Published online Sep 24, 2020. doi: 10.5306/wjco.v11.i9.747
Peer-review started: April 3, 2020
First decision: April 18, 2020
Revised: August 5, 2020
Accepted: August 24, 2020
Article in press: August 24, 2020
Published online: September 24, 2020
Processing time: 168 Days and 19.1 Hours
The adjuvant treatment for patients with resected pancreatic cancer (PC) is not yet standardized. Because the prognosis differs according to the American Joint Committee on Cancer (AJCC) stage, a tailored approach to establish more aggressive treatment plans in high-risk patients is necessary. However, studies comparing the efficacy of adjuvant treatment modalities according to the AJCC stage are largely lacking.
To compare the efficacy of chemotherapy and chemoradiation therapy according to AJCC 8th staging system in patients with PC who underwent surgical resection.
A total of 335 patients who underwent surgical resection and adjuvant treatment for PC were included. Patients were divided into three groups: Chemoradiation therapy (CRT) group, systemic chemotherapy (SCT) group and combined treatment of chemoradiation plus chemotherapy therapy (CRT-SCT) group. The primary outcomes were differences in overall survival (OS) between the three groups. The secondary outcomes were differences in recurrence-free survival, recurrence pattern and adverse events between the three groups.
Patients received CRT (n = 65), SCT (n = 62) and CRT-SCT (n = 208). Overall median OS was 33.3 mo (95% confidence interval (CI): 27.4-38.6). In patients with stage I/II, the median OS was 27.0 mo (95%CI: 2.06-89.6) in the CRT group, 35.8 mo (95%CI: 26.9-NA) in the SCT group and 38.6 mo (95%CI: 33.3-55.7) in the CRT-SCT group. Among them, there was no significant difference in OS between the three groups. In 59 patients with stage III, median OS in the SCT group [19.0 mo (95%CI: 12.6-NA)] and the CRT-SCT group [23.4 mo (95%CI: 22.0-44.4)] was significantly longer than that in the CRT group [17.7 mo (95%CI: 6.8-NA); P = 0.011 and P < 0.001, respectively]. There were no significant differences in incidence of locoregional and distant recurrences between the three groups (P = 0.158 and P = 0.205, respectively). Incidences of grade 3 or higher hematologic adverse events were higher in the SCT and CRT-SCT groups than in the CRT group.
SCT and CRT-SCT showed significantly longer OS and recurrence-free survival than CRT in patients with AJCC stage III, while there was no significant difference in OS between the CRT, SCT and CRT-SCT groups in patients with AJCC stage I/II. Different adjuvant therapy according to AJCC stage can be applied in patients with PC.
Core Tip: We retrospectively reviewed 335 patients who underwent surgical resection and adjuvant treatment for pancreatic cancer. Patients were divided into three groups; chemoradiation therapy (CRT), systemic chemotherapy (SCT) and CRT-SCT. In patients with stage III cancer, median overall survival in the SCT and CRT-SCT groups was significantly longer than that in the CRT group. On the other hand, there was no significant difference in overall survival between CRT, SCT and CRT-SCT in those with stage I/II. SCT with or without CRT might be a reasonable choice preferentially over CRT in patients with American Joint Committee on Cancer stage III.
- Citation: You MS, Ryu JK, Huh G, Chun JW, Paik WH, Lee SH, Kim YT. Comparison of efficacy between adjuvant chemotherapy and chemoradiation therapy for pancreatic cancer: AJCC stage-based approach. World J Clin Oncol 2020; 11(9): 747-760
- URL: https://www.wjgnet.com/2218-4333/full/v11/i9/747.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i9.747
Worldwide, pancreatic cancer occurs in 458918 people annually[1]. The 5-year survival rate has been lower than 7% over the past 30 years, and now is 8.5%, which is still low compared to other cancers[2]. Currently, it is the fourth leading cause of cancer-related deaths and is expected to rank second by 2030[3]. The only curative treatment is surgery. However, only 15%-20% of the patients are diagnosed as resectable state at initial diagnosis[4]. Furthermore, even after curative resection, over 80% of the patients experience relapse[5].
Because curative resection is not sufficient for complete resolution of pancreatic cancer, adjuvant therapy after surgery has become a standard treatment[6]. Various regimens including chemoradiation therapy and chemotherapy are implemented in real clinical practice[7-9]. In a clinical trial in 2010, there was no significant difference in efficacy between 5-fluorouracil plus leucovorin (FL) and gemcitabine alone chemotherapy[10]. More recently, combination chemotherapy with fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) and gemcitabine plus capecitabine have shown significantly longer survival than gemcitabine alone chemotherapy[8,11]. The definite standard chemotherapy regimen has not been established, and there are several ongoing clinical trials regarding various adjuvant chemotherapy regimens[7].
In comparison with the chemotherapy, efficacy of adjuvant chemoradiation therapy is debatable[12]. In 1985, chemoradiation therapy was first reported to increase overall survival in a clinical trial[6]. Recently, Rutter et al[13] reported that chemoradiation therapy is more effective than chemotherapy alone in resected pancreatic adenocarcinoma. Chemoradiation therapy is also known to reduce locoregional recurrence significantly[14]. On the other hand, a recent clinical trial in Europe reported no significant difference in efficacy between chemoradiation therapy and chemotherapy groups[9]. Another study demonstrated that the efficacy of adjuvant chemoradiation therapy was restricted to the patients with a surgical margin ≤ 1 mm[15]. To date, several studies regarding the efficacy of chemoradiation therapy have not shown consistent results.
Although there are many well-known prognostic factors for resected pancreatic cancer, the methods to further stratify patients for specific adjuvant therapies or intensified treatment modalities remain unclear[16,17]. Current guidelines recommend a variety of adjuvant regimens, regardless of the expected prognosis of the patient[18]. Meanwhile, American Joint Committee on Cancer (AJCC) 8th staging subdivided N1 into N1 and N2. When there are more than three lymph node metastasis, it is defined as N2 and is included in stage III[19]. Because the prognosis differs according to the AJCC stage, a tailored approach to establish more aggressive treatment plans in high-risk patients is necessary. Studies comparing the efficacy of adjuvant treatment modalities according to the AJCC stage are largely lacking.
There is a lack of studies comparing the efficacy of chemoradiation therapy (CRT), systemic chemotherapy (SCT) or a combination of both therapies (CRT-SCT). We aimed to evaluate the efficacy of the three treatment modalities according to the AJCC 8th staging system in patients with pancreatic cancer who underwent R0 resection.
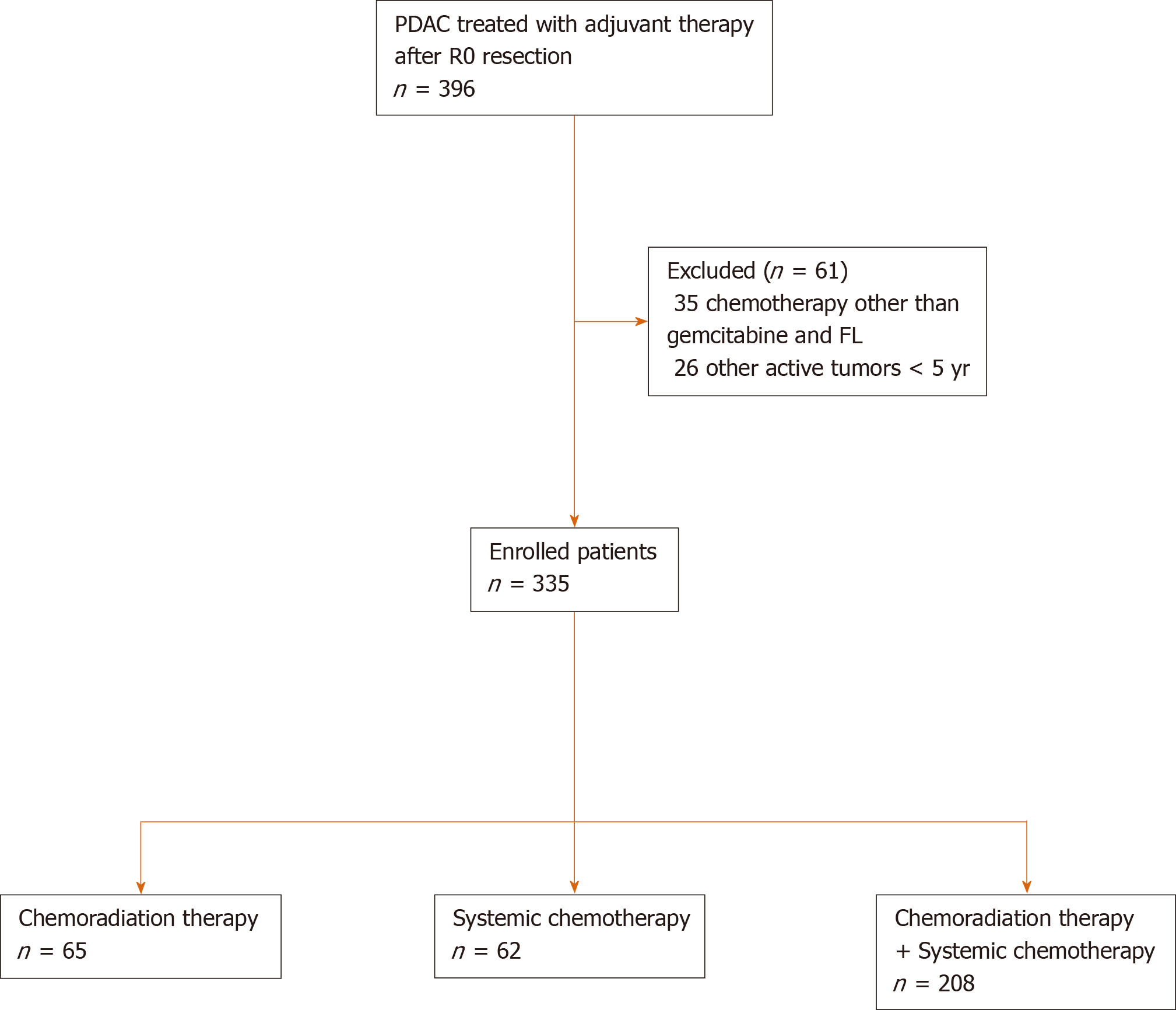
Medical records of patients who underwent complete microscopic resection for pancreatic ductal adenocarcinoma at Seoul National University Hospital from September 2005 to December 2017 were reviewed. R0 resection was defined as microscopic absence of tumor cells at a definite resection margin. After exclusion of patients who received preoperative chemotherapy and/or radiotherapy and who were treated with other active tumors within 5 years, 370 patients were analyzed. Additionally, 35 patients who received chemotherapeutic agents other than gemcitabine and FL were excluded. Finally, 335 patients were included in this study (Figure 1). This study was approved by Institutional Review Board of the Seoul National University Hospital, Seoul, South Korea (1609-015-789).
All the operations were carried out in accordance with standardized protocols. Lymph node groups that were resected in pancreatoduodenectomy included regional lymph nodes to the right side of the celiac and superior mesenteric artery and all the tissues in the hepatoduodenal ligament except for the portal vein and hepatic artery[20]. Patients were evaluated for recurrence at 1 mo after R0 resection. If not recurred, adjuvant treatment was initiated within 4 mo after surgery. Decisions regarding the type of treatment were discussed in a multidisciplinary team, where clinic-pathological characteristics including age, comorbidity, recovery from surgery, primary tumor extent, lymph node involvement and surgical margin status were comprehensively reviewed. The adjuvant treatment modalities were categorized into three groups based on the receipt of adjuvant SCT and/or CRT: CRT group, those receiving adjuvant CRT alone; SCT group, those receiving adjuvant SCT alone; and CRT-SCT group, those receiving both adjuvant CRT and adjuvant SCT. CRT consisted of 45-55 Gy over 5 to 8 wk or 20 Gy for 10 consecutive days two times repeatedly with chemotherapeutic agents: 5-fluorouracil (500 mg/m2 on each first 3 d of radiation therapy), gemcitabine (weekly 300-1000 mg/m2) or capecitabine (1600 mg/m2 daily with weekend breaks)[21,22]. Radiotherapy was delivered to tumor bed, surgical anastomosis sites and adjacent lymph node basins. The SCT group received either gemcitabine or FL combination therapy; gemcitabine (1000 mg/m2) on days 1, 8 and 15 every 4 wk or leucovorin (20 mg/m2) and fluorouracil (425 mg/m2) on days 1-5 every 4 wk. The CRT-SCT group included patients who received sequential treatment (CRT followed by SCT or SCT followed by CRT) and those who received sandwich CRT (SCT followed by CRT and then by SCT). During the first 2 years after surgery, patients were followed up at 3 mo to 6 mo intervals. In the absence of recurrence in the first 2 years, patients were evaluated every 6 mo.
The primary outcome was OS in the CRT, SCT and CRT-SCT groups according to the AJCC staging system. OS was defined as the time from surgery to death from any cause. Patients alive at the point of final analysis were censored at the date last seen alive. The secondary outcomes were recurrence-free survival (RFS), recurrence pattern and adverse events in the CRT, SCT and CRT-SCT groups. RFS was defined from the date of surgery to tumor recurrence, which was confirmed histologically and/or radiographically. Patients alive without tumor recurrence at the last date of follow-up or dead without evidence of tumor recurrence were censored for RFS. Locoregional recurrence was defined as recurrence limited to remnant pancreas, retroperitoneum, periadventitial tissues around the resected pancreas and regional lymph nodes. Distant recurrence was defined as the recurrence beyond the locoregional area.
During adjuvant treatment, patients were monitored closely for the occurrence of toxicity. The adverse events were assessed using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.03. Preoperatively, patient demographics, underlying medical conditions and tumor marker levels were evaluated. Pathologic findings included tumor location, diameter, differentiation, AJCC 8th edition tumor node metastasis stage, a surgical margin from tumor and lymphovascular and perineural invasion. Mortality data were collected from the database of Korean Ministry of the Interior and Safety.
Categorical variables were expressed as the number of patients and percentages. Continuous variables were presented as mean ± standard deviation. Categorical data between two groups were compared using the χ2 test. Analysis of the variance was performed for comparisons between three groups. Survival analysis was based on Kaplan–Meier method with median and 95%CI. Associations between several variables and OS were evaluated using Cox proportional hazard regression models calculating hazard ratio and 95%CI. Using the variables that showed P < 0.1 in the univariable analysis, multivariable-adjusted Cox proportional hazard regression analysis was performed. Survival differences between groups were compared using the log-rank test. All statistics were evaluated using R version 3.5.0 for Windows (Institute for Statistics and Mathematics, Vienna, Austria; URL: http://www.R-project.org).
Baseline characteristics are summarized in Table 1. The mean age of patients was 63.5 ± 9.1 years, and 98 (29.3%) patients were older than 70. Tumors were most commonly located in the head/uncinate in 215 (64.2%) patients. Mean Charlson’s comorbidity index was 4.6 ± 1.3, and it was more than or equal to 6 in 78 (23.3%) patients. One hundred and twenty-six (37.6%) patients had a safety margin of less than or equal to 0.1 cm. Lymph node metastasis was found in 187 patients (55.8%). The mean duration to initial adjuvant treatment after surgery was 47.5 ± 17.7 d, and the mean follow-up duration was 32.2 ± 28.6 mo.
| Variables | n = 335 |
| Age | 63.5 ± 9.1 |
| Female/male | 143 (42.7)/192 (57.3) |
| ECOG | |
| 0 | 275 (82.1) |
| 1 | 60 (17.9) |
| Charlson's comorbidity index | 4.6 ± 1.3 |
| Tumor location, n (%) | |
| Head/uncinate | 215 (64.2) |
| Body | 54 (16.1) |
| Tail | 54 (16.1) |
| Overlapping | 12 (3.6) |
| Surgery type, n (%) | |
| Pancreaticoduodenectomy | 222 (66.3) |
| Distal pancreatectomy | 102 (30.4) |
| Total pancreatectomy | 7 (2.1) |
| Subtotal pancreatectomy | 4 (1.2) |
| Differentiation, n (%) | |
| WD | 24 (7.2) |
| MD | 268 (80.0) |
| PD | 29 (8.7) |
| UD | 3 (0.9) |
| Unknown | 11 (3.3) |
| AJCC stage, n (%) | |
| IA | 46 (13.7) |
| IB | 90 (26.9) |
| IIA | 12 (3.6) |
| IIB | 128 (38.2) |
| III | 59 (17.6) |
| Lymphovascular invasion, n (%) | 196 (58.9) |
| Perineural invasion, n (%) | 280 (83.6) |
| Preoperative laboratory findings | |
| WBC as × 103 cells/µL | 6.1 ± 1.8 |
| Hb in g/dL | 12.9 ± 1.5 |
| AST in IU/L | 43.4 ± 49.6 |
| ALT in IU/L | 66.4 ± 93.7 |
| ALP in IU/L | 159.3 ± 165.1 |
| Albumin in mg/dL | 4.2 ± 3.5 |
| Total bilirubin in mg/dL | 2.2 ± 3.6 |
| Creatinine in mg/dL | 0.8 ± 0.2 |
| CA 19-9 in U/mL | 623.2 ± 1491.9 |
| CEA in ng/mL | 22.4 ± 307.2 |
| Duration to initial adjuvant treatment in d | 47.5 ± 17.7 |
| Follow-up duration in mo | 32.2 ± 28.6 |
In total, median OS was 33.3 mo (95%CI: 27.4-38.6). In univariable Cox proportional hazard regression analysis, variables that showed P < 0.1 included safety margin ≤ 0.1 cm, poorly differentiated or undifferentiated histology, high AJCC stage, preoperative CA19-9 ≥ 100 U/mL and lymphovascular and perineural invasion. In multivariable-adjusted Cox proportional hazard regression analysis, lymphovascular invasion, AJCC stage and preoperative CA19-9 level were the independent prognostic factors. The number of patients and OS classified by each variable are summarized in Table 2.
| Number of patients, n = 335 | Median OS, mo (95%CI) | Univariable | Multivariable | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |||
| Age ≥ 70 | 0.145 | 0.218 | ||||
| No | 237 | 35.1 (28.5-44.4) | 1.00 | 1.00 | ||
| Yes | 98 | 26.9 (22.2-39.2) | 1.24 (0.91-1.70) | 1.29 (0.84-1.99) | ||
| Sex | 0.226 | 0.394 | ||||
| Female | 143 | 33.3 (27.1-46.1) | 1.00 | 1.00 | ||
| Male | 192 | 32.3 (25.7-39.8) | 1.21 (0.90-1.62) | 1.14 (0.83-1.55) | ||
| ECOG | 0.469 | 0.731 | ||||
| 0 | 275 | 34.2 (27.8-42.4) | 1.00 | 1.00 | ||
| 1 | 60 | 27.4 (19.1-39.8) | 1.16 (0.79-1.69) | 1.11 (0.74-1.65) | ||
| Charlson’s index ≥ 6 | 0.222 | 0.876 | ||||
| No | 257 | 34.0 (28.5-39.8) | 1.00 | 1.00 | ||
| Yes | 78 | 26.0 (22.2-50.8) | 1.21 (0.87-1.68) | 1.07 (0.68-1.67) | ||
| Safety margin ≤ 0.1 cm | 0.032 | 0.345 | ||||
| No | 209 | 35.9 (30.7-47.0) | 1.00 | 1.00 | ||
| Yes | 126 | 26.9 (22.3-34.8) | 1.41 (1.04-1.91) | 1.18 (0.86-1.62) | ||
| Histology | 0.077 | 0.038 | ||||
| WD/MD | 292 | 33.9 (27.8-39.6) | 1.00 | 1.00 | ||
| PD/UD | 32 | 18.0 (16.2-NA) | 1.50 (0.94-2.40) | 1.71 (1.04-2.82) | ||
| Lymphovascular invasion | < 0.001 | < 0.001 | ||||
| No | 137 | 60.9 (44.4-89.6) | 1.00 | 1.00 | ||
| Yes | 196 | 25.3 (21.5-28.5) | 2.14 (1.61-2.85) | 1.91 (1.36-2.69) | ||
| Perineural invasion | < 0.001 | 0.590 | ||||
| No | 55 | 59.3 (42.4-114.6) | 1.00 | 1.00 | ||
| Yes | 280 | 30.6 (26.0-35.7) | 1.73 (1.16-2.59) | 1.17 (0.75-1.82) | ||
| AJCC stage | ||||||
| I | 136 | 59.3 (39.6-82.1) | 1.00 | < 0.001 | 0.003 | |
| II | 140 | 27.6 (23.0-34.8) | 1.83 (1.32-2.54) | 1.36 (0.96-1.94) | ||
| III | 59 | 22.0 (16.3-27.4) | 2.84 (1.93-4.18) | 1.97 (1.29-3.01) | ||
| CA19-9 ≥ 100 U/mL | < 0.001 | 0.002 | ||||
| No | 159 | 47.0 (34.0-90.6) | 1.00 | 1.00 | ||
| Yes | 175 | 26.9 (22.5-33.6) | 1.74 (1.30-2.33) | 1.67 (1.21-2.30) | ||
Table 3 summarizes differences in baseline characteristics between CRT, SCT and CRT-SCT groups. Patients were significantly younger and showed lower Eastern Cooperative Oncology Group performance in CRT-SCT group than in the other two groups. The proportion of patients with safety margin ≤ 0.1 cm was highest in the SCT group. Overall, 65 patients received adjuvant CRT; the most frequent chemotherapeutic agent for CRT was 5-fluorouracil (52/65, 80.0%), followed by gemcitabine (8/65, 12.3%) and capecitabine (5/65, 7.7%). The median OS between the three CRT agents were not significantly different [26.0 mo (95%CI 19.4-47.0) in 5-fluorouracil, 16.7 mo (95%CI: 16.3-NA) in gemcitabine and 19.1 mo (95%CI: 11.8-NA) in capecitabine; P = 0.579]. Adjuvant SCT was performed in 62 patients including 44 patients receiving gemcitabine and 18 patients receiving FL. Mean cycle of gemcitabine and FL was 4.9 ± 1.6 and 5.5 ± 1.3, respectively (P = 0.089). Median OS in the gemcitabine group and the FL group was 47.4 mo (95%CI: 19.0-NA) and 31.1 mo (95%CI 24.7-NA), respectively (P = 0.858). In CRT-SCT group, gemcitabine-based chemotherapy was most commonly used (122/193, 63.2%) for maintenance SCT followed by 5-fluorouracil-based chemotherapy (71/193, 36.8%). Treatment duration was longest in the CRT-SCT group (194.8 ± 48.2 d) followed by the SCT group (143.2 ± 47.5 d) and the CRT group (39.8 ± 9.9 d).
| Variables | Group A, n = 65 | Group B, n = 62 | Group C, n = 208 | P value |
| Female, n (%) | 23 (35.4) | 27 (43.5) | 93 (44.7) | 0.410 |
| Age ≥ 70, n (%) | 24 (36.9) | 26 (41.9) | 48 (23.1) | 0.005 |
| ECOG, n (%) | 0.009 | |||
| 0 | 47 (72.3) | 47 (75.8) | 181 (87.0) | |
| 1 | 18 (27.7) | 15 (24.2) | 27 (13.0) | |
| Histology, n (%) | 0.160 | |||
| WD/MD | 52 (80.0) | 55 (88.7) | 185 (88.9) | |
| PD/UD | 10 (15.4) | 7 (11.3) | 15 (7.2) | |
| Unknown | 3 (4.6) | 0 (0.0) | 8 (3.8) | |
| Charlson's index ≥ 6, n (%) | 17 (26.2) | 20 (32.3) | 41 (19.7) | 0.101 |
| Safety margin ≤ 0.1 cm, n (%) | 26 (40.0) | 32 (51.6) | 68 (32.7) | 0.024 |
| CA19-9 ≥ 100 U/mL, n (%) | 26 (40.0) | 36 (58.1) | 113 (54.6) | 0.074 |
| Lymphovascular invasion, n (%) | 27 (41.5) | 34 (54.8) | 87 (42.0) | 0.435 |
| Perineural invasion, n (%) | 54 (83.1) | 56 (90.3) | 170 (81.7) | 0.275 |
| Surgery type, n (%) | 0.388 | |||
| Pancreaticoduodenectomy | 42 (64.6) | 38 (61.3) | 142 (68.3) | |
| Distal pancreatectomy | 19 (29.2) | 24 (38.7) | 59 (28.4) | |
| Subtotal pancreatectomy | 1 (1.5) | 0 (0.0) | 3 (1.4) | |
| Total pancreatectomy | 3 (4.6) | 0 (0.0) | 4 (1.9) | |
| AJCC stage, n (%) | 0.080 | |||
| IA/IB | 19 (29.2) | 28 (45.2) | 89 (42.8) | |
| IIA/IIB | 37 (56.9) | 21 (33.9) | 82 (39.4) | |
| III | 9 (13.8) | 13 (21.0) | 37 (17.8) | |
| Duration to initial adjuvant treatment in d | 46.8 ± 15.3 | 43.9 ± 18.4 | 48.8 ± 18.0 | 0.223 |
| Treatment duration | 39.8 ± 9.9 | 143.2 ± 47.5 | 194.8 ± 48.2 | < 0.001 |
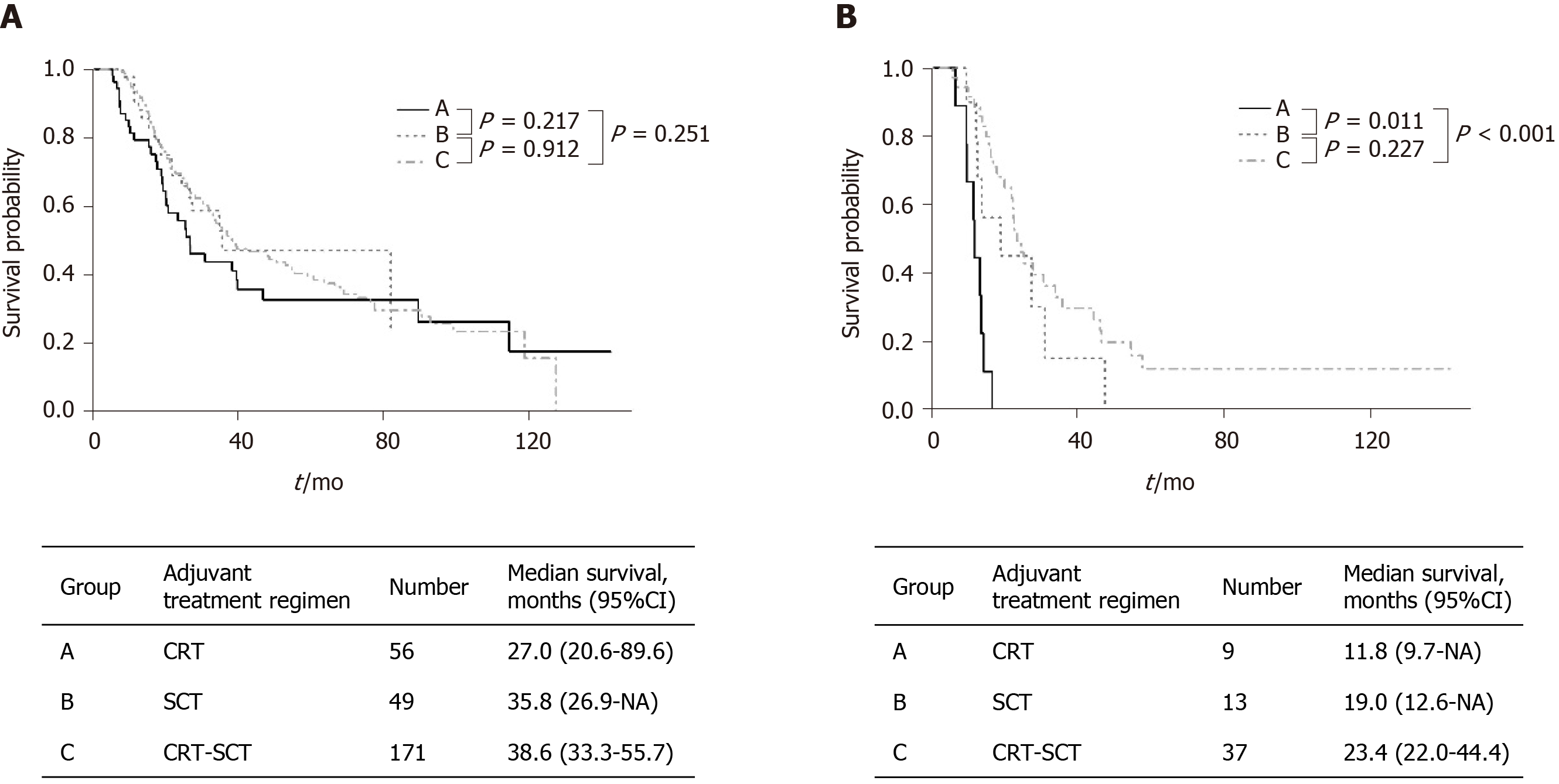
In patients with stage I/II, CRT-SCT (171/276, 62.0%) was most frequently implemented followed by CRT (56/276, 20.3%) and SCT (49/276, 17.8%). In stage III, patients underwent CRT-SCT (37/59, 62.7%) most frequently followed by SCT (13/59, 22.0%) and CRT (9/59, 15.3%). In patients with stage I/II, the median OS was 27.0 mo (95%CI: 2.06-89.6) in the CRT group, 35.8 mo (95%CI: 26.9-NA) in the SCT group and 38.6 mo (95%CI: 33.3-55.7) in the CRT-SCT group (Figure 2A). Among them, there was no significant difference in the OS between the three groups. In 59 patients with stage III (Figure 2B), median OS in the SCT group [19.0 mo (95%CI: 12.6-NA)] and the CRT-SCT group [23.4 mo (95%CI: 22.0-44.4)] was significantly longer than that in the CRT group [17.7 mo (95%CI: 6.8-NA); P = 0.011 and P < 0.001, respectively].
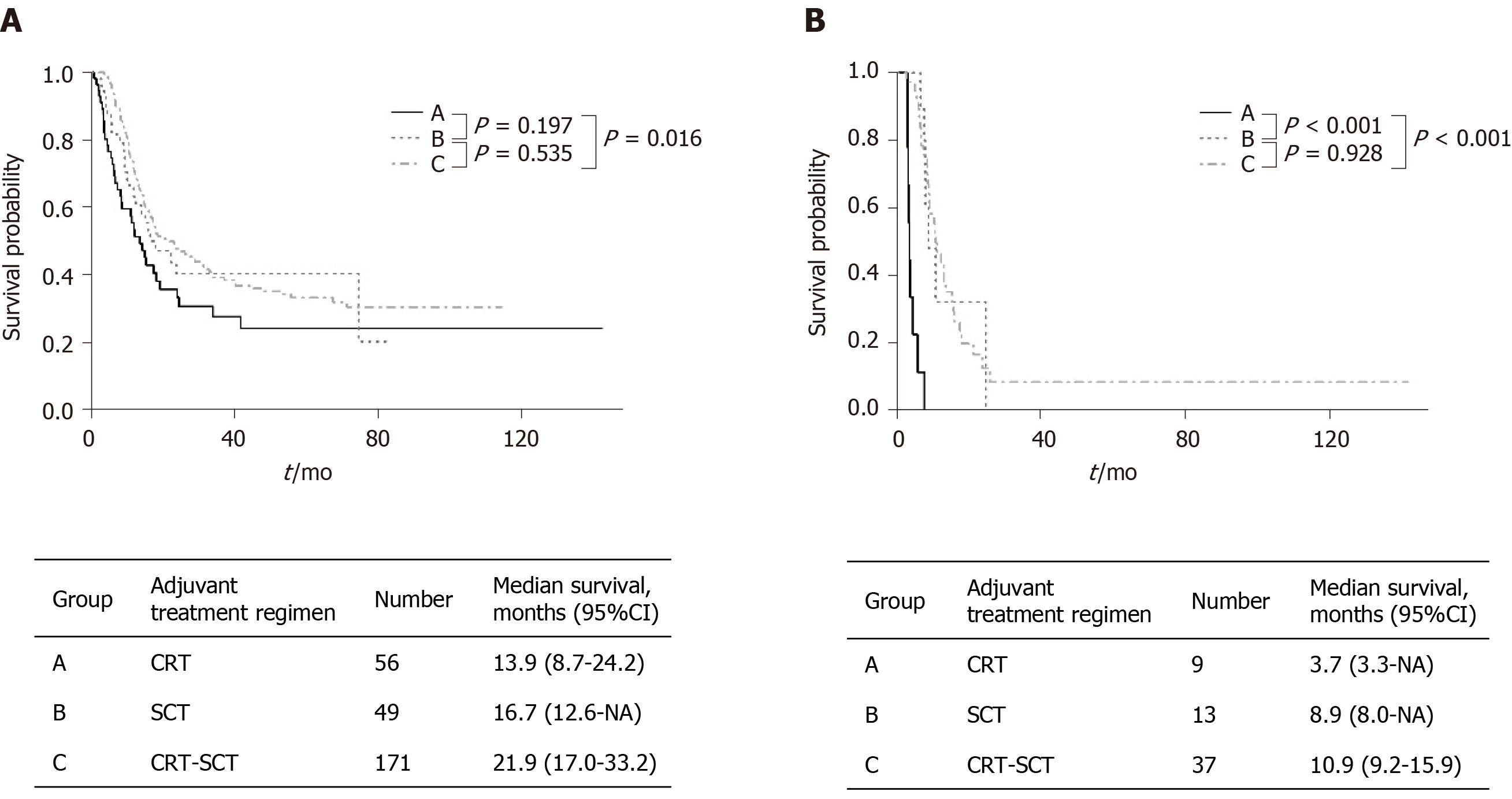
During follow-up, recurrence of 53.7% in stage I, 65.0% in II and 79.7% in III were identified (P = 0.002). Median RFS was 15.9 mo (95%CI: 14.2-18.3) in the overall cohort. In patients with Stage I/II, RFS was significantly longer in the CRT-SCT group [21.9 mo (95%CI: 17.0-33.2)] than in the CRT group [13.9 mo (95%CI: 8.7-24.2)], while there was no significant difference in RFS between the CRT and SCT groups (Figure 3A). Patients with AJCC stage III revealed a median RFS in the CRT, SCT and CRT-SCT groups as 3.7 mo (95%CI: 3.3-NA), 8.9 mo (95%CI: 8.0-NA) and 10.9 mo (95%CI: 9.2-15.9), respectively (Figure 3B). RFS was significantly longer in the CRT-SCT and SCT groups than in the CRT group (both P < 0.001). Additionally, when subgroup analysis was performed only in patients with a free margin ≤ 0.1 cm, there was no significant differences in RFS and OS between the three treatment modalities (P = 0.367 and P = 0.389, respectively).
Differences in the recurrence pattern between the CRT, SCT and CRT-SCT groups are summarized in Table 4. Recurrence was confirmed in 46 patients (70.8%) in the CRT group, 33 patients (53.2%) in the SCT group and 132 patients (63.5%) in the CRT-SCT group (P = 0.119). There were no significant differences in incidence of locoregional and distant recurrences between the three groups (P = 0.158 and P = 0.205). Among distant recurrence, liver was the most common site of recurrence in all groups. There was no significant difference in the number of patients who were able to undergo further line of treatment after relapse between the CRT, SCT and CRT-SCT groups (58.5%, 41.9% and 49.5%, respectively; P = 0.175).
| Recurrence site | Group A, n = 65 | Group B, n = 62 | Group C, n = 208 | P value |
| Remnant pancreas | 2 (3.1) | 2 (3.2) | 8 (3.8) | 0.945 |
| Operation bed | 16 (24.6) | 17 (27.4) | 47 (22.5) | 0.728 |
| Liver | 24 (36.9) | 13 (21.0) | 53 (25.4) | 0.089 |
| Peritoneum | 9 (13.8) | 3 (4.8) | 29 (13.9) | 0.144 |
| Lung | 8 (12.3) | 4 (6.5) | 19 (9.1) | 0.521 |
| Lymph node | 3 (4.6) | 8 (12.9) | 17 (8.1) | 0.238 |
| Local only | 9 (19.1) | 12 (36.4) | 29 (22.0) | 0.158 |
| Distant | 38 (80.9) | 21 (63.6) | 100 (75.8) | 0.205 |
| Multiple site | 13 (20.0) | 11 (17.7) | 36 (17.3) | 0.884 |
Details of adverse events more than or equal to moderate grade during treatment are summarized in Table 5. There were no somatic adverse events that showed significant differences between the three groups. In terms of hematologic adverse events, however, the incidence of neutropenia, anemia and thrombocytopenia was higher in the SCT and CRT-SCT groups than in the CRT group. Granulocyte-colony stimulating factor was employed in patients with neutropenia grade II (5/93, 5.4%), grade III (24/86, 27.9%) and grade IV (18/32, 56.2%).
| Complications | Group A, n = 65 | Group B, n = 62 | Group C, n = 208 | P value | ||||||
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | ||
| Febrile neutropenia | - | - | - | - | - | - | - | 1 (0.5) | - | 0.736 |
| Neutropenia | 15 (23.1) | 3 (4.6) | 5 (7.7) | 15 (24.2) | 15 (24.2) | 6 (9.7) | 63 (30.3) | 68 (32.7) | 21 (10.1) | < 0.001 |
| Anemia | 14 (21.5) | 1 (1.5) | 1 (1.5) | 19 (30.6) | 2 (3.2) | 1 (1.6) | 84 (40.4) | 17 (8.2) | 1 (0.5) | 0.001 |
| Thrombocytopenia | - | 1 (1.5) | - | 5 (8.1) | 2 (3.2) | 1 (1.6) | 16 (7.7) | 7 (3.4) | 2 (1.0) | 0.037 |
| Hypertransaminasemia | 3 (4.6) | 1 (1.5) | - | 3 (4.8) | 1 (1.6) | - | 24 (11.5) | 12 (5.8) | 3 (1.4) | 0.006 |
| N/V | 4 (6.2) | - | - | 5 (8.1) | 2 (3.2) | - | 10 (4.8) | 4 (1.9) | - | 0.441 |
| Diarrhea | 4 (6.2) | - | - | 3 (4.8) | 1 (1.6) | - | 16 (7.7) | 2 (1.0) | - | 0.735 |
| Anorexia | 5 (7.7) | 1 (1.5) | - | 4 (6.5) | - | - | 14 (6.7) | - | - | 0.770 |
| Abdominal pain | 3 (4.6) | - | - | 3 (4.8) | - | - | 10 (4.8) | 1 (0.5) | - | 0.973 |
| GERD | 4 (6.2) | - | - | 1 (1.6) | - | - | 7 (3.4) | - | - | 0.374 |
| Fatigue | 5 (7.7) | 2 (3.1) | - | 2 (3.2) | 1 (1.6) | - | 19 (9.1) | 2 (1.0) | - | 0.408 |
| Mucositis | 1 (1.5) | - | - | 2 (3.2) | 1 (1.6) | - | 5 (2.4) | 1 (0.5) | - | 0.545 |
| Peripheral neuropathy | - | - | - | - | 1 (1.6) | - | - | 3 (1.4) | - | 0.611 |
A wide variety of regimens have been studied as adjuvant treatment in pancreatic cancer patients[7-9]. However, the adjuvant treatment for pancreatic cancer is not yet standardized. There are discrepancies among previous studies comparing the efficacy of adjuvant CRT and SCT[6,9,13]. In this study, RFS and OS were significantly longer in the SCT and CRT-SCT groups than in the CRT group in patients with AJCC stage III. Meanwhile, the increase in OS in the SCT and CRT-SCT groups was not statistically significant compared to that in the CRT group in patients with AJCC stage I/II. There were no significant differences in the recurrence pattern between the three groups, and hematologic adverse events occurred more frequently in the SCT and CRT-SCT groups than in the CRT group.
Previous studies comparing SCT and CRT have not shown consistent results. CRT did not show a significant increase in OS compared with the control group in ESPAC-1 and EORTC clinical trials[9,23,24]. On the other hand, recent population-based studies using a national cancer registry database showed that CRT gave better survival than SCT[13,25]. However, they were limited by potential inherent biases, and the findings should be carefully interpreted. In the current study, SCT and SCT-CRT showed more favorable therapeutic outcomes than CRT, especially in patients with stage III. There is no consensus on whether adjuvant treatment plans should be subdivided according to the AJCC stage yet. Based on the findings in our study, we suggest that SCT with or without CRT should be preferred over CRT in high-risk patients, such as in patients with AJCC stage III.
CRT is known to lower the risk of locoregional relapse whereas SCT reduces distant metastasis[26,27]. A previous study reported approximately 20% of local only recurrence in resected pancreatic cancer[28]. In this study, local only recurrence was more frequent in the SCT group, but the difference was not statistically significant, which is consistent with a previous clinical trial[24] indicating conventional CRT may not be sufficient for improving locoregional relapse. Currently, there are many types of radiation therapies available for pancreatic cancer, including new modalities such as stereotactic body radiation therapy[29,30]. Efforts should be made to find the best suitable radiotherapy that can reduce local recurrence when combined with SCT.
A previous study showed that there was no significant difference in efficacy between gemcitabine and FL chemotherapy[10]. Based on the clinical trial, this study included patients receiving gemcitabine or FL in the chemotherapy group, and there was no significant difference in OS between gemcitabine and FL. Most recently, FOLFIRINOX showed a significant increase in OS compared with gemcitabine single therapy, and adverse events more than or equal to severe grade occurred in approximately 75% of the patients[11]. Meanwhile, nab-paclitaxel, which has recently shown significant therapeutic effect in metastatic pancreatic cancer, is underway to confirm its effectiveness in resected pancreatic cancer[7]. Because various adjuvant treatment regimens have a different spectrum of efficacy and toxicity, appropriate patient stratification is essential to establish a proper treatment plan.
Surgical margin, lymph node metastasis, tumor differentiation, high level of preoperative CA19-9 and perineural, lymphatic and venous invasion are well-known prognostic factors in resected pancreatic cancer[31]. In accordance with the previous studies, this study demonstrated several independent prognostic factors for OS based on the Cox proportional hazard regression model. It is necessary to thoroughly evaluate patients on the basis of an elaborate prognostication model and establish an active treatment plan in high-risk patients. It is important to classify patients through appropriate risk stratification models to establish active treatment plans, such as intensive systemic chemotherapy or clinical trials.
The limitations of this study are as follows. First, there can be an inherent selection bias of a single-center retrospective study design. Due to the nature of the study, the baseline characteristics of the groups were different. Compared with other groups, the CRT-SCT group had a higher proportion of young patients and Eastern Cooperative Oncology Group performance status of zero with a lower proportion of patients with a free surgical margin ≤ 1 mm. Patients with better performance status might have been selected for CRT-SCT, which could potentially bias the results in favor of CRT-SCT. Furthermore, it is now well known that radiation therapy increases the local recurrence-free survival in patients with a surgical margin ≤ 1 mm. However, the proportion of these patients were highest in the SCT group, which may be a major selection bias. Second, the statistical power can be weak because the total number of patients was relatively small in this study. Moreover, because the incidence of tumor recurrence and death was few in stage I, we were statistically unable to further evaluate OS and RFS in stages I and II separately. Third, this study excluded patients treated with adjuvant chemotherapy with regimens other than FL or gemcitabine. Recently, modified FOLFIRINOX showed its superiority compared to gemcitabine alone in the adjuvant settings and is a preferred in fit patients. However, the use of modified FOLFIRINOX as an adjuvant treatment is limited in Korea because it has not been approved for reimbursement by the Korean healthcare system. Because this study is limited by a small number of patients and retrospective design, a well-designed clinical trial that prospectively compares SCT and/or CRT should be followed.
In R0-resected pancreatic cancer, there was no significant difference in OS between CRT, SCT and CRT-SCT groups in patients with AJCC stage I/II, while SCT and CRT-SCT showed significantly longer OS and RFS than CRT in patients with AJCC stage III. SCT with or without CRT is a reasonable choice over CRT, especially in patients with AJCC stage III. Close monitoring for the occurrence of hematologic adverse events is essential during the treatment. In pancreatic cancer treated with R0 resection, a tailored approach based on AJCC stage can help establish an active treatment plan in high-risk patients and develop stratified treatment protocols in future clinical trials.
The adjuvant treatment for pancreatic cancer is not yet standardized. There are discrepancies among previous studies comparing the efficacy of adjuvant chemoradiation therapy (CRT) and systemic chemotherapy (SCT).
Recently, the American Joint Committee on Cancer (AJCC) 8th subdivided N1 into N1 and N2. Because the prognosis differs according to the AJCC stage, a tailored approach to establish more aggressive treatment plans in high-risk patients is necessary. However, studies comparing the efficacy of adjuvant treatment modalities according to the AJCC stage are largely lacking.
This retrospective study was conducted to compare the efficacy of CRT, SCT and a combination of both therapies (CRT-SCT) according to the AJCC 8th staging system in patients with resected pancreatic cancer.
Data on 335 patients who underwent surgical resection and adjuvant treatment for pancreatic cancer between September 2005 and December 2017 were retrospectively reviewed. Patients were divided into three groups: CRT group, SCT group and CRT-SCT group. The primary outcome was overall survival (OS) in the three groups.
Patients received CRT (n = 65), SCT (n = 62) and CRT-SCT (n = 208). Overall median OS was 33.3 mo (95%CI: 27.4-38.6). In patients with stage I/II, the median OS was 27.0 mo (95%CI: 2.06-89.6) in the CRT group, 35.8 mo (95%CI: 26.9-NA) in the SCT group and 38.6 mo (95%CI: 33.3-55.7) in the CRT-SCT group. Among them, there was no significant difference in OS between the three groups. In 59 patients with stage III, median OS in the SCT group [19.0 mo (95%CI: 12.6-NA)] and CRT-SCT group [23.4 mo (95%CI: 22.0-44.4)] was significantly longer than in the CRT group [17.7 mo(95%CI: 6.8-NA); P = 0.011 and P < 0.001, respectively].
The study has concluded that SCT with or without CRT might be a reasonable choice preferentially over CRT in patients with AJCC stage III.
The adjuvant treatment for pancreatic cancer is not yet standardized. In this study, SCT and CRT-SCT showed significantly longer OS and recurrence-free survival than CRT in patients with AJCC stage III, while there was no significant difference in OS between CRT, SCT and CRT-SCT groups in patients with AJCC stage I/II. Different adjuvant therapy according to AJCC stage can be applied in patients with PC.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labori KJ, Polistina F S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | SEER. Surveillance, Epidemiology, and End Results Program. SEER Stat Facts Sheets: Pancreas Cancer, Bethesda, MD, United States. 2018. |
| 3. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5134] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 4. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 5. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1343] [Article Influence: 149.2] [Reference Citation Analysis (2)] |
| 6. | Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 951] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 7. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 805] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 8. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1394] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 9. | Van Laethem JL, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, Peeters M, Polus M, Praet M, Mauer M, Collette L, Budach V, Lutz M, Van Cutsem E, Haustermans K. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol. 2010;28:4450-4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 11. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1943] [Article Influence: 277.6] [Reference Citation Analysis (0)] |
| 12. | Twombly R. Adjuvant chemoradiation for pancreatic cancer: few good data, much debate. J Natl Cancer Inst. 2008;100:1670-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Rutter CE, Park HS, Corso CD, Lester-Coll NH, Mancini BR, Yeboa DN, Johung KL. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: An analysis of the National Cancer Data Base. Cancer. 2015;121:4141-4149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Ocuin LM, Miller-Ocuin JL, Zenati MS, Vargo JA, Singhi AD, Burton SA, Bahary N, Hogg ME, Zeh HJ, Zureikat AH. A margin distance analysis of the impact of adjuvant chemoradiation on survival after pancreatoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Oncol. 2017;8:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA, Samra JS, Gill AJ, Kench JG, Merrett ND, Das A, Musgrove EA, Sutherland RL, Biankin AV; NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 17. | Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, Büchler MW, Werner J. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20:2188-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | National Comprehensive Cancer Network. NCCN Guidelines Insights: Pancreatic adenocarcinoma (Version 1, 2019). Available from: https://jnccn.org/view/journals/jnccn/17/3/article-p202.xml?rskey=J9zK6q&result=5. |
| 19. | Song M, Yoon SB, Lee IS, Hong TH, Choi HJ, Choi MH, Lee MA, Jung ES, Choi MG. Evaluation of the prognostic value of the new AJCC 8th edition staging system for patients with pancreatic adenocarcinoma; a need to subclassify stage III? Eur J Cancer. 2018;104:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Kang MJ, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Comparison of the long-term outcomes of uncinate process cancer and non-uncinate process pancreas head cancer: poor prognosis accompanied by early locoregional recurrence. Langenbecks Arch Surg. 2010;395:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Chuong MD, Boggs DH, Patel KN, Regine WF. Adjuvant chemoradiation for pancreatic cancer: what does the evidence tell us? J Gastrointest Oncol. 2014;5:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 753] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 23. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1908] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 24. | Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, Wils J. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-82; discussion 782-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 896] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 25. | Hsieh MC, Chang WW, Yu HH, Lu CY, Chang CL, Chow JM, Chen SU, Cheng Y, Wu SY. Adjuvant radiotherapy and chemotherapy improve survival in patients with pancreatic adenocarcinoma receiving surgery: adjuvant chemotherapy alone is insufficient in the era of intensity modulation radiation therapy. Cancer Med. 2018;7:2328-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1710] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 27. | Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol. 2011;2:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 28. | Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, Evans RG. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Goldsmith C, Plowman PN, Green MM, Dale RG, Price PM. Stereotactic ablative radiotherapy (SABR) as primary, adjuvant, consolidation and re-treatment option in pancreatic cancer: scope for dose escalation and lessons for toxicity. Radiat Oncol. 2018;13:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Zhong J, Patel K, Switchenko J, Cassidy RJ, Hall WA, Gillespie T, Patel PR, Kooby D, Landry J. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123:3486-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Will U, Müller AK, Fueldner F, Wanzar I, Meyer F. Endoscopic papillectomy: data of a prospective observational study. World J Gastroenterol. 2013;19:4316-4324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |