore M, Franceschini D, Pasello G, Perrino M, Schiavon M, Pruneri G, Dei Tos AP, Sangalli C, Garassino MC, Berardi R, Alessi A, Calareso G, Petrini I, Scorsetti M, Scotti V, Rosso L, Rea F, Pastorino U, Casali PG, Ramella S, Ricardi U, Abate-Daga L, Torri V, Trama A, Palmieri G, Marino M, Zucali PA; TYME network collaborators. Best practices for the management of thymic epithelial tumors: A position paper by the Italian collaborative group for ThYmic MalignanciEs (TYME). Cancer Treat Rev. 2018;71:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 33. | Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, Yu E, Smith CA, McNair S, Ung YC, Evans WK; Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 34. | Yang Y, Dong J, Huang Y. Thoracoscopic thymectomy vs open thymectomy for the treatment of thymoma: A meta-analysis. Eur J Surg Oncol. 2016;42:1720-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Agatsuma H, Yoshida K, Yoshino I, Okumura M, Higashiyama M, Suzuki K, Tsuchida M, Usuda J, Niwa H. Video-Assisted Thoracic Surgery Thymectomy Versus Sternotomy Thymectomy in Patients With Thymoma. Ann Thorac Surg. 2017;104:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Xie A, Tjahjono R, Phan K, Yan TD. Video-assisted thoracoscopic surgery vs open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg. 2015;4:495-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 37. | Zhang H, Lu N, Wang M, Gu X, Zhang D. Postoperative radiotherapy for stage I thymoma: a prospective randomized trial in 29 cases. Chin Med J (Engl). 1999;112:136-138. [PubMed] |
| 38. | Forquer JA, Rong N, Fakiris AJ, Loehrer PJ Sr, Johnstone PA. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys. 2010;76:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg. 2006;81:2328-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Maggi G, Giaccone G, Donadio M, Ciuffreda L, Dalesio O, Leria G, Trifiletti G, Casadio C, Palestro G, Mancuso M. Thymomas. A review of 169 cases, with particular reference to results of surgical treatment. Cancer. 1986;58:765-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Singhal S, Shrager JB, Rosenthal DI, LiVolsi VA, Kaiser LR. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg. 2003;76:1635-41; discussion 1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Haniuda M, Miyazawa M, Yoshida K, Oguchi M, Sakai F, Izuno I, Sone S. Is postoperative radiotherapy for thymoma effective? Ann Surg. 1996;224:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Rena O, Papalia E, Oliaro A, Ruffini E, Filosso P, Novero D, Maggi G, Casadio C. Does adjuvant radiation therapy improve disease-free survival in completely resected Masaoka stage II thymoma? Eur J Cardiothorac Surg. 2007;31:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Ogawa K, Uno T, Toita T, Onishi H, Yoshida H, Kakinohana Y, Adachi G, Itami J, Ito H, Murayama S. Postoperative radiotherapy for patients with completely resected thymoma: a multi-institutional, retrospective review of 103 patients. Cancer. 2002;94:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Korst RJ, Kansler AL, Christos PJ, Mandal S. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Omasa M, Date H, Sozu T, Sato T, Nagai K, Yokoi K, Okamoto T, Ikeda N, Tanaka F, Maniwa Y; Japanese Association for Research on the Thymus. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer. 2015;121:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Curran WJ Jr, Kornstein MJ, Brooks JJ, Turrisi AT 3rd. Invasive thymoma: the role of mediastinal irradiation following complete or incomplete surgical resection. J Clin Oncol. 1988;6:1722-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 189] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Hamaji M, Shah RM, Ali SO, Bettenhausen A, Lee HS, Burt BM. A Meta-Analysis of Postoperative Radiotherapy for Thymic Carcinoma. Ann Thorac Surg. 2017;103:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Lim YJ, Kim E, Kim HJ, Wu HG, Yan J, Liu Q, Patel S. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | D'Angelillo RM, Trodella L, Ramella S, Cellini N, Balducci M, Mantini G, Cellini F, Ciresa M, Fiore M, Evoli A, Sterzi S, Russo P, Grozio A, Cesario A, Granone P. Novel prognostic groups in thymic epithelial tumors: assessment of risk and therapeutic strategy selection. Int J Radiat Oncol Biol Phys. 2008;71:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Rimner A, Yao X, Huang J, Antonicelli A, Ahmad U, Korst RJ, Detterbeck F, Gomez DR. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol. 2016;11:1785-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | Loehrer PJ Sr, Kim K, Aisner SC, Livingston R, Einhorn LH, Johnson D, Blum R. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol. 1994;12:1164-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 197] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 53. | Hirai F, Yamanaka T, Taguchi K, Daga H, Ono A, Tanaka K, Kogure Y, Shimizu J, Kimura T, Fukuoka J, Iwamoto Y, Sasaki H, Takeda K, Seto T, Ichinose Y, Nakagawa K, Nakanishi Y; West Japan Oncology Group. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol. 2015;26:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, Comber H, Dimitrova N, Leinonen MK, Siesling S, van der Zwan JM, Van Eycken L, Visser O, Žakelj MP, Anderson LA, Bella F, Kaire I, Otter R, Stiller CA, Trama A; RARECAREnet working group. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18:1022-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 55. | Travis WD, Brambilla A, Burke AP, Marx A, Nicholson AG. Clasification of tumours of the lung, pleura, thymus and heart. World Health Organization. 4th ed. 2015. |
| 56. | Lee CY, Bae MK, Park IK, Kim DJ, Lee JG, Chung KY. Early Masaoka stage and complete resection is important for prognosis of thymic carcinoma: a 20-year experience at a single institution. Eur J Cardiothorac Surg. 2009;36:159-62; discussion 163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Evans AG, French CA, Cameron MJ, Fletcher CD, Jackman DM, Lathan CS, Sholl LM. Pathologic characteristics of NUT midline carcinoma arising in the mediastinum. Am J Surg Pathol. 2012;36:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Ahmad U, Yao X, Detterbeck F, Huang J, Antonicelli A, Filosso PL, Ruffini E, Travis W, Jones DR, Zhan Y, Lucchi M, Rimner A. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015; 149: 95-100, 101.e1-101. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Hwang Y, Park IK, Park S, Kim ER, Kang CH, Kim YT. Lymph Node Dissection in Thymic Malignancies: Implication of the ITMIG Lymph Node Map, TNM Stage Classification, and Recommendations. J Thorac Oncol. 2016;11:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Bhora FY, Chen DJ, Detterbeck FC, Asamura H, Falkson C, Filosso PL, Giaccone G, Huang J, Kim J, Kondo K, Lucchi M, Marino M, Marom EM, Nicholson AG, Okumura M, Ruffini E, Van Schil P; Staging and Prognostic Factors Committee; Advisory Boards. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol. 2014;9:S88-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878-84; discussion 884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 62. | Giaccone G, Rajan A, Ruijter R, Smit E, van Groeningen C, Hogendoorn PC. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol. 2009;4:1270-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, Lee MJ, Lee S, Ling A, Spittler AJ, Carter CA, Guha U, Wang Y, Szabo E, Meltzer P, Steinberg SM, Trepel JB, Loehrer PJ, Giaccone G. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 64. | Zucali PA, De Pas T, Palmieri G, Favaretto A, Chella A, Tiseo M, Caruso M, Simonelli M, Perrino M, De Vincenzo F, Toffalorio F, Damiano V, Pasello G, Garbella E, Ali M, Conforti F, Ottaviano M, Cioffi A, De Placido S, Giordano L, Bertossi M, Destro A, Di Tommaso L, Santoro A. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol. 2018;36:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 65. | Gomez D, Komaki R. Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol. 2010;5:S336-S343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Fuller CD, Ramahi EH, Aherne N, Eng TY, Thomas CR Jr. Radiotherapy for thymic neoplasms. J Thorac Oncol. 2010;5:S327-S335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Gomez D, Komaki R, Yu J, Ikushima H, Bezjak A; International Thymic Malignancy Interest Group. [Radiation therapy definitions and reporting guidelines for thymic malignancies]. Zhongguo Fei Ai Za Zhi. 2014;17:110-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1173] [Article Influence: 78.2] [Reference Citation Analysis (1)] |
| 69. | Willmann J, Rimner A. The expanding role of radiation therapy for thymic malignancies. J Thorac Dis. 2018;10:S2555-S2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Gomez D, Komaki R, Yu J, Ikushima H, Bezjak A. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol. 2011;6:S1743-S1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 71. | Fan C, Feng Q, Chen Y, Zhai Y, Zhou Z, Chen D, Xiao Z, Zhang H, Li J, Hui Z, Liang J, Lv J, Mao Y, Wang L, He J. Postoperative radiotherapy for completely resected Masaoka stage III thymoma: a retrospective study of 65 cases from a single institution. Radiat Oncol. 2013;8:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Liao ZX, Komaki RR, Thames HD Jr, Liu HH, Tucker SL, Mohan R, Martel MK, Wei X, Yang K, Kim ES, Blumenschein G, Hong WK, Cox JD. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 73. | Shaikh F, Zauderer MG, von Reibnitz D, Wu AJ, Yorke ED, Foster A, Shi W, Zhang Z, Adusumilli PS, Rosenzweig KE, Krug LM, Rusch VW, Rimner A. Improved Outcomes with Modern Lung-Sparing Trimodality Therapy in Patients with Malignant Pleural Mesothelioma. J Thorac Oncol. 2017;12:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Liao J, Liu T, Zhang H, Cai F, Chen J, Dang J. The role of postoperative radiation therapy for completely resected stage III thymoma and effect of higher heart radiation dose on risk of cardiovascular disease: A retrospective cohort study. Int J Surg. 2018;53:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Kumar V, Garg M, Goyal A, Chaudhary N, Soni P, Binod Chandra A. Changing pattern of secondary cancers among patients with malignant thymoma in the USA. Future Oncol. 2018;14:1943-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Pan CC, Chen PC, Wang LS, Chi KH, Chiang H. Thymoma is associated with an increased risk of second malignancy. Cancer. 2001;92:2406-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Travis LB, Boice JD Jr, Travis WD. Second primary cancers after thymoma. Int J Cancer. 2003;107:868-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Zhu HJ, Hoppe BS, Flampouri S, Louis D, Pirris J, Nichols RC, Henderson RH, Mercado CE. Rationale and early outcomes for the management of thymoma with proton therapy. Transl Lung Cancer Res. 2018;7:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Parikh RR, Rhome R, Hug E, Tsai H, Cahlon O, Chon B, Goenka A. Adjuvant Proton Beam Therapy in the Management of Thymoma: A Dosimetric Comparison and Acute Toxicities. Clin Lung Cancer. 2016;17:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Vogel J, Lin L, Litzky LA, Berman AT, Simone CB 2nd. Predicted Rate of Secondary Malignancies Following Adjuvant Proton Versus Photon Radiation Therapy for Thymoma. Int J Radiat Oncol Biol Phys. 2017;99:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Franceschini D, Cozzi L, Loi M, Franzese C, Reggiori G, Mancosu P, Clivio A, Fogliata A, Scorsetti M. Volumetric modulated arc therapy vs intensity-modulated proton therapy in the postoperative irradiation of thymoma. J Cancer Res Clin Oncol. 2020;146:2267-2276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Haefner MF, Verma V, Bougatf N, Mielke T, Tonndorf-Martini E, König L, Rwigema JM, Simone CB 2nd, Uhlmann L, Eichhorn F, Winter H, Grosch H, Haberer T, Herfarth K, Debus J, Rieken S. Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol. 2018;57:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Vogel J, Berman AT, Lin L, Pechet TT, Levin WP, Gabriel P, Khella SL, Singhal S, Kucharczuk JK, Simone CB 2nd. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol. 2016;118:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Xu C, Feng QF, Fan CC, Zhai YR, Chen YD, Zhang HX, Xiao ZF, Liang J, Chen DF, Zhou ZM, Wang LH, He J. Patterns and predictors of recurrence after radical resection of thymoma. Radiother Oncol. 2015;115:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Mizuno T, Okumura M, Asamura H, Yoshida K, Niwa H, Kondo K, Horio H, Matsumura A, Yokoi K; Japanese Association for Research on Thymus. Surgical management of recurrent thymic epithelial tumors: a retrospective analysis based on the Japanese nationwide database. J Thorac Oncol. 2015;10:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Wright CD, Wain JC, Wong DR, Donahue DM, Gaissert HA, Grillo HC, Mathisen DJ. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg. 2005;130:1413-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 87. | Sandri A, Cusumano G, Lococo F, Alifano M, Granone P, Margaritora S, Cesario A, Oliaro A, Filosso P, Regnard JF, Ruffini E. Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol. 2014;9:1796-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Bott MJ, Wang H, Travis W, Riely GJ, Bains M, Downey R, Rusch V, Huang J. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg. 2011;92:1984-91; discussion 1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Matsuguma H, Furuta M, Tsukiyama I, Kamiya N, Sawafuji M, Yokoi K. Endobronchial brachytherapy for recurrent thymoma showing endobronchial polypoid growth. Am J Clin Oncol. 1999;22:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Shah AP, Strauss JB, Kirk MC, Chen SS, Kroc TK, Zusag TW. Upright 3D treatment planning using a vertical CT. Med Dosim. 2009;34:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Wu KL, Jiang GL, Qian H, Wang LJ, Yang HJ, Fu XL, Zhao S. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. Int J Radiat Oncol Biol Phys. 2003;57:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | McAvoy S, Ciura K, Wei C, Rineer J, Liao Z, Chang JY, Palmer MB, Cox JD, Komaki R, Gomez DR. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys. 2014;90:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 93. | Badiyan SN, Rutenberg MS, Hoppe BS, Mohindra P, Larson G, Hartsell WF, Tsai H, Zeng J, Rengan R, Glass E, Katz S, Vargas C, Feigenberg SJ, Simone CB 2nd. Clinical Outcomes of Patients With Recurrent Lung Cancer Reirradiated With Proton Therapy on the Proton Collaborative Group and University of Florida Proton Therapy Institute Prospective Registry Studies. Pract Radiat Oncol. 2019;9:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | McAvoy SA, Ciura KT, Rineer JM, Allen PK, Liao Z, Chang JY, Palmer MB, Cox JD, Komaki R, Gomez DR. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol. 2013;109:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 95. | Ho JC, Nguyen QN, Li H, Allen PK, Zhang X, Liao Z, Zhu XR, Gomez D, Lin SH, Gillin M, Komaki R, Hahn S, Chang JY. Reirradiation of thoracic cancers with intensity modulated proton therapy. Pract Radiat Oncol. 2018;8:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Lara PN Jr, Bonomi PD, Faber LP. Retreatment of recurrent invasive thymoma with platinum, doxorubicin, and cyclophosphamide. Chest. 1996;110:1115-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Merveilleux du Vignaux C, Dansin E, Mhanna L, Greillier L, Pichon E, Kerjouan M, Clément-Duchêne C, Mennecier B, Westeel V, Robert M, Quantin X, Zalcman G, Thiberville L, Lena H, Molina T, Calcagno F, Fournel P, Mazières J, Besse B, Girard N. Systemic Therapy in Advanced Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol. 2018;13:1762-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 98. | Hirai F, Edagawa M, Shimamatsu S, Toyozawa R, Toyokawa G, Nosaki K, Yamaguchi M, Seto T, Twakenoyama M, Ichinose Y. c-kit mutation-positive advanced thymic carcinoma successfully treated as a mediastinal gastrointestinal stromal tumor: A case report. Mol Clin Oncol. 2016;4:527-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Girard N. Targeted therapies for thymic malignancies. Thorac Surg Clin. 2011;21:115-123, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Kim SH, Kim YJ, Ock C, Kim M, Keam B, Kim TM, Kim D, Heo D, Lee JS. OA11.05 Phase II Study of Sunitinib in Patients with Thymic Carcinoma Previously Treated with Platinum-Based Chemotherapy (KOSMIC Trial). J Thorac Oncol. 2018;13:346-347. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Itoh S, Satouchi M, Sato J, Okuma Y, Niho S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, Nagasaka Y, Kawasaki M, Yamada T, Kuchiba A, Yamamoto N. Durable anti-tumor activity of the multi-targeted inhibitor lenvatinib in patients with advanced or metastatic thymic carcinoma: Preliminary results from a multicenter phase II (REMORA) trial. Ann Oncol. 2019;30: Suppl 5:748-749. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, Manning M, Mogg R, Blumenschein WM, Tan MT, Subramaniam DS, Liu SV, Kaplan IM, McCutcheon JN. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 103. | Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol. 2019;37:2162-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 104. | Katsuya Y, Horinouchi H, Seto T, Umemura S, Hosomi Y, Satouchi M, Nishio M, Kozuki T, Hida T, Sukigara T, Nakamura K, Kuchiba A, Ohe Y. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer. 2019;113:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |