Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.854
Peer-review started: September 11, 2020
First decision: September 24, 2020
Revised: October 8, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: November 24, 2020
Processing time: 67 Days and 22.3 Hours
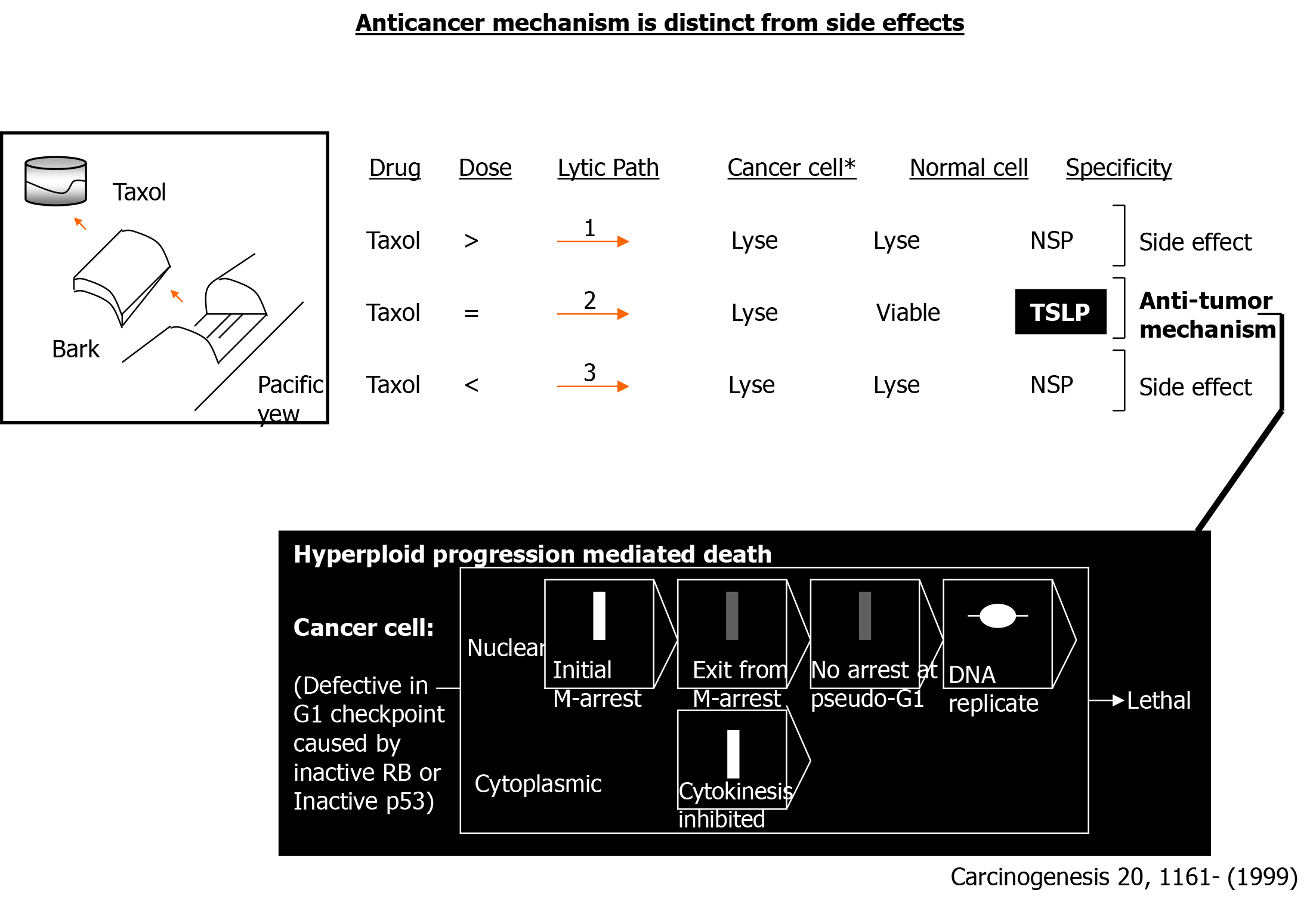
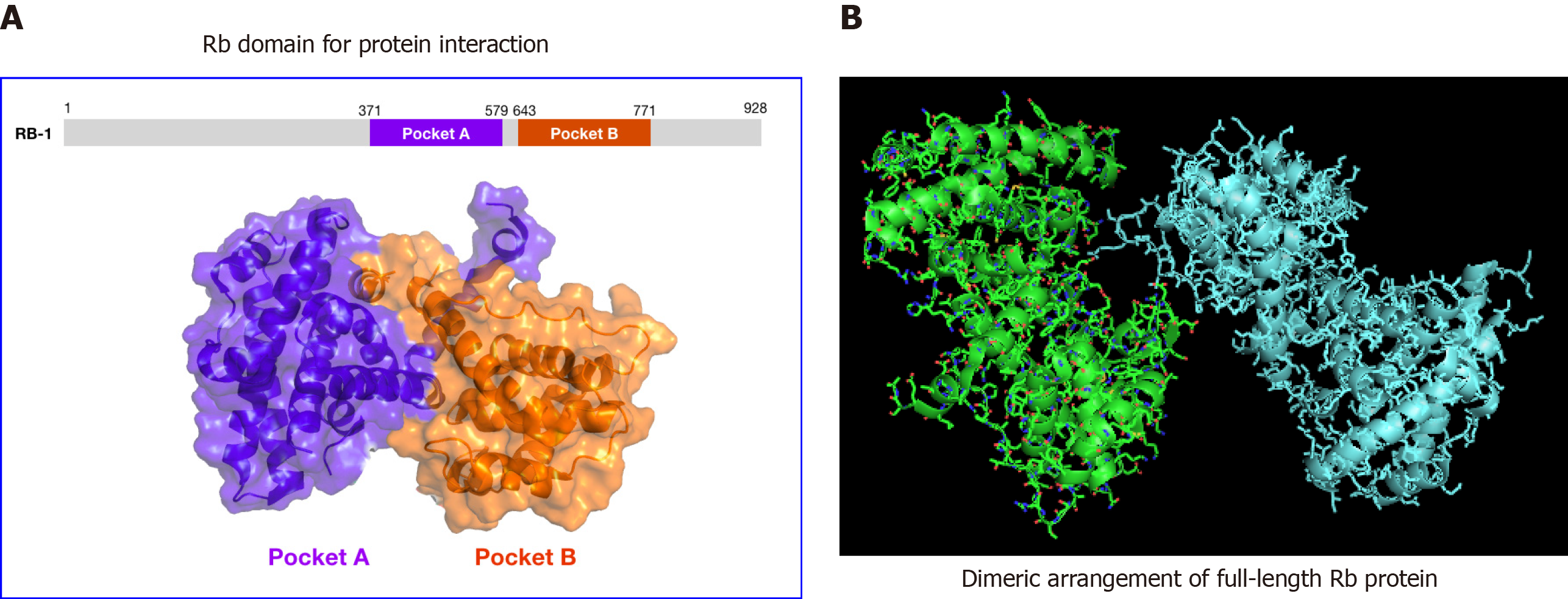
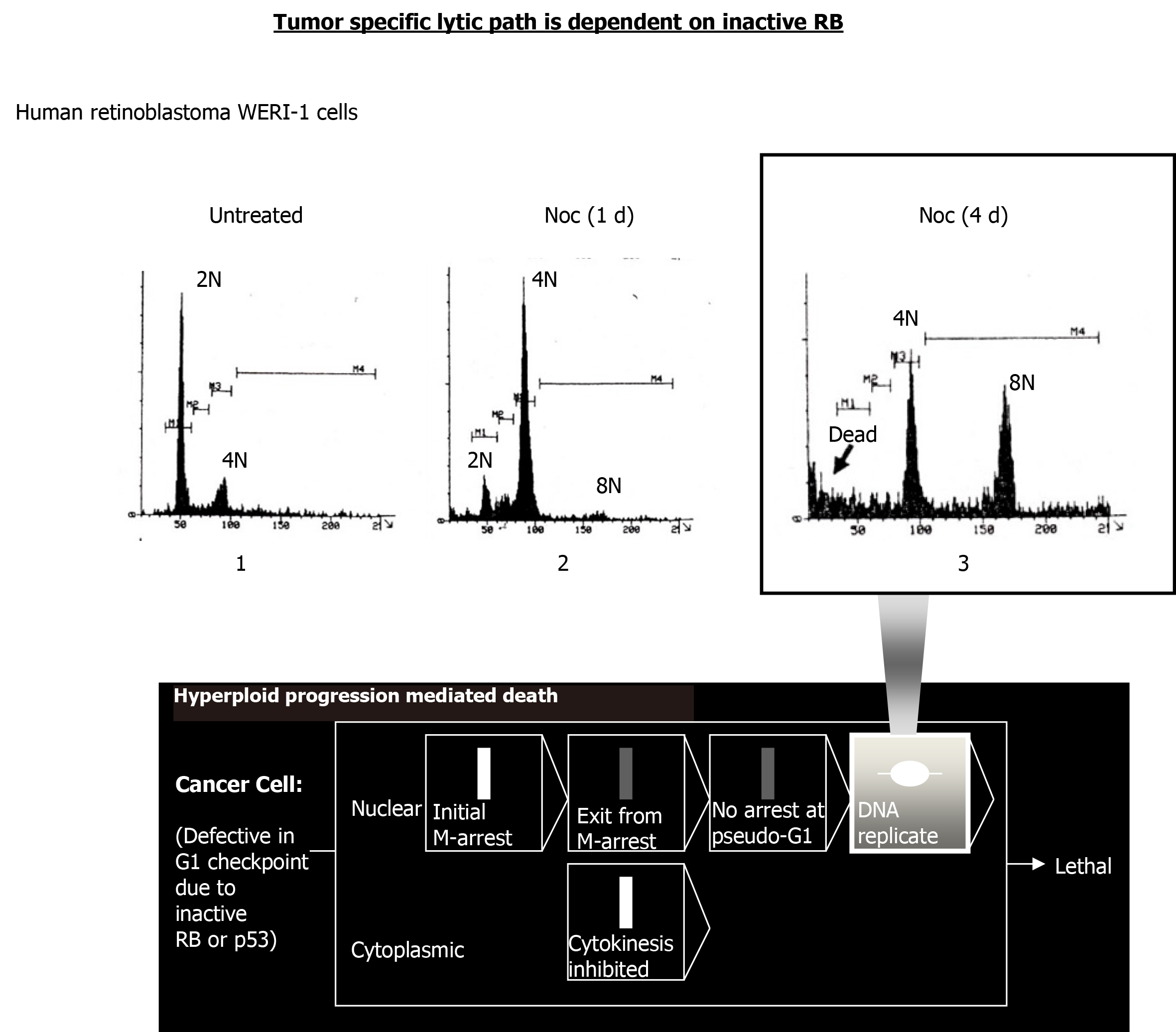
A major advance was made to reduce the side effects of cancer therapy via the elucidation of the tumor-specific lytic path “hyperploid progression-mediated death” targeting retinoblastoma (Rb) or p53-mutants defective in G1 DNA damage checkpoint. The genetic basis of human cancers was uncovered through the cloning of the tumor suppressor Rb gene. It encodes a nuclear DNA-binding protein whose self-interaction is regulated by cyclin-dependent kinases. A 3D-structure of Rb dimer is shown, confirming its multimeric status. Rb assumes a central role in cell cycle regulation and the “Rb pathway” is universally inactivated in human cancers. Hyperploidy refers to a state in which cells contain one or more extra chromosomes. Hyperploid progression occurs due to continued cell-cycling without cytokinesis in G1 checkpoint-defective cancer cells. The evidence for the triggering of hyperploid progression-mediated death in RB-mutant human retinoblastoma cells is shown. Hence, the very genetic mutation that predisposes to cancer can be exploited to induce lethality. The discovery helped to establish the principle of targeted cytotoxic cancer therapy at the mechanistic level. By triggering the lytic path, targeted therapy with tumor specificity at the genetic level can be developed. It sets the stage for systematically eliminating side effects for cytotoxic cancer therapy.
Core Tip: Side effect remains a major impediment to achieving a cure. An important advance has been made to establish the principle of cytotoxic cancer therapy at the mechanistic level. It concerns the discovery of the tumor-specific lytic path “hyperploid progression mediated death” targeting retinoblastoma (or p53) mutants defective in G1 DNA damage checkpoint. By triggering the lytic path, tumor specificity can be achieved at the genetic level for cytotoxic drugs.
- Citation: Hong F, Castro M, Linse K. Tumor-specific lytic path “hyperploid progression mediated death”: Resolving side effects through targeting retinoblastoma or p53 mutant. World J Clin Oncol 2020; 11(11): 854-867
- URL: https://www.wjgnet.com/2218-4333/full/v11/i11/854.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i11.854
Frank-Un Hong (a.k.a. Frank Un, Frank D. Hong) is an established cancer researcher and research scientist at Bio-synthesis, Inc. While serving as a consultant in Beckman Research Institute of City of Hope National Medical Center, it became the top cancer center in the western half of the United States. He also served as a consultant in the highly regarded School of Pharmacy at the University of Southern California. As faculty at the top-ranked University of Texas M. D. Anderson Cancer Center, he contributed editorially for research journals, e.g. Gene therapy and Molecular Biology, Mini-reviews in Medical Chemistry. His works have been presented at the University of California at Los Angeles Keystone conference, National Eye Institute conference, the American Association of Cancer Research symposium, esteemed Cold Spring Harbor Symposium on Quantitative Biology on several occasions, and covered by news media.
Of historic relevance, T. Svedberg, who invented ultracentrifuge (Nobel prize 1926), assisted A. Tiselius in developing electrophoresis (Nobel prize 1948) and supported the “Brownian motion” proposed by A. Einstein (Nobel prize 1921). At the University of Uppsala (Sweden), Svedberg was succeeded by S. Claesson (Nobel committee member for chemistry), who also taught at the Baylor University, under whom F. Hong studied physical chemistry in 1980. He also studied polymer chemistry under M. Dole in 1981, who discovered the “Dole effect”, which led to electrospray ionization mass spectrometry (Nobel prize 2002, J. Fenn, K. Tanaka). In 1979-1980, F. Hong worked on the organic synthesis of oligodeoxynucleotides using the phosphoramidite chemistry with W. Lunsford, a former colleague of W. Letsinger—a field to which H. Khorana (Nobel prize 1968, tRNAs encode amino acids) contributed. In 1983-1984, at the American Bio-Nuclear of F. Hoffmann-La Roche, F. Hong achieved the chemical synthesis of protected purine deoxynucleotide for oligonucleotide synthesis.
In 1981-1982, at the Johns Hopkins University, F. Hong worked on the cloning of phosphate transferase system-encoding genes with its discoverer S. Roseman, who uncovered that phosphate transferase system governs diauxie, whose prior interpretation by J. Monod led to the ‘‘operon’’ concept (Nobel prize 1965). In 1982, F. Hong worked on “transdetermination” with A. Shearn, which is related to altered embryonic phenotype caused by the mutant homeotic gene (Nobel prize 1995, E. Lewis, C. Nusslein-Volhard, E. Wieschaus).
In 1985, at the Salk Institute founded by J. Salk (developed polio vaccine), F. Hong determined the genomic sequence of the avian infectious bronchitis virus strain M41-positive-sense single-stranded RNA coronavirus (belongs to the identical family as SARS or coronavirus disease 2019 coronavirus but distinct genus). This was done to identify the neutralizing antibody epitopes on spike protein with W. Spaan (University of Utrecht, The Netherlands), which was reported in Virus Research[1]. Vaccines prepared for the subsequent epidemic by “SARS coronavirus” likewise target the spike protein. In 1984-1985, F. Hong worked on the intracellular transport mechanism (of Vesicular stomatitis virus’s G protein) with J. Rose, a former colleague of D. Baltimore (Nobel prize 1975, reverse transcriptase), which is implicated in diabetes or mental disorder (Nobel prize 2013, Scheckman, Sudhof, Rothman).
In 1985-1986, at the scripps research institute, F. Hong cloned/determined the first eukaryotic cDNA sequence of human muscle 6-phosphofructokinase gene with S. Vora of E. Beutler’s group (parent of B. Beutler, Nobel prize 2011, Toll-like receptor), which was reported in Biochem. Biophys. Res. Comm[2]. Phosphofructokinase-M is linked to Tarui disease, cancer metabolism, and represents one of the earliest susceptibility genes to be identified for human heart disease (cardiac hypertrophy).
In 1986-1987, at the University of California at San Diego, F. Hong identified/cloned human retinoblastoma susceptibility gene Rb, proving the genetic basis of human cancers, which was published in Science[3]. This work was done with W. Lee, a former colleague of P. Duesberg (discovered v-Src oncogene of Rous sarcoma virus, which led to c-Src discovery; Nobel prize 1989, M Bishop, H Varmus). Mutation in tumor suppressor gene RB was also found in osteosarcoma, breast cancer, small cell lung cancer, colon cancer, and glioma. It inspired the cloning of other tumor suppressor genes: Example, p53, breast cancer 1, phosphatase and tensin homolog, neurofibromatosis type 1, Wilms' tumour 1.
In 1986-1987, F. Hong determined the cDNA sequence of human RB [the N-terminus that are GC-rich via the Maxam-Gilbert method[4] and the C-terminal region via Sanger’s chain termination method[3], which contained cyclin-dependent kinase (CDK) phosphorylation motifs. It led to the subsequent identification of CDK4/6 as the regulator of Rb, which governs cell cycle progression across “restriction point” at the G1 phase. This was followed by the development of the CDK4/6 inhibitor (example, palbociclib by Pfizer Inc.), which was approved by the United States Food and Drug Administration to treat advanced-stage breast cancer.
In 1987, F. Hong discovered Rb’s DNA binding property that revealed Rb’s role in regulating transcription or DNA replication, which was reported in Nature[4]. It instilled the view that Rb may function as a transcription factor—example, by interacting with E2F or other associating proteins. Subsequently, he documented a stable complex formed by purified Rb protein and double-stranded DNA, which indicated that Rb interacts with DNA directly[5].
In 1987-1988, he observed that SV40 promoter-driven Rb overexpression causes cells to arrest as enlarged cells in cell culture (unpublished), which preceded the subsequent discovery of Rb’s function in DNA damage checkpoint in G1 or S phase.
In 1988-1989, F. Hong identified/characterized the Rb gene promoter and found its regulatory elements reminiscent of “housekeeping” genes, which was reported in Proc Natl. Acad. of Sci. United States[6]. In 1990, he reported the discovery of the first Rb mutation in human prostate cancer, i.e. defective promoter, which was published in Proc Natl. Acad. of Sci. United States[7]. It led to the finding that mutant Rb triples the mortality risk of prostate cancer patients and that Rb loss confers resistance to androgen receptor antagonizing therapy.
In 1989, he found a similarity between the Rb polypeptide sequence and the neurofilament subunit NF-L, indicating that Rb may function in the structural aspect of chromosomes, which was reported in Bioscience Report[8]. In 1990-1991, he discovered Rb’s oligomerizing property, which was published in the Journal of Biological Chemistry[9]. It suggested that Rb may form a higher-ordered structure like nuclear matrix (“corral hypothesis” presented at Cold spring Harbor Symposium on Quantitative Biology in 1991[10] and 1994[11] with the latter organized by J. Watson; Nobel prize 1962, double helix) to assume its function—i.e. modulating chromatin to affect DNA condensation, histone modification, heterochromatin, DNA replication. It led to the finding that Rb’s N-terminus interacts with its C-terminus, which was subsequently confirmed. Further, it revealed that CDKs negatively regulate the Rb-to-Rb self-interaction (presented at the 1994 Cold Spring Harbor Symposium on Quantitative Biology)[11].
In 1993, at the Fred Hutchinson Cancer Research Center, F. Hong worked on the human APC gene, whose inactivation causes familial adenomatous polyposis with its discoverer G. Joslyn, a former colleague of R. White (University of Utah).
In 1993-1995, at the Salk Institute, F. Hong worked on growth factors, Alzheimer’s disease, and aging disorders with D. Schubert, a former colleague of J. Monod (Pasteur Institute, France).
In 1996-1997, F. Hong discovered the tumor-specific lytic path “hyperploid progression mediated death” targeting Rb or p53 mutant cancers defective in G1 DNA damage checkpoint while investigating the mechanism through which Taxol induces chromosomal aneuploidy in human brain cancer cells, which was published in Carcinogenesis[12]. This work was done with P. Nisen, a former colleague of S. Cohen (Nobel prize 1986, epidermal growth factor) who worked on gene therapy of brain cancer. In 2010, the events that led to its discovery were chronicled in the book, “Multiple Drug Resistance”[13]. The lytic path has been confirmed globally.
In 2005-2006, at the City of Hope National Medical Center’s Beckman Research Institute, F. Hong discovered the mechanism through which the sensitivity of resistant cancers to the antimetabolite drug hydroxyurea could be restored by modulating the Rb-associating transcription factor ICBP90 (UHRF1), which was reported in Anticancer Research[14]. In 2007, F. Hong reported the discovery that Rb protein mediates the cytotoxicity of the DNA crosslinking drug cisplatin in G1 DNA damage checkpoint-retaining human cancers in Anticancer Drugs[15].
In 2015-2018, at the University of Southern California, F. Hong worked on the role of monoamine oxidase (MAO), which degrades serotonin (5-HT) and other neurotransmitters, in human cancer with J. Shih (part of a delegation sponsored by the United States National Academy of Science to open diplomatic relation with China on the scientific front during the Nixon administration), who discovered MAO isoenzymes A and B via cloning, the role of MAO in autism, and the genetic regulation of behavior.
Advances in medicine come slowly. The discipline of cancer therapy is no exception as there has been little increase in survival within the last fifty years for certain human cancers despite extensive research. One of the key issues standing in the way of achieving a cure is side effects as it undermines dose escalation or prolonged treatment. The problem is well documented for chemotherapy, which nevertheless remains one of the major treatment modalities. This is because chemotherapeutics can induce cancer cell death (cytotoxic) rather than merely arrest cycling cells (cytostatic), which represents an important therapeutic asset. Here, a major advance made to solve the problem of side effects is described.
The basic tenet underlying chemoprevention is to reverse the course of cellular changes occurring during a normal to cancerous state transition through the pharmacological intervention. In several cases, the precise stages that a normal, well-differentiated cell undergoes to become cancerous have been well documented. One such example is colon cancer, where the transition of a normal cell through the “polyp” stage in route to becoming cancerous has been clearly documented. In the case of an intestinal tumor, this scenario has been confirmed using an Apc (adenomatous polyposis coli)-gene inactivated murine model[16]. By understanding the molecular mechanism underlying this process, several targets can be identified for pharmacological intervention.
In the case of colon carcinogenesis, overexpression of the gene encoding cyclooxygenase (COX), which is responsible for the production of prostaglandin from arachidonic acid, is an early and central event. This has led to the development of COX-inhibiting nonsteroidal anti-inflammatory drugs such as sulindac, or more selective COX-2-specific drugs.
In the case of breast carcinogenesis, the estrogen pathway has been targeted for chemoprevention given estrogen’s role as a tumor promoter. Tamoxifen is an analog of estrogen used for suppressing mammary carcinogenesis. Raloxifene represents another agent with a similar potential. Development of selective estrogen receptor modulators, which function as estrogen agonists in tissues where estrogen is beneficial yet as antagonists in tissues where estrogen may promote carcinogenesis (breast, uterus, ovary) remains the goal.
For cancers of the head and neck as well as the lung, the retinoid pathway has been targeted for chemoprevention. Retinoids are essential for proper differentiation of lung and upper airway epithelium, and the loss of retinoic acid receptor (RAR) has been associated with premalignant lesions of the oral epithelium and the formation of lung cancer[17]. Consequently, (nuclear) receptors for retinoids represent critical molecular targets for chemoprevention. The nuclear receptors function as transcription factors for specific genes, whose promoters contain their responsive elements. Administration of 9-cis-retinoic acid, which represents an agonist for all 6 types of retinoid receptors, has been shown to restore the expression of RAR, and reverse the development of cancerous lesions[18]. Those binding to retinoid X receptors but not to RARs such as targretin may represent more selective chemopreventive agents for these cancers[19].
The majority of previously developed anti-cancer drugs were designed to interfere with various aspects of DNA metabolism. The underlying rationale is that interfering with the propagation of genetic material will undermine the continuous cell division necessary for tumor formation. The targeted areas of DNA metabolism include DNA replication, DNA repair, chromosome segregation, DNA mutagenesis, etc.
One class of such anti-cancer drugs includes agents that form noncovalent complexes with DNA. These include anthracyclines, mitoxantrone, dactinomycin, bleomycin, and plicamycin. Doxorubicin (adriamycin) and daunorubicin, isolated from different species of streptomyces, belong to the category of anthracyclines. In the case of doxorubicin, it intercalates with double-stranded DNA and causes local uncoiling. This happens due to the separation of the stacked bases by the intercalated doxorubicin molecule. Doxorubicin treatment of cells results in the cleavage of their DNA. Cleavage of the intracellular double-stranded DNA is thought to occur due to the inhibition of topoisomerase II. Cleavage of single-stranded DNA is thought to occur due to the formation of the drug-Fe-DNA complex, which induces the generation of hydroxyl radicals due to hydrogen peroxide[20].
In contrast, cisplatin belongs to a different class of anti-cancer drugs that interact directly with DNA, through forming crosslinks. The ability of cisplatin to form adducts with DNA has been exploited for the treatment of head and neck cancer.
Other types of anticancer drugs that do not interact with DNA directly, yet affect its metabolism indirectly, include methotrexate, which inhibits the synthesis of nucleotides necessary for DNA replication. They also include Taxol and vincristine, the antimicrotubule drugs that interfere with the DNA metabolism at the level of sister chromatid segregation (which occurs during metaphase-to-anaphase transition of mitosis) by blocking the formation of mitotic spindles.
Certain anticancer drugs such as etoposide function by inhibiting enzymes involved in the repair of DNA breaks, such as topoisomerase II.
The occurrence of side effects continues to pose a problem for chemoprevention and chemotherapy. Side effects associated with chemopreventive drugs include the following. In the case of the COX-inhibitors, their nonselective aspect can lead to serious side effects including gastrointestinal ulceration and bleeding. The side effects have mainly been attributed to the inhibition of the COX-1 enzyme, which is involved in generating prostaglandin. Prostaglandin functions to maintain stomach lining integrity, regulate blood flow within the kidneys, and balance platelet function. This has prompted the development of more selective drugs, i.e. inhibitors of COX-2 enzymes whose level increases in response to diet, stress, and injury. However, treatment with COX-2 inhibitors has been associated with an increased risk for heart attack and stroke[21].
The use of tamoxifen to treat breast cancer has been associated with the occurrence of endometrial cancer and thromboembolism. Raloxifene, an estrogenic agent that maintains bone mass in postmenopausal women to prevent osteoporosis, is used for breast cancer chemoprevention and has a similar degree of risk as tamoxifen for developing thromboembolism[22].
The use of retinoids has been associated with various toxicities including skin dryness, cheilitis, hypertriglyceridemia, and conjunctivitis.
For the majority of drugs used in chemotherapy, side effects remain a major problem. As most currently used drugs were designed to interfere with various aspects of DNA metabolism necessary for rapidly replicating cancer cells, it undoubtedly has a negative effect on normal cells that also divide frequently, ex. white blood cells. The lack of tumor-specificity may result in side effects including weight loss, infection (due to decreased immunity), fatigue, and pain.
Among various main-line therapies, chemotherapy utilizing Taxol has emerged as one of the most potent treatments for breast, ovarian, and lung cancers. But treatment with Taxol results in various side effects, including dyspnea with bronchospasm, urticaria, hypotension, neutropenia, and peripheral neuropathy[23].
For the crosslinking drug cisplatin, nephrotoxicity is its chief dose-limiting side effect[24]. For anthracyclines like doxorubicin, side effects include cardiac toxicity, bone marrow suppression, and gastrointestinal and hepatic effects[25]. The side effects, resulting from their lack of tumor-specificity, remain a major hurdle as it undermines the effort to attain the dose necessary for tumor destruction.
Another significant area of concern for DNA-targeting anticancer drugs is their mutagenic nature. Because drugs such as cisplatin or alkylating drugs interact directly with DNA, its mutagenic nature can contribute to the development of secondary malignancies.
The problem facing current cancer therapy is not the lack of cytotoxic drugs per se but the lack of cytotoxic drugs that are tumor-specific. Indeed, the vast majority of drugs administered today as part of chemotherapy are capable of lysing cancer cells. Yet, upon administration, their propensity to cause side effects presents a major impediment as it incurs a debilitating effect and preempts dose-escalation necessary to eliminate recurrent cancers. The prevailing view is that the cytotoxicity results from their ability to interfere with cell cycle progression—hence, leading to the death of cancer cells as well as normal cells that divide.
Yet, this view may have oversimplified the scenario. First, pharmacologically, many of these drugs were isolated based on their ability to lyse cancer cells, not normal cells. The key amongst them is Taxol, which was isolated after screening various plant extracts for a tumor suppressing potential. Second, physiologically, many of these drugs represent a part of the host's defense repertoire against invading microorganisms—hence, were not designed to inflict damage on normal cells. Third, clinically, these drugs can achieve a cure in a minor subset of cancer patients despite that both the responders and nonresponders suffer side effects. These discrepancies have led to the speculation that the anti-tumor mechanism of these drugs may be distinct from the mechanism underlying their side effects, which ultimately led to the elucidation of a tumor-specific lytic path.
During 1996-1999, while working on the mechanism through which antimicrotubule drugs like taxol induce chromosomal aneuploidy, a tumor-specific lytic path targeting G1 checkpoint defective human cancers was discovered by serendipity (Figure 1). The tumor-specific lytic path ‘‘hyperploid progression mediated death’’ is specific for RB or p53 genetic mutants that are defective in DNA damage checkpoint for the G1 phase[12]. Through its elucidation, the principle of tumor-specific cytotoxic therapy, which is based on the genetics of human cancer, was established. Previously, the lytic path was mentioned in a biological context[26]. Subsequently, the events that led to its discovery were chronicled in the book “Multiple Drug Resistance”[13]. More significantly, for cancer therapy, it provided a mechanistic framework for developing cytotoxic drugs devoid of side effects by conferring tumor specificity at the genetic level.
The genetic basis of human cancers was elucidated through the identification of the prototypic tumor suppressor gene Rb, whose inactivation predisposes to the development of retinoblastoma[27]. The underlying genetic mechanism was proposed by A. Knudsen, who suggested that its dominant pattern of inheritance could be explained through the late inactivation of the remaining wild-type allele in a hereditary case harboring a mutant allele[28]. It also suggested that the sporadic cases may take a longer period to develop tumors due to the time it takes to acquire mutations in both wild-type alleles.
The retinoblastoma susceptibility gene was identified through molecular cloning, which was conducted in several laboratories[3,29,30]. The human Rb gene consists of 27 exons and the germline and somatic mutations may occur throughout its coding region as well as the promoter[6,31]. The delineation of the RB gene structure has significantly advanced our ability to manage retinoblastoma clinically through genetic diagnosis[32]. Subsequent investigations documented the occurrence of Rb gene mutation in various human cancer types including breast cancer, prostate cancer, osteosarcoma, non-small cell lung cancer (> 80%), and brain cancer[6,33-35].
Rb protein (also known as RB1) is a key component of mammalian DNA damage checkpoint that monitors the integrity of DNA and blocks cell cycle progression past the G1 (or S) phase in the event of DNA damage. The arrest at G1 is critical as it renders time to repair damaged DNA and avoid replicating mutated DNA. The step mediated by Rb represents a focal point where growth regulatory signals transduced through mitogen-activated protein kinase/ extracellular signal-regulated kinase or phosphatase and tensin homolog/AKT pathway as well as mitogenic signals initiated by epidermal growth factor receptor or ERBB2 (also known as Her2) converge to modulate cell proliferation--hence, giving rise to the concept that Rb represents the center of cell cycle control.
Nevertheless, the exact molecular mechanism through which Rb executes G1 arrest remains incompletely understood[36]. The binding of Rb to DNA-affinity columns suggested that Rb’s intracellular function may be regulatory in nature, which was confirmed by its role in regulating gene transcription[4,37,38].
Critical insight into the Rb function was obtained upon uncovering its propensity to form oligomers through self-interaction[8,9]. The observation that cellular Rb exists as a dimer in the cell lysate confirmed its oligomerization potential[10]. A 3-dimensional model showing Rb protein dimer as observed in the asymmetric crystal unit for RbPL–P is shown (Figure 2). The crystal structure (2.0 Å) of an Rb construct containing “pocket domain” with the phosphoserine-mimetic (S608E) and a shortened RbPL (large loop within the pocket domain) was solved by Burke et al[39] via crystallizing RbPL–P (representing Rb380–787Δ616–642/S608E/S612A/S780A) that binds E2FTD (transactivating domain of E2F) with a lesser affinity, suggesting that the glutamate substitution mimics phosphorylated S608. Rb's post-translational modification via phosphorylation, which was uncovered through the identification of cyclin-dependent kinase recognition motifs in the Rb polypeptide sequence, was shown to negatively regulate Rb self-interaction[11]. These findings suggested that Rb may form a higher-ordered structure in vivo to execute G1 arrest.
The central role of Rb in regulating cell cycling stems from the post-translational modification of Rb by various cyclin-dependent kinases, which occurs progressively as cells transit from the G1 to the M phase. These include Cdk4 and Cdk6 in the G1 phase and Cdk2 in the S phase whose activities require associating with distinct cyclins. The activities of specific Cdks are further modulated through complexing with other factors such as p14Art and p16, with the latter representing a distinct tumor suppressor. The activity of Cdk2 is inhibited by p21Cip1/Waf1, whose transcription is regulated by p53, which is mutated in nearly 50% of all human cancers. Additionally, the oncogenic proteins encoded by viruses such as papillomavirus and adenovirus target Rb to promote cell proliferation. As many as 200 cellular proteins may interact with Rb, including E2F regulating the transcription of genes involved in S phase activities that bind to the pocket domain. The above “Rb pathway” is inactivated in nearly all human cancers.
Hyperploid progression occurs due to continued cell cycle progression without cytokinesis. Briefly, disruption of the mitotic spindle by antimicrotubule drugs activates spindle checkpoint, a component of M phase DNA damage checkpoint, to induce M arrest. After a transient M-arrest, the treated cells re-enter the cell cycle (due to mitotic slippage) without cytokinesis to eventually become re-arrested at a “G1-like” phase in Rb-retaining cells. In cells lacking Rb, however, the treated cells continue with DNA replication, resulting in hyperploidy. Continued treatment with the antimicrotubule drug leads to the death of the hyperploid cells.
Experimental evidence for the triggering of hyperploid progression mediated death in RB-mutant human cancer cells is shown. WERI-1 is a human retinoblastoma cell line lacking both RB alleles[40]. Continued treatment of WERI-1 cells with the antimicrotubule drug nocodazole led to the death of hyperploid cells induced (Figure 3).
After the initial report by Hong et al[12] in July of 1999, multiple other reports followed providing additional evidence for hyperploid progression mediated death. The first category of such reports used antimicrotubule drugs as the inducer.
In August of the same year, Casenghi et al[41] of the University of La Sapienza in Italy reported the propensity of K562 cells lacking p53 to undergo hyperploid progression before dying following the nocodazole treatment. In the report, polyploidization was confirmed via in situ hybridization using chromosome-specific pericentromeric probes. Their exit after a transient M arrest was confirmed by assessing the cyclin B1 or MPM-2 level and the re-initiation of DNA replication was detected by flow cytometric analysis of bromodeoxyuridine (BrdUrd)-incorporated cells.
In September of the same year, Verdoodt et al[42] of Vrije Universiteit Brussel in Belgium reported that a positive correlation exists between the extent of polyploidization induced by nocodazole and the level of apoptosis.
In 2001, Tsuiki et al[43] of Kumamoto University in Japan reported that U251MG cells containing mutant p53 undergo hyperploid progression (> 4N or 8N peak appeared) before dying (sub-2N or 0-1N peak detected) as monitored by flow cytometry. The authors also showed that enhancing hyperploid progression through the broad-range protein kinase inhibitor Staurosporine causes a greater extent of lethality in U251MG cells.
In the same year, Cassinelli et al[44] of instituto Nazionale per lo studio e la Cura dei tumori in Italy also reported that treatment of p53-mutant human ovarian cancer IGROV-1/Pt1 cells, p53-deficient human prostate carcinoma P3 cells, or Saos-2 with taxol or its analog integrated digital network 5109 led to hyperploid progression and death.
In a separate report by Lanzi et al[45] of Instituto Nazionale per lo studio e la Cura dei tumori in Italy, the death of PC3 cells undergoing hyperploid progression following taxol treatment was directly shown using the TUNEL/PI double-staining method.
In 2002, Emory University reported that altering microtubule by noscapine caused murine melanoma B16S9 cells to undergo hyperploid progression before dying.
In 2012, Qi et al[46] of Showa Pharmaceutical University in Japan showed that pseudolaric acid B induces hyperploid progression, resulting in mitotic catastrophe before apoptosis in murine fibrosarcoma L929 cells. Pseudolaric acid B is a microtubule destabilizing agent found in the bark of pseudolarix kaempferi Gorden (pinaceae) tree in central China, which was used for treating fungal infection.
The second category of reports used antimitotic agents other than antimicrotubule drugs as the inducer of hyperploid progression mediated death. In 2003, Ditchfield et al[47] of the University of Manchester and AstraZeneca Pharmaceuticals in England reported that ZM447439-treated HeLa cells undergo hyperploid progression and die. ZM447439 is an inhibitor of Aurora B kinase.
In 2004, Harrington et al[48] of Vertex Pharmaceuticals in England reported that treatment of HeLa cells or human breast cancer MCF-7 cells with VX-680 (tozasertib), which targets aurora kinase (see below) causes them to undergo hyperploid progression before dying. The exit from M phase of VX-680 treated cancer cells were monitored by assessing the cyclin B1 level. DNA replication occurring without the prior cytokinesis, leading to > 4N cells, was also described.
In the same year, Gizatullin et al[49] of Dana Farber Cancer Institute at Harvard Medical School reported that VX-680 triggers hyperploid progression before death in human non-small cell lung cancer A549 cells with a deficient level of p53-induced p21cip1/waf1. They also were able to document that cells undergoing hyperploid progression were dying via apoptosis[49].
In 2005, Tao et al[50] of Merck Research Laboratories reported that treating human ovarian cancer A2780 cells with KSP-IA causes hyperploid progression and death. KSP-IA is an inhibitor of Eg5, a member of the kinesin-5 family that plays a critical role in chromosome segregation by maintaining spindle bipolarity. Eg5 functions as a molecular motor that slides along microtubule tracks within cells.
In 2006, Dijkhuis et al[51] of University Medical Center Gronigen in the Netherlands reported that treating with PDMP (D,L-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol) sensitizes neuroblastoma cells to paclitaxel by triggering hyperploidy. PDMP is an inhibitor of glucosylceramide synthase that suppresses sphingolipid biosynthesis.
In the same year, Chin et al[52] of the DNAX Research Institute of Molecular and Cellular Biology Research Institute reported that treating M checkpoint-suppressed HeLa cells with monasterol causes hyperploid progression and death. Monasterol is an inhibitor of kinesin-5.
In 2007, New York Medical College reported that treatment of human prostate cancer PC-3, CWR22Rv1, or DU-145 cells with Reversine causes polyploidy via suppressing cyclin B or Cdk1, resulting in growth arrest.
In 2008, D’Alise et al[53] of the European Institute of Oncology in Italy reported that the synthetic purine reversine inhibits the aurora kinase and induces hyperploid progression and death in human colon cancer HCT116 cells.
In the same year, Hauf et al[54] of Research Institute of Molecular Pathology in Austria reported that treating HeLa cells with hesperadin, which also targets aurora kinase, causes them to undergo hyperploid progression before dying. Flow cytometry showed the emergence of hyperploidy peak (8N and 16N) preceding the appearance of dead cells[54].
In 2018, Cheng et al[55] of Peking University in China demonstrated that the treatment of human renal carcinoma cells with Reversine led to polyploidy formation and caspase-dependent cell death.
In the case of p53, its mutant form may impart oncogenic properties to the affected cell. Nevertheless, various molecularly targeted approaches for treating p53-mutant cancers have been developed[56]. For RB, its loss is associated with enhanced efficacy for ionizing radiation therapy, chemotherapeutics such as cisplatin or adriamycin[57].
In 2018, Knudsen et al[57] of Thomas Jefferson University screened for therapeutic agents that may exhibit synthetic lethality with Rb loss. They found that drugs inhibiting checkpoint kinase1 (CHK1) or polo-like kinase 1 (PLK1) kinase exhibit greater lethality in Rb mutant tumors. The finding raises the potential of treating triple-negative breast cancers with dysfunctional Rb[58]. CHK1 encodes a serine/threonine kinase whose activation initiates cell cycle arrest in response to DNA damage during S, G2, and M phases. PLK1 is a serine/threonine kinase that activates cdc25C, which in turn activates cyclin B/cdc2 complex through dephosphorylation, as well as anaphase-promoting complex to transit from G2 to M phase.
In 2019, Gomaa et al[59] of the University of Miami Miller School of Medicine showed that reconstituting the microRNA, miR-4715--3p, reduced aurora kinase A level in MKN45 gastric cancer cells, resulting in chromosome polyploidy and cell death. miR-4715--3p is an epigenetic regulator that downregulates aurora kinase A expression by binding to the 3'-untranslated region of its mRNA for degradation[59].
In the same year, Sun et al[60] of the National Cancer Institute demonstrated that genetically silencing INCENP (Inner centromere protein) in neuroblastoma cells induces polyploidy and apoptosis. The INCENP gene encodes a key scaffolding component of chromosomal passenger complex consisting of survivin, aurora kinase B, borealin, and INCENP, which oversees proper alignment and segregation of chromosomes and cytokinesis during mitosis.
Additionally, Zheng et al[61] of the University of Texas M. D. Anderson Cancer Center showed that treating lung cancer cells with a highly selective tyrosine threonine kinase inhibitor, CFI-402257, causes aneuploidy and apoptosis. TTK (also called Mps1) or tyrosine threonine kinase is a component of mammalian spindle assembly checkpoint, which is integral to maintaining chromosome integrity.
In 2020, Simon Serrano et al[62] of the Lund University in Sweden reported that inhibiting Mps1 kinase (mitotic kinase monopolar spindle 1) induces hyperploid progression mediated death in neuroblastoma cells. The mechanism of death in Mps1 inhibited cells involves transiting through polyploidization/aneuploidization before the onset of mitotic catastrophe[62].
In 2010, Charité-Universitätsmedizin Berlin in Germany determined that combining the aurora kinase inhibitor ZM447439 significantly improves the antiproliferative effects of the chemotherapeutic drug cisplatin and streptozocin. For the study, the authors employed gastroenteropancreatic neuroendocrine tumor cells.
In 2017, Czech Academy of Sciences in the Czech Republic examined the effect of combining CHK1 kinase inhibitor SCH900776 with the DNA crosslinking drug cisplatin or platinum (IV)-LA-12 complexes in treating colon cancer. In p53 or p21 deficient cells, the combination therapy increased mitotic slippage, leading to polyploidy. Further, the delayed death caused by the drug combination in p53 deficient cells was accelerated by p21 deficiency[63].
In the same year, Bressy et al[64] of Université Paris-Saclay in France assessed the therapeutic efficacy of combining oncolytic adenovirus containing delta-24 deletion in the E1A gene with valproic acid, a histone deacetylase inhibitor for colon carcinoma. Previously, it was shown that E1A interacts with Rb; thus, the recombinant virus may selectively replicate in Rb-deficient cancer cells but not in normal cells expressing wild-type Rb. The co-treatment led to polyploidy with increased H2AX phosphorylation indicative of DNA damage, as well as elevated cell death[64].
In 2018, Kawakami et al[65] of the University of Texas M. D. Anderson Cancer Center reported that treating with CFI-400945, which inhibits polo-like kinase 4 regulating centriole duplication, causes polyploidy and mitotic defect, resulting in the death of lung cancer cells. Further, it was shown to cooperate with the Cdk2 inhibitor seliciclib[65].
In 2019, Gong et al[66] of Eli Lilly and Company showed that cell cycle inhibitors targeting aurora kinase B exhibit synthetic lethality with Rb mutant. LY3295668 is an inhibitor of Aurora kinase but exhibits with > 1000-fold selectivity against Aurora kinase B. Further, prolonged treatment was possible due to little toxicity against bone marrow[66].
A recent report in 2020 by Liu et al[67] of Kaohsiung Medical University in Taiwan showed that treating non-small-cell lung cancer cells with 4-HPPP [4-(4-(4-hydroxyphenoxy) phenoxy) phenol] caused polyploidy-specific cell death. The treatment resulted in cellular aneuploidization accompanied by the activation of double-strand DNA break markers such as Ataxia-telangiectasia-mutated and Rad3-related and gamma-H2AX. Previously, the phenoxyphenol derivatives have been suggested to sensitize the lung cancer cells to the topoisomerase inhibitor camptothecin by reducing the apoptosis-inducing threshold[68].
In the same year, Jemaà et al[69] of Lund University in Sweden described that treating tetraploid colon cancer cells with PLK1 inhibitor caused mitotic slippage, followed by apoptosis. Further, combining PLK1 inhibitor with vincristine or colchicine resulted in greater lethality, demonstrating a synergistic effect.
With the event of targeted therapy, an enormous capital has been spent by pharmaceutical industries on developing drugs that inhibit oncogenic signaling pathways. Equally staggering is the amount of federal funds allocated to assess their clinical efficacy. Yet, for the most part, benefits have been incremental. In the case of anti-ErbB2 drugs, only a modest gain in overall survival was achieved after combining with chemotherapy. With the PI3K inhibiting drugs, a similar outcome was observed upon combining with anti-hormone therapy for advanced-stage breast cancer. Besides, the cytostatic nature of these drugs may require perennial treatment, which may not be ideal as cancer cells could acquire genetic mutations to become resistant. Against this backdrop, the elucidation of “hyperploid progression mediated death” targeting Rb or p53 mutants assumes greater significance as it provides an opportunity to develop cytotoxic drugs with tumor specificity at the genetic level, thereby eliminating side effects.
We are grateful to Tri Ngo for assistance with computer-based molecular modeling. Also acknowledged are the faculties at Johns Hopkins University, U. C. Berkeley, Baylor University, Davidson College (alma mater of United States president Woodrow Wilson, Nobel Prize 1919), Hollywood High School (alma mater of Hollywood classics actors, ex. Judy Garland in ‘‘The Wizard of Oz’’), clinical staff (U. C. San Francisco), and actress in 'The Flying Nun’ (Warner Bros. Pictures, Hollywood). City of Hope National Medical Center (National Comprehensive Cancer Network member) was established partly through the assistance of Hollywood's film industries (ex. Warner Bros. Pictures), supported by Hollywood’s renown figures (Frank Sinatra, Bob Hope, Ella Fitzgerald, etc.), visited by United States First Lady Eleanor (Franklin D. Roosevelt presidency), United States presidential candidate Robert F. Kennedy, Queen Elizabeth II (United Kingdom), graced by Pope John Paul II (Vatican, Italy), and supported by numerous distinguished individuals/industries. Its Beckman Research Institute was established by A. Beckman (California Institute of Technology; Beckman Instruments).
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Q S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Niesters HG, Lenstra JA, Spaan WJ, Zijderveld AJ, Bleumink-Pluym NM, Hong F, van Scharrenburg GJ, Horzinek MC, van der Zeijst BA. The peplomer protein sequence of the M41 strain of coronavirus IBV and its comparison with Beaudette strains. Virus Res. 1986;5:253-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Vora S, Hong F, Olender E. Isolation of a cDNA for human muscle 6-phosphofructokinase. Biochem Biophys Res Commun. 1986;135:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987;235:1394-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 945] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 4. | Lee WH, Shew JY, Hong FD, Sery TW, Donoso LA, Young LJ, Bookstein R, Lee EY. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987;329:642-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 470] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Hong FD. Retinoblastoma protein has properties similar to the characteristics associated with structural proteins. Thesis, University of California at San Diego, 1992. Available from: https://www.proquest.com/products-services/dissertations/Milestone-Editions-for-Authors.html. |
| 6. | Hong FD, Huang HJ, To H, Young LJ, Oro A, Bookstein R, Lee EY, Lee WH. Structure of the human retinoblastoma gene. Proc Natl Acad Sci USA. 1989;86:5502-5506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Bookstein R, Rio P, Madreperla SA, Hong F, Allred C, Grizzle WE, Lee WH. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci USA. 1990;87:7762-7766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 212] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Hong F, Lee WH. Sequence similarity between part of human retinoblastoma susceptibility gene product and a neurofilament protein subunit. Biosci Rep. 1991;11:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Hensey CE, Hong F, Durfee T, Qian YW, Lee EY, Lee WH. Identification of discrete structural domains in the retinoblastoma protein. Amino-terminal domain is required for its oligomerization. J Biol Chem. 1994;269:1380-1387. [PubMed] |
| 10. | Lee WH, Hollingsworth RE, Qian YW, Chen PL, Hong F, Lee EY. RB protein as a cellular "corral" for growth-promoting proteins. Cold Spring Harb Symp Quant Biol. 1991;56:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Lee WH, Xu Y, Hong F, Durfee T, Mancini MA, Ueng YC, Chen PL, Riley D. The corral hypothesis: a novel regulatory mode for retinoblastoma protein function. Cold Spring Harb Symp Quant Biol. 1994;59:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Hong FD, Chen J, Donovan S, Schneider N, Nisen PD. Taxol, vincristine or nocodazole induces lethality in G1-checkpoint-defective human astrocytoma U373MG cells by triggering hyperploid progression. Carcinogenesis. 1999;20:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 13. | Un F, Meszaros A, Balogh G. Finding the genetic solution for cancer in the mechanism of Taxol cytotoxicity. Meszaros A, Balogh G. Multiple Drug Resistance. New York: Nova Science Publishers 2010; 219-223. |
| 14. | Un F, Qi C, Prosser M, Wang N, Zhou B, Bronner C, Yen Y. Modulating ICBP90 to suppress human ribonucleotide reductase M2 induction restores sensitivity to hydroxyurea cytotoxicity. Anticancer Res. 2006;26:2761-2767. [PubMed] |
| 15. | Un F. G1 arrest induction represents a critical determinant for cisplatin cytotoxicity in G1 checkpoint-retaining human cancers. Anticancer Drugs. 2007;18:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Prescott SM, White RL. Self-promotion? Intimate connections between APC and prostaglandin H synthase-2. Cell. 1996;87:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, Kurie JM, Hong WK, Lotan R. Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst. 1997;89:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, Fofonoff S, Byers R, Atkinson EN, Vaughan C. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 539] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 19. | Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 261] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Pratt WB. The Anticancer Drugs. New York: Oxford University Press, 1994: 155-182. |
| 21. | Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 345] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Cosman F. Selective estrogen-receptor modulators. Clin Geriatr Med. 2003;19:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 529] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 24. | Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 709] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 25. | Richardson DS, Johnson SA. Anthracyclines in haematology: preclinical studies, toxicity and delivery systems. Blood Rev. 1997;11:201-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 511] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 27. | Berry JL, Polski A, Cavenee WK, Dryja TP, Murphree AL, Gallie BL. The RB1 Story: Characterization and Cloning of the First Tumor Suppressor Gene. Genes (Basel). 2019;10:879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Hino O, Kobayashi T. Mourning Dr. Alfred G. Knudson: the two-hit hypothesis, tumor suppressor genes, and the tuberous sclerosis complex. Cancer Sci. 2017;108:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1992] [Cited by in RCA: 1885] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 30. | Lipsick J. A History of Cancer Research: Tumor Suppressor Genes. Cold Spring Harb Perspect Biol. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Tomar S, Sethi R, Sundar G, Quah TC, Quah BL, Lai PS. Mutation spectrum of RB1 mutations in retinoblastoma cases from Singapore with implications for genetic management and counselling. PLoS One. 2017;12:e0178776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Gudiseva HV, Berry JL, Polski A, Tummina SJ, O'Brien JM. Next-Generation Technologies and Strategies for the Management of Retinoblastoma. Genes (Basel). 2019;10:1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Burkhart DL, Morel KL, Sheahan AV, Richards ZA, Ellis L. The Role of RB in Prostate Cancer Progression. Adv Exp Med Biol. 2019;1210:301-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Chung PED, Gendoo DMA, Ghanbari-Azarnier R, Liu JC, Jiang Z, Tsui J, Wang DY, Xiao X, Li B, Dubuc A, Shih D, Remke M, Ho B, Garzia L, Ben-David Y, Kang SG, Croul S, Haibe-Kains B, Huang A, Taylor MD, Zacksenhaus E. Modeling germline mutations in pineoblastoma uncovers lysosome disruption-based therapy. Nat Commun. 2020;11:1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Bhateja P, Chiu M, Wildey G, Lipka MB, Fu P, Yang MCL, Ardeshir-Larijani F, Sharma N, Dowlati A. Retinoblastoma mutation predicts poor outcomes in advanced non-small cell lung cancer. Cancer Med. 2019;8:1459-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev. 2016;30:1492-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 37. | Kitajima S, Li F, Takahashi C. Tumor Milieu Controlled by RB Tumor Suppressor. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Vélez-Cruz R, Johnson DG. The Retinoblastoma (RB) Tumor Suppressor: Pushing Back against Genome Instability on Multiple Fronts. Int J Mol Sci. 2017;18:1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Burke JR, Hura GL, Rubin SM. Structures of inactive retinoblastoma protein reveal multiple mechanisms for cell cycle control. Genes Dev. 2012;26:1156-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Schwermer M, Hiber M, Dreesmann S, Rieb A, Theißen J, Herold T, Schramm A, Temming P, Steenpass L. Comprehensive characterization of RB1 mutant and MYCN amplified retinoblastoma cell lines. Exp Cell Res. 2019;375:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Casenghi M, Mangiacasale R, Tuynder M, Caillet-Fauquet P, Elhajouji A, Lavia P, Mousset S, Kirsch-Volders M, Cundari E. p53-independent apoptosis and p53-dependent block of DNA rereplication following mitotic spindle inhibition in human cells. Exp Cell Res. 1999;250:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Verdoodt B, Decordier I, Geleyns K, Cunha M, Cundari E, Kirsch-Volders M. Induction of polyploidy and apoptosis after exposure to high concentrations of the spindle poison nocodazole. Mutagenesis. 1999;14:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Tsuiki H, Nitta M, Tada M, Inagaki M, Ushio Y, Saya H. Mechanism of hyperploid cell formation induced by microtubule inhibiting drug in glioma cell lines. Oncogene. 2001;20:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Cassinelli G, Supino R, Perego P, Polizzi D, Lanzi C, Pratesi G, Zunino F. A role for loss of p53 function in sensitivity of ovarian carcinoma cells to taxanes. Int J Cancer. 2001;92:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Lanzi C, Cassinelli G, Cuccuru G, Supino R, Zuco V, Ferlini C, Scambia G, Zunino F. Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells. Prostate. 2001;48:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Qi M, Yao G, Fan S, Cheng W, Tashiro S, Onodera S, Ikejima T. Pseudolaric acid B induces mitotic catastrophe followed by apoptotic cell death in murine fibrosarcoma L929 cells. Eur J Pharmacol. 2012;683:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 950] [Cited by in RCA: 961] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 48. | Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, Miller KM. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 765] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 49. | Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI. The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res. 2006;66:7668-7677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Tao W, South VJ, Zhang Y, Davide JP, Farrell L, Kohl NE, Sepp-Lorenzino L, Lobell RB. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 51. | Dijkhuis AJ, Klappe K, Jacobs S, Kroesen BJ, Kamps W, Sietsma H, Kok JW. PDMP sensitizes neuroblastoma to paclitaxel by inducing aberrant cell cycle progression leading to hyperploidy. Mol Cancer Ther. 2006;5:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Chin GM, Herbst R. Induction of apoptosis by monastrol, an inhibitor of the mitotic kinesin Eg5, is independent of the spindle checkpoint. Mol Cancer Ther. 2006;5:2580-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | D'Alise AM, Amabile G, Iovino M, Di Giorgio FP, Bartiromo M, Sessa F, Villa F, Musacchio A, Cortese R. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol Cancer Ther. 2008;7:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 922] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 55. | Cheng L, Wang H, Guo K, Wang Z, Zhang Z, Shen C, Chen L, Lin J. Reversine, a substituted purine, exerts an inhibitive effect on human renal carcinoma cells via induction of cell apoptosis and polyploidy. Onco Targets Ther. 2018;11:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Zhao D, Tahaney WM, Mazumdar A, Savage MI, Brown PH. Molecularly targeted therapies for p53-mutant cancers. Cell Mol Life Sci. 2017;74:4171-4187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Knudsen ES, Wang JY. Targeting the RB-pathway in cancer therapy. Clin Cancer Res. 2010;16:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 58. | Witkiewicz AK, Chung S, Brough R, Vail P, Franco J, Lord CJ, Knudsen ES. Targeting the Vulnerability of RB Tumor Suppressor Loss in Triple-Negative Breast Cancer. Cell Rep. 2018;22:1185-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 59. | Gomaa A, Peng D, Chen Z, Soutto M, Abouelezz K, Corvalan A, El-Rifai W. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci Rep. 2019;9:16970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 60. | Sun M, Veschi V, Bagchi S, Xu M, Mendoza A, Liu Z, Thiele CJ. Targeting the Chromosomal Passenger Complex Subunit INCENP Induces Polyploidization, Apoptosis, and Senescence in Neuroblastoma. Cancer Res. 2019;79:4937-4950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Zheng L, Chen Z, Kawakami M, Chen Y, Roszik J, Mustachio LM, Kurie JM, Villalobos P, Lu W, Behrens C, Mino B, Solis LM, Silvester J, Thu KL, Cescon DW, Rodriguez-Canales J, Wistuba II, Mak TW, Liu X, Dmitrovsky E. Tyrosine Threonine Kinase Inhibition Eliminates Lung Cancers by Augmenting Apoptosis and Polyploidy. Mol Cancer Ther. 2019;18:1775-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Simon Serrano S, Sime W, Abassi Y, Daams R, Massoumi R, Jemaà M. Inhibition of mitotic kinase Mps1 promotes cell death in neuroblastoma. Sci Rep. 2020;10:11997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Herůdková J, Paruch K, Khirsariya P, Souček K, Krkoška M, Vondálová Blanářová O, Sova P, Kozubík A, Hyršlová Vaculová A. Chk1 Inhibitor SCH900776 Effectively Potentiates the Cytotoxic Effects of Platinum-Based Chemotherapeutic Drugs in Human Colon Cancer Cells. Neoplasia. 2017;19:830-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Bressy C, Majhen D, Raddi N, Jdey W, Cornilleau G, Zig L, Guirouilh-Barbat J, Lopez BS, Bawa O, Opolon P, Grellier E, Benihoud K. Combined therapy of colon carcinomas with an oncolytic adenovirus and valproic acid. Oncotarget. 2017;8:97344-97360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Kawakami M, Mustachio LM, Zheng L, Chen Y, Rodriguez-Canales J, Mino B, Kurie JM, Roszik J, Villalobos PA, Thu KL, Silvester J, Cescon DW, Wistuba II, Mak TW, Liu X, Dmitrovsky E. Polo-like kinase 4 inhibition produces polyploidy and apoptotic death of lung cancers. Proc Natl Acad Sci USA. 2018;115:1913-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 66. | Gong X, Du J, Parsons SH, Merzoug FF, Webster Y, Iversen PW, Chio LC, Van Horn RD, Lin X, Blosser W, Han B, Jin S, Yao S, Bian H, Ficklin C, Fan L, Kapoor A, Antonysamy S, Mc Nulty AM, Froning K, Manglicmot D, Pustilnik A, Weichert K, Wasserman SR, Dowless M, Marugán C, Baquero C, Lallena MJ, Eastman SW, Hui YH, Dieter MZ, Doman T, Chu S, Qian HR, Ye XS, Barda DA, Plowman GD, Reinhard C, Campbell RM, Henry JR, Buchanan SG. Aurora A Kinase Inhibition Is Synthetic Lethal with Loss of the RB1 Tumor Suppressor Gene. Cancer Discov. 2019;9:248-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 67. | Liu W, Wu CY, Lu MJ, Chuang YJ, Tsai EM, Leu S, Lin IL, Ko CJ, Chiu CC, Chang WT. The Phenoxyphenol Compound 4-HPPP Selectively Induces Antiproliferation Effects and Apoptosis in Human Lung Cancer Cells through Aneupolyploidization and ATR DNA Repair Signaling. Oxid Med Cell Longev. 2020;2020:5167292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Chou HL, Fong Y, Wei CK, Tsai EM, Chen JY, Chang WT, Wu CY, Huang HW, Chiu CC. A Quinone-Containing Compound Enhances Camptothecin-Induced Apoptosis of Lung Cancer Through Modulating Endogenous ROS and ERK Signaling. Arch Immunol Ther Exp (Warsz). 2017;65:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Jemaà M, Kifagi C, Serrano SS, Massoumi R. Preferential Killing of Tetraploid Colon Cancer Cells by Targeting the Mitotic Kinase PLK1. Cell Physiol Biochem. 2020;54:303-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |