Published online Feb 24, 2019. doi: 10.5306/wjco.v10.i2.86
Peer-review started: July 30, 2018
First decision: September 11, 2018
Revised: September 14, 2018
Accepted: December 4, 2018
Article in press: December 5, 2018
Published online: February 24, 2019
Processing time: 207 Days and 10.7 Hours
To investigate infused hematopoietic cell doses and their interaction with conditioning regimen intensity +/- total body irradiation (TBI) on outcomes after peripheral blood hematopoietic cell transplant (PBHCT).
Our retrospective cohort included 247 patients receiving a first, T-replete, human leukocyte antigen-matched allogeneic PBHCT and treated between 2001 and 2012. Correlations were calculated using the Pearson product-moment correlation coefficient. Overall survival and progression free survival curves were generated using the Kaplan-Meier method and compared using the log-rank test.
Neutrophil engraftment was significantly faster after reduced intensity TBI based conditioning [reduced intensity conditioning (RIC) + TBI] and > 4 × 106 CD34+ cells/kg infused. A higher total nucleated cell dose led to a higher incidence of grade II-IV acute graft-versus-host disease in the myeloablative + TBI regimen group (P = 0.03), but no significant difference in grade III-IV graft-versus-host disease. A higher total nucleated cell dose was also associated with increased incidence of moderate/severe chronic graft-versus-host disease, regardless of conditioning regimen. Overall and progression-free survival were significantly better in patients with a RIC + TBI regimen and total nucleated cell dose > 8 × 108/kg (3 years, overall survival: 70% vs 38%, P = 0.02, 3 years, progression free survival: 64% vs 38%, P = 0.02).
TBI and conditioning intensity may alter the relationship between infused cell doses and outcomes after PBHCT. Immune cell subsets may predict improved survival after unmanipulated PBHCT.
Core tip: This study investigated infused hematopoietic cell doses and their interaction with conditioning regimen intensity on outcomes after peripheral blood hematopoietic cell transplant. Our retrospective cohort included 247 patients receiving a first, T-replete, human leukocyte antigen-matched allogeneic peripheral blood hematopoietic cell transplant. Neutrophil engraftment was significantly faster after reduced intensity total body irradiation and > 4 × 106 CD34+ cells/kg infused. Overall and progression-free survival was significantly better in patients with a reduced intensity conditioning and total body irradiation regimen and total nucleated cell dose > 8 × 108/kg.
- Citation: Burns M, Singh AK, Hoefer CC, Zhang Y, Wallace PK, Chen GL, Platek A, Winslow TB, Iovoli AJ, Choi C, Ross M, McCarthy PL, Hahn T. Impact of conditioning regimen on peripheral blood hematopoietic cell transplant. World J Clin Oncol 2019; 10(2): 86-97
- URL: https://www.wjgnet.com/2218-4333/full/v10/i2/86.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i2.86
Peripheral blood hematopoietic cell transplant (PBHCT) is the most commonly used allogeneic hematopoietic cell source due to its faster rate of neutrophil engraftment[1-4]. The optimal CD34+ cell dose range to minimize time to neutrophil and platelet recovery without increasing risk of acute graft-versus-host disease (GvHD) is 4-10 × 106/kg[5-11]. Some studies have reported a higher CD34+ cell dose yields improved overall survival (OS)[9,12-14], while others have found no significant association[7,10,15-18].
A higher total nucleated cell (TNC) dose has been reported to improve survival after PBHCT[14,16], but analyses of specific T-cell subsets (CD4+, CD8+, natural killer cells) have been inconsistent[17-19]. Factors such as T-cell depletion, conditioning regimen intensity, use of total body irradiation (TBI), and donor age may be interacting with graft cell doses to generate different effects on PBHCT outcomes. In addition, flow cytometric enumeration of cell doses are not standardized (except for CD34+ cell dose) and may also lead to differences in results between studies.
In our retrospective study, we explored whether the collected and infused CD34+, CD3+, CD4+, CD8+, or TNC dose influenced engraftment, OS, progression free survival (PFS), and incidence of acute and chronic GvHD, and whether the results were affected by conditioning regimen intensity or use of TBI.
This retrospective cohort study included 247 consecutive adult (≥ 18 years old) patients receiving their first allogeneic PBHCT between January 2001 and September 2012. Patients receiving syngeneic, human leukocyte antigen (HLA)-mismatched, T-cell depleted, or bone marrow transplants were excluded from this analysis. This study was reviewed and approved by the Institutional Review Board of Roswell Park Cancer Institute.
Four conditioning regimen groups were defined a priori as (1) myeloablative (MA) without TBI (MA-noTBI), (2) myeloablative with TBI (MA + TBI), (3) reduced intensity conditioning (RIC) without TBI (RIC-noTBI) and (4) RIC with TBI (RIC + TBI). These are described in Table 1. Conditioning regimens were assigned based on institutional standards including: (1) patients aged ≥ 60 years: received RIC regimens, (2) patients aged 41-59 years: a RIC regimen was preferred for patients with any of the following criteria: HLA mismatch, Karnofsky Performance Score (KPS) < 70, extensive co-morbidities, recent smoking history, (3) patients aged 19-40 years: a myeloablative regimen was preferred unless the patient had an HLA mismatched donor, KPS < 70, severe co-morbidity, and (4) patients aged ≤ 40 years with acute lymphoid leukemia: TBI regimen.
| Conditioning regimen | Number of patients | Protocol |
| Myeloablative without TBI (MA-noTBI) | 38 | Bu 12.8 mg/kg intravenous total dose and Cy 120 mg/kg total dose |
| Myeloablative with TBI (MA + TBI) | 51 | Cy 120 mg/kg total dose and TBI 1000-1350 cGy |
| Reduced intensity conditioning without TBI (RIC-noTBI) | 118 | Flu 125 mg/m2 total dose and Mel 140 mg/m2 total dose |
| Reduced intensity conditioning with TBI (RIC + TBI) | 40 | Flu 160 mg/m2 total dose, Mel 50-75 mg/m2 total dose, and TBI 400 cGy |
Donor marrow was stimulated with 10 mg/kg of granulocyte-colony stimulating factor for a minimum of 2 d and continued until white blood cell count was > 8000 × 109/L; the attending bone marrow transplant physician provided a target CD34+ cell dose to be collected and, for related donors, approved the final dose collected and the end of apheresis. Most donors underwent apheresis for 1 d.
Apheresis product cell doses were determined using multi-parameter flow cytometry. CD34+ cell counts were obtained using the ISHAGE protocol[20], substituting 7-aminoactinomycin D with TO-PRO. CD3+, CD4+, and CD8+ cell counts used standard methodology[21]. TNC doses were determined by multiplying the white blood cell count (× 108/mL) on the day of apheresis by the volume of the product. Each cell count in the final infused product was divided by the actual recipient weight in kilograms measured within 2 d of the start of conditioning regimen to calculate the cell dose infused.
CD34+ cell dose was analyzed using previously published categories of < 4, 4-8, > 8 × 106/kg. CD3+, CD4+, and CD8+ cell doses were analyzed above and below the respective median cell doses in the study population. TNC dose was analyzed as above and below the median cell doses and also with various doses ranging from 7-10 × 108 cells/kg to determine an optimal TNC dose threshold.
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count > 0.5 × 109/L. Platelet engraftment was defined as the first date with a platelet count > 20 × 109/L after 7 consecutive days with no platelet transfusions. PFS was calculated as the time from PBHC infusion to date of first disease progression post-PBHCT or date of death from any cause; survivors without disease progression were censored at date of last follow-up. OS was calculated as the time from PBHC infusion to date of death from any cause with survivors censored at date of last follow-up. Acute and chronic GvHD were graded using standard definitions[22-23].
The statistical methods of this study were reviewed by Yali Zhang from Roswell Park Comprehensive Cancer Center. Correlations between TNC dose and CD3+ dose, CD4+ dose, CD8+ dose, and CD34+ dose were calculated using the Pearson product-moment correlation coefficient. The cumulative incidence of acute and chronic GvHD was analyzed adjusting for the competing risk of disease relapse. Univariable analysis of OS and PFS were analyzed as time-to-event; survival curves were generated using the Kaplan-Meier method and were compared using the log-rank test. Multivariable analyses tested each cell dose while adjusting for significant factors in the univariate analysis, first in all patients and then stratified by the four conditioning regimen groups. Variables included in the multivariable analyses were age (≥/< 40 years), KPS (≥/< 80) at time of transplant, and BMI (≥/< 35 kg/m2). All analyses were performed using SAS version 9.4.
The cohort consisted of 135 sibling and 112 unrelated donor transplant recipients. Sibling donors were 6/6 HLA-matched at HLA-A, -B, and -DRB1. Unrelated donors were 10/10 HLA-matched at HLA-A, -B, -C, -DRB1, and DQB1 (3 patients were 8/8 HLA-matched at HLA-A, -B, -C, -DRB1). Patients who received a MA regimen were significantly younger, had a higher KPS, more commonly had a sibling donor, tacrolimus/methotrexate GvHD prophylaxis regimen, and were treated for different diseases than patients who received a RIC regimen (Table 2).
| MA-noTBI (n = 38) | MA+TBI (n = 51) | RIC-noTBI (n = 118) | RIC+TBI (n = 40) | P | |
| Age at BMT | < 0.0001 | ||||
| Median-years (range) | 47 (26-58) | 36 (19-51) | 54 (23-73) | 61 (23-71) | |
| < 40 | 6 (16) | 29 (57) | 22 (19) | 3 (8) | |
| ≥ 40 | 32 (84) | 22 (43) | 96 (81) | 37 (93) | |
| Gender | NS | ||||
| Female | 21 (55) | 19 (37) | 45 (38) | 22 (55) | |
| Male | 17 (45) | 32 (63) | 73 (62) | 18 (45) | |
| Diagnosis | < 0.0001 | ||||
| ALL | 0 | 20 (39) | 7 (6) | 6 (15) | |
| AML | 11 (29) | 28 (55) | 58 (49) | 14 (35) | |
| CML | 7 (18) | 1 (2) | 3 (3) | 1 (3) | |
| MDS/MPD | 11 (29) | 1 (2) | 23 (19) | 10 (25) | |
| NHL/CLL/PLL | 8 (21) | 1 (2) | 25 (21) | 8 (20) | |
| Other | 1 (3) | 0 | 2 (2) | 1 (3) | |
| Karnofsky Performance Status | 0.03 | ||||
| ≤ 70 | 8 (21) | 8 (16) | 35 (30) | 13 (33) | |
| 80 | 13 (34) | 15 (29) | 51 (43) | 14 (35) | |
| ≥ 90 | 17 (45) | 28 (55) | 32 (27) | 13 (33) | |
| BMT Regimen | < 0.0001 | ||||
| BuCy | 36 (95) | 0 | 0 | 0 | |
| CyTBI | 0 | 47 (92) | 0 | 0 | |
| FluCy | 0 | 0 | 12 (10) | 0 | |
| FluMel | 0 | 0 | 102 (86) | 0 | |
| FluMelTBI | 0 | 0 | 0 | 40 (100) | |
| Other | 2 (5) | 4 (8) | 4 (3) | 0 | |
| Sex Match | NS | ||||
| Matched | 24 (63) | 30 (59) | 70 (59) | 27 (68) | |
| Mismatched | 14 (37) | 21 (41) | 48 (41) | 13 (33) | |
| Donor | < 0.0001 | ||||
| HLA Matched Related | 33 (87) | 31 (61) | 54 (46) | 17 (43) | |
| HLA Matched Unrelated | 5 (13) | 20 (39) | 64 (54) | 23 (58) | |
| GvHD Prophylaxis | < 0.0001 | ||||
| TacMtx | 18 (47) | 35 (69) | 33 (28) | 0 | |
| TacMMF | 4 (11) | 2 (4) | 18 (15) | 0 | |
| TacmMtxMMF | 15 (39) | 7 (14) | 64 (54) | 40 (100) | |
| Single Agent | 1 (3) | 7 (14) | 3 (3) | 0 | |
| CMV Status | NS | ||||
| R+D+ | 6 (16) | 8 (16) | 28 (24) | 9 (23) | |
| R+D- | 14 (37) | 12 (24) | 35 (30) | 12 (30) | |
| R-D+ | 0 | 8 (16) | 18 (15) | 4 (10) | |
| R-D- | 18 (47) | 23 (45) | 37 (31) | 15 (38) | |
| BMI kg/m2 | NS | ||||
| Normal (< 30) | 15 (39) | 25 (49) | 34 (29) | 13 (33) | |
| Overweight (25-< 30) | 12 (32) | 14 (27) | 40 (34) | 15 (38) | |
| Obese (≥ 30-< 35) | 7 (18) | 7 (14) | 28 (24) | 7 (18) | |
| Morbid (≥ 35) | 4 (11) | 5 (10) | 16 (14) | 5 (13) |
Median (range) cell doses for the whole cohort were 264.3 (10.4-1137.5) × 106/kg for CD3+, 166.2 (8.3-590.9) × 106/kg for CD4+, 103.7 (2.2-590.9) × 106/kg for CD8+, 6.5 (0.9-27.6) × 106/kg for CD34+, and 8.3 (1.4-21.4) × 108/kg for TNC. Graft composition for conditioning subgroups are detailed in Supplementary Table 1.
The cumulative incidence of neutrophil engraftment was 99% at day 28 post-PBHCT. Six patients died on days 3, 5, 12, 20, 26, and 36 before neutrophil engraftment. Overall, patients who received a CD34+ cell dose > 4 × 106/kg experienced faster neutrophil engraftment (median 13 d vs 15 d, P = 0.05) as compared to patients who received a CD34+ cell dose < 4 × 106/kg. Analysis by conditioning regimen demonstrated significantly faster neutrophil engraftment for an infused CD34+ cell dose > 4 × 106/kg in the RIC + TBI group (median 15 d vs 18 d, P = 0.01) and no statistically significant differences by CD34+ cell dose for the other three conditioning regimen groups (Table 3). There were no significant differences in time to neutrophil engraftment by CD3+, CD4+, CD8+, and TNC dose either overall or in any conditioning subgroup (Supplementary Table 2).
| Conditioning group | ||||
| MA-noTBI (n = 38) | MA + TBI (n = 51) | RIC-noTBI (n = 118) | RIC + TBI (n = 40) | |
| Median days to engraftment (range) | ||||
| Absolute neutrophil count > 500/mm3 | ||||
| CD34+ dose > 4 × 106/kg | 14 (10-22) | 14 (9-28) | 14 (3-36) | 15 (10-22) |
| CD34+ dose < 4 × 106/kg | 14.5 (12-19) | 19 (13-28) | 15 (10-21) | 18 (16-24) |
| P | NS | NS | NS | 0.01 |
| Platelet count > 20000/mm3 | ||||
| CD34+ dose > 4 × 106/kg | 17 (3-171) | 20 (13-81) | 17 (3-1515) | 17 (10-866) |
| CD34+ dose < 4 × 106/kg | 19.5 (15-32) | 34 (20-228) | 22 (14-275) | 18 (11-20) |
| P | NS | 0.001 | 0.01 | NS |
Five patients did not nadir their platelet count below 20000/mm3 post-PBHCT and were excluded from the analysis of platelet engraftment. The cumulative incidence of platelet engraftment was 89% at day 40 post-PBHCT. One patient failed to engraft platelets and had a second transplant on day 44. Ten patients died before day 40, three patients died between days 41 to 100, and one patient died 6 mo post-PBHCT without platelet engraftment. Overall, patients who received a CD34+ cell dose > 4 × 106/kg experienced significantly faster platelet engraftment (median 16 d vs 20 d, P = 0.001) as compared to patients with a CD34+ cell dose < 4 × 106/kg. Analysis by conditioning regimen demonstrated significantly faster platelet engraftment in patients with a CD34+ cell dose > 4 × 106/kg for the MA + TBI group (median 20 d vs 34 d, P = 0.001), and the RIC-noTBI group (median 17 d vs 22 d, P = 0.01), but no statistically significant differences in time to platelet engraftment by CD34+ cell dose for the other two conditioning regimen groups (Table 3). Platelet engraftment was significantly faster in patients who received a higher CD3+ or CD8+ cell dose in the RIC-noTBI group, but not in any of the other conditioning regimen groups. CD4+ and TNC cell doses were not significant (Supplementary Table 2).
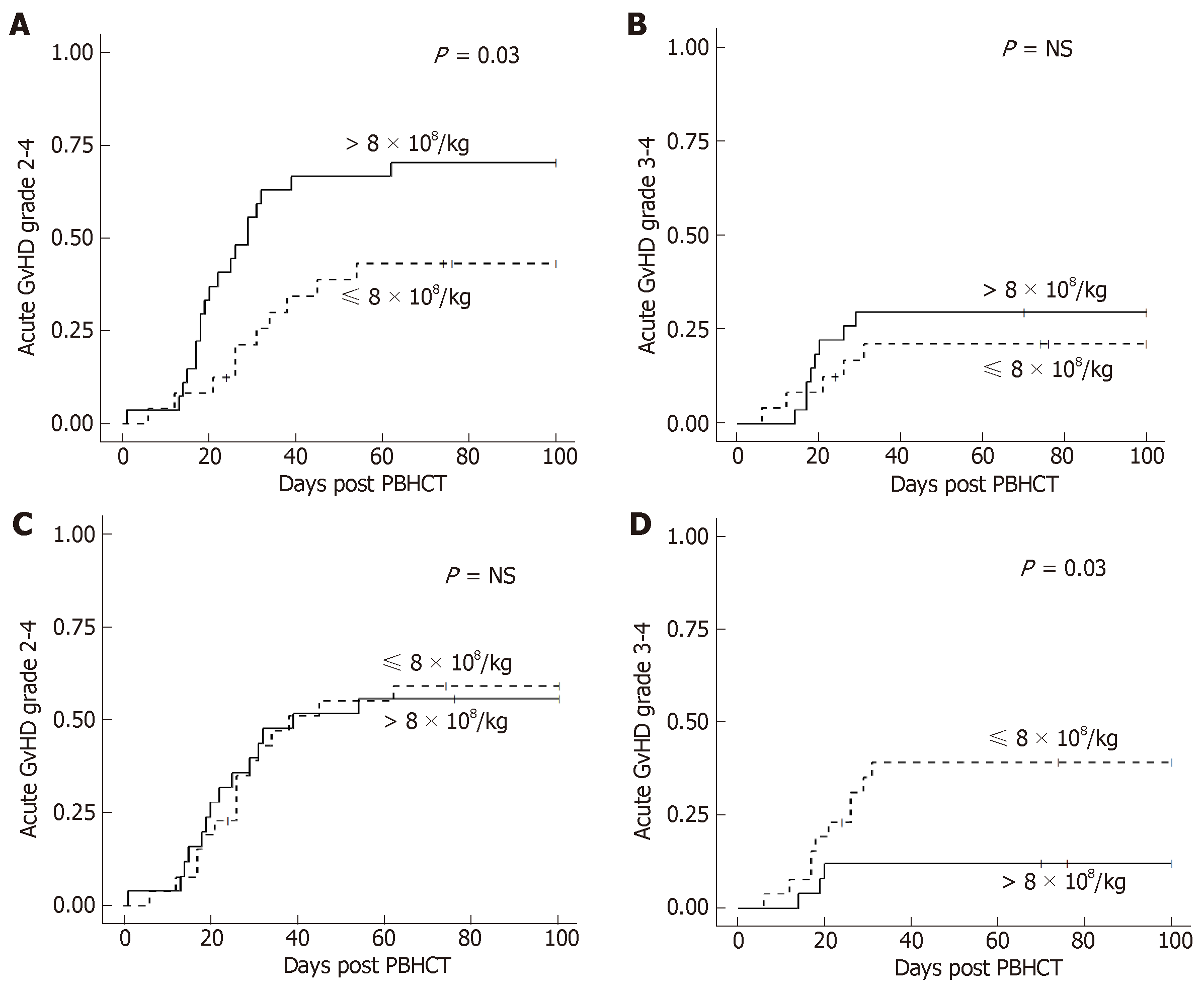
In the MA + TBI conditioning regimen group, there was a higher incidence of grade II-IV acute GvHD in patients who received a TNC dose > 8 × 108/kg, however there was no difference in grade III-IV acute GvHD (Figure 1A and 1B). Conversely, there was a higher incidence of grade III-IV acute GvHD in patients who received a lower CD34+ cell dose (≤ 8 × 106/kg), however there was no difference in grade II-IV acute GvHD by CD34+ cell dose (Figure 1C and 1D). These effects with TNC and CD34+ dose in MA + TBI were not seen in any of the other conditioning regimen groups. There were no statistically significant associations of CD3+, CD4+, or CD8+ dose with acute GvHD overall or in any conditioning regimen subgroup.
There was no significant association of chronic GvHD incidence with a TNC dose of > 8 × 108/kg either overall or by conditioning regimen. There was a significantly higher incidence of moderate to severe chronic GvHD in all patients who received a TNC dose > 9 × 108/kg (P = 0.004) but was not statistically significant in any conditioning regimen subgroup. There was no association of CD34+, CD3+, CD4+, or CD8+ cell dose with chronic GvHD either overall or in any conditioning regimen group.
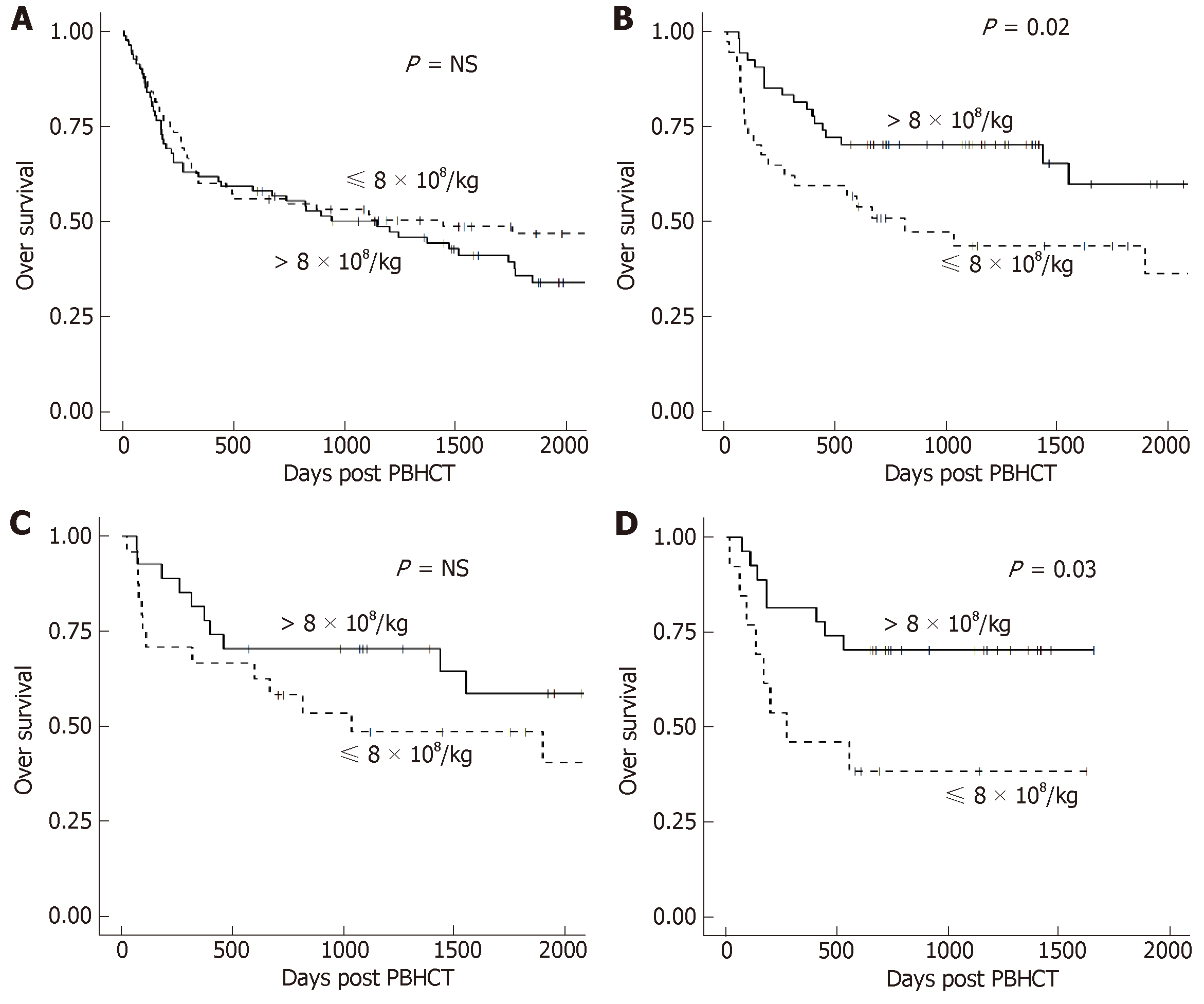
Median follow-up in all patients was 4.8 years (range 1.6-12 years). CD34+, CD3+, CD4+, and CD8+ cell doses were not associated with either OS or PFS in all patients or stratified by conditioning regimen. TNC dose showed no significant difference in OS or PFS when analyzed in all patients (Figure 2A). However, a significant improvement in OS was seen in patients with TBI-based conditioning regimens who received higher (> 8 × 108/kg) TNC doses (Figure 2B). Further analysis showed this effect was restricted to the RIC + TBI (Figure 2D) group with no significant difference in the MA + TBI group (Figure 2C). Similar results were found with PFS: a higher (> 8 × 108/kg) TNC dose was associated with improved PFS in patients who received TBI-based conditioning regimens, which was driven by the RIC + TBI subgroup.
Based on the univariate analysis, age, KPS, and BMI were included as covariates in the multivariable analysis of each cell dose with OS, and KPS and BMI were included as covariates in the multivariable analysis of each cell dose with PFS (Table 4). Similar to the univariate analysis, TNC dose > 8 × 108 cells/kg was associated with improved OS and PFS in patients who received TBI-based conditioning regimens. However, upon further stratification, this finding was statistically significant only in the RIC + TBI conditioning group.
| Variable | Overall survival1 | Progression free survival2 | ||||||||
| All patients (n = 247) | MA-noTBI (n = 38) | MA + TBI (n = 51) | RIC-noTBI (n = 118) | RIC + TBI (n = 40) | All patients (n = 247) | MA-noTBI (n = 38) | MA + TBI (n = 51) | RIC-noTBI (n = 118) | RIC + TBI (n = 40) | |
| CD3+ cell dose | ||||||||||
| < Median HR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| > Median HR | 1.1 | 1.0 | 0.5 | 1.3 | 0.8 | 1.0 | 1.1 | 0.4 | 1.4 | 0.6 |
| 95%CI | 0.8-1.5 | 0.4-2.6 | 0.2-1.1 | 0.8-2.1 | 0.3-2.2 | 0.7-1.4 | 0.4-2.6 | 0.2-1.0 | 0.8-2.2 | 0.2-1.7 |
| P | NS | NS | NS | NS | NS | NS | NS | 0.05 | NS | NS |
| CD4+ cell dose | ||||||||||
| < Median HR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| > Median HR | 1.2 | 1.1 | 0.6 | 1.2 | 2.0 | 1.1 | 1.1 | 0.5 | 0.3 | 1.5 |
| 95%CI | 0.8-1.7 | 0.4-2.6 | 0.2-1.3 | 0.8-1.0 | 0.7-6.0 | 0.8-1.6 | 0.5-2.6 | 0.2-1.2 | 0.8-2.1 | 0.5-4.3 |
| P | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| CD8+ cell dose | ||||||||||
| < Median HR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| > Median HR | 1.2 | 1.5 | 1.0 | 1.3 | 0.5 | 1.1 | 1.1 | 0.9 | 1.2 | 0.7 |
| 95%CI | 0.8-1.6 | 0.5-4.2 | 0.4-2.3 | 0.8-2.1 | 0.2-1.8 | 0.8-1.5 | 0.5-2.8 | 0.4-2.0 | 0.8-2.0 | 0.2-2.0 |
| P | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| TNC dose | ||||||||||
| < Median HR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| > Median HR | 0.8 | 1.0 | 0.7 | 0.9 | 0.2 | 0.8 | 1.1 | 0.7 | 1.1 | 0.2 |
| 95%CI | 0.6-1.2 | 0.4-2.4 | 0.3-1.9 | 0.5-1.5 | 0.1-0.8 | 0.6-1.2 | 0.4-2.5 | 0.3-1.6 | 0.6-1.8 | 0.1-0.8 |
| P | NS | NS | NS | NS | 0.02 | NS | NS | NS | NS | 0.02 |
| CD34+ cell dose | ||||||||||
| < 4 × 106/kg HR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4-8 × 106/kg HR | 1.0 | 0.5 | 1.3 | 1.1 | 0.6 | 0.8 | 0.7 | 1.2 | 1.0 | 0.6 |
| 95%CI | 0.6-1.5 | 0.2-1.4 | 0.4-4.4 | 0.6-1.9 | 0.1-2.3 | 0.6-1.4 | 0.2-2.1 | 0.4-4.0 | 0.6-1.8 | 0.2-2.6 |
| P | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| > 8 × 106/kg HR | 1.0 | 1.3 | 0.7 | 1.1 | 0.5 | 0.9 | 1.5 | 0.6 | 1.1 | 0.5 |
| 95%CI | 0.6-1.5 | 0.4-4.0 | 0.2-2.3 | 0.5-2.0 | 0.1-2.4 | 0.6-1.4 | 0.5-4.6 | 0.2-2.0 | 0.6-2.0 | 0.1-2.6 |
| P | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
To investigate potential correlations between cell types, Table 5 summarizes the matrix of Pearson correlations between cell doses. While most cell doses are significantly and positively correlated with the others (P < 0.001), most correlation coefficients were low. Pearson r2 < 0.5 means < 50% of the difference between cell doses can be explained by the linear relationship between the two. CD3+ cell dose is correlated with CD4+, CD8+, and TNC cell doses (r2: 0.5-0.83, Table 4), however CD34+ cell dose is not correlated with any of the other cell types (r2 < 0.05) and is thus an independent cell type.
| CD3+ dose | CD4+ dose | CD8+ dose | CD34+ dose | ||
| CD4+ dose | |||||
| R | 0.91 | ||||
| R2 | 0.83 | ||||
| P | < 0.0001 | ||||
| CD8+ dose | |||||
| R | 0.81 | 0.55 | |||
| R2 | 0.66 | 0.30 | |||
| P | < 0.0001 | < 0.0001 | |||
| CD34+ dose | |||||
| R | 0.20 | 0.21 | 0.11 | ||
| R2 | 0.04 | 0.04 | 0.01 | ||
| P | 0.0012 | 0.0009 | NS | ||
| Total nucleated cell dose | |||||
| R | 0.71 | 0.64 | 0.56 | 0.38 | |
| R2 | 0.50 | 0.41 | 0.31 | 0.14 | |
| P | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
The effect of infused cell dose on post-transplant outcomes is complex. Our single center study is the first to analyze the relationship of conditioning regimen intensity and use of TBI with infused cell doses. A recent study demonstrated that in reduced intensity transplant without TBI, TNC dose was associated with improved PFS and OS similar to our results in reduced intensity conditioning with or without TBI[24]. Martin et al[24] reported that higher TNC dose was also associated with decreased relapse and increased incidence of chronic GvHD.
Our results indicate that overall CD34+ cell dose is not associated with OS or PFS in our patient population as observed in other studies[9,11-13,24]. This differs from a study in T-cell depleted transplants after myeloablative TBI conditioning, which reported CD34+ doses between 4-8 × 106/kg were optimal for OS, and anything above or below this range resulted in increased mortality[25]. Gorin et al[16] demonstrated RIC + TBI patients receiving a TNC dose > 9.1 × 108/kg had improved PFS, which was similar to our results.
There was no association in patients with higher CD3+, CD4+, CD8+, or CD34+ doses and OS or GvHD, however recent studies indicated that an optimal CD34+ cell dose can lead to improved survival, less GvHD, and improved engraftment[5-11]. Our results demonstrated an association with TNC dose, which could indicate there is another graft cell subset that may better predict these outcomes. One candidate is the natural killer (NK) cell. A low donor NK cell dose was associated with significantly longer time to engraftment and worse OS[16]. NK cells have also been implicated as an important modulator of GvHD and the graft vs leukemia effect[26]. It is possible that increasing the donor NK cell dose could allow for a more robust graft vs leukemia effect without increasing risk of GvHD[27]. Focusing on the recipient, previous work demonstrated that host NK cells are relatively radiation-resistant and may decrease the incidence and severity of GvHD[28-30]. Thus, in the setting of low dose TBI, host NK cells could be preserved and mediate a decrease in GvHD while allowing for an improved graft vs leukemia effect, translating into an improved PFS/OS.
Further confirmation of our results in a larger, multi-center registry study could be performed. In addition, analysis of other cell populations (i.e., NK cell dose) could explain our findings. Further understanding of the impact of graft composition on post-transplant outcomes, and their potential interactions with conditioning regimens could allow physicians to better target certain cell doses in order to improve post-transplant survival outcomes.
Peripheral blood hematopoietic cell transplant (PBHCT) is the most commonly used allogeneic hematopoietic cell source due to its quick rate of neutrophil engraftment. A higher total nucleated cell (TNC) dose has been reported to improve survival after PBHCT, but analyses of specific T-cell subsets have been inconsistent. Factors such as T-cell depletion, conditioning regimen intensity, use of total body irradiation (TBI), and donor age may be interacting with graft cell doses to generate different effects on PBHCT outcomes. In addition, flow cytometric enumeration of cell doses are not standardized and may also lead to differences in results between studies.
While the optimal CD34+ cell dose range to minimize time to neutrophil and platelet recovery without increasing risk of acute graft-versus-host disease has been found to be 4-10 × 106/kg, some studies have reported a higher CD34+ cell dose yields improved overall survival while others have found no significant association. Further understanding of the impact of graft composition on post-transplant outcomes, and their potential interactions with conditioning regimens, could allow physicians to better target certain cell doses in order to improve post-transplant survival outcomes.
The objectives of this study were to examine whether the collected and infused CD34+, CD3+, CD4+, CD8+ or TNC dose influenced engraftment, overall survival, progression free survival, and incidence of acute and chronic GvHD, and whether the results were affected by conditioning regimen intensity or use of TBI.
Four conditioning regimen groups were defined a priori as (1) myeloablative (MA) without TBI (MA-noTBI), (2) myeloablative with TBI (MA + TBI), (3) Reduced intensity conditioning (RIC) without TBI (RIC-noTBI) and (4) RIC with TBI (RIC + TBI). Correlations between TNC dose and CD3+ dose, CD4+ dose, CD8+ dose, CD34 dose were calculated using the Pearson product-moment correlation coefficient. The cumulative incidence of acute and chronic GvHD was analyzed adjusting for the competing risk of disease relapse. Univariable analysis of OS and progression free survival (PFS) were analyzed as time-to-event; survival curves were generated using the Kaplan-Meier method and were compared using the log-rank test. Multivariable analyses tested each cell dose while adjusting for significant factors in the univariate analysis, first in all patients and then stratified by the four conditioning regimen groups. These analyses allowed us to explore the interaction of conditioning regimen and allogeneic donor apheresis product composition in relation to outcomes after unmanipulated peripheral blood hematopoietic cell transplantation.
The cohort consisted of 135 sibling and 112 unrelated donor transplant recipients. Overall, patients who received a CD34+ cell dose > 4 × 106/kg experienced faster neutrophil engraftment and platelet engraftment as compared to patients who received a CD34+ cell dose < 4 × 106/kg. Analysis by conditioning regimen demonstrated significantly faster neutrophil engraftment for an infused CD34+ cell dose > 4 × 106/kg in the RIC + TBI group. Overall and progression-free survival was significantly better in patients with a RIC + TBI regimen and TNC dose > 8 × 108/kg. Our results indicated that overall CD34+ cell dose is not associated with OS or PFS in our patient population, similar to other studies. We did find an overall and progression-free survival benefit in patients with a RIC + TBI regimen and TNC dose > 8 × 108/kg, which could indicate there is another graft cell subset that may better predict these outcomes.
Our single center study is the first to analyze the relationship of conditioning regimen intensity and use of TBI with infused cell doses. Neutrophil engraftment was significantly faster after reduced intensity TBI based conditioning and > 4 × 106 CD34+ cells/kg infused. In addition, overall and progression-free survival were significantly better in patients with a RIC + TBI regimen and TNC dose > 8 × 108/kg. Our study suggested that TBI and conditioning intensity may alter the relationship between infused cell doses and outcomes after PBHCT. Our results demonstrated that immune cell subsets may predict improved survival after unmanipulated PBHCT.
This study suggests that TBI and conditioning intensity may alter the relationship between infused cell doses and outcomes after PBHCT. Further confirmation of our results in a larger, multi-center registry study could be performed. In addition, analysis of other cell populations, such as NK cell dose, could explain our findings. Further understanding of the impact of graft composition on post-transplant outcomes, and their potential interactions with conditioning regimens could allow physicians to better target certain cell doses in order to improve post-transplant survival outcomes.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vikey AK, Zhou J S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. In Center for International Blood Marrow Transplant Research Conference, 2014. . |
| 2. | Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J, Shepherd V, Rowland A, Sirohi B, Tait D, Horton C, Long S, Singhal S. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000;355:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 265] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, Ehninger G, Johnston L, Maziarz RT, Pulsipher MA, Porter DL, Mineishi S, McCarty JM, Khan SP, Anderlini P, Bensinger WI, Leitman SF, Rowley SD, Bredeson C, Carter SL, Horowitz MM, Confer DL; Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells vs bone marrow from unrelated donors. N Engl J Med. 2012;367:1487-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 681] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 4. | Singhal S, Powles R, Kulkarni S, Treleaven J, Sirohi B, Millar B, Shepherd V, Saso R, Rowland A, Long S, Cabral S, Horton C, Mehta J. Comparison of marrow and blood cell yields from the same donors in a double-blind, randomized study of allogeneic marrow vs blood stem cell transplantation. Bone Marrow Transplant. 2000;25:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Remberger M, Ringdén O, Blau IW, Ottinger H, Kremens B, Kiehl MG, Aschan J, Beelen DW, Basara N, Kumlien G, Fauser AA, Runde V. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Dhédin N, Prébet T, De Latour RP, Katsahian S, Kuentz M, Piard N, Réa D, Norol F, Jouet JP, Ribeil JA, Tabrizi R, Rio B, Lioure B, Tiberghien P, Bourhis JH, Sirvent A, Bordigoni P, Blaise D, Michallet M, Vernant JP. Extensive chronic GVHD is associated with donor blood CD34+ cell count after G-CSF mobilization in non-myeloablative allogeneic PBSC transplantation. Bone Marrow Transplant. 2012;47:1564-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Zaucha JM, Gooley T, Bensinger WI, Heimfeld S, Chauncey TR, Zaucha R, Martin PJ, Flowers ME, Storek J, Georges G, Storb R, Torok-Storb B. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98:3221-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Remberger M, Mattsson J, Hassan Z, Karlsson N, LeBlanc K, Omazic B, Okas M, Sairafi D, Ringdén O. Risk factors for acute graft-versus-host disease grades II-IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors: a single centre study. Bone Marrow Transplant. 2008;41:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Pulsipher MA, Chitphakdithai P, Logan BR, Leitman SF, Anderlini P, Klein JP, Horowitz MM, Miller JP, King RJ, Confer DL. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114:2606-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Tsirigotis P, Shapira MY, Or R, Bitan M, Samuel S, Gesundheit B, Ackerstein A, Abdul-Hai A, Slavin S, Resnick IB. The number of infused CD34+ cells does not influence the incidence of GVHD or the outcome of allogeneic PBSC transplantation, using reduced-intensity conditioning and antithymocyte globulin. Bone Marrow Transplant. 2010;45:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Remberger M, Törlén J, Ringdén O, Engström M, Watz E, Uhlin M, Mattsson J. Effect of Total Nucleated and CD34(+) Cell Dose on Outcome after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Mehta J, Mehta J, Frankfurt O, Altman J, Evens A, Tallman M, Gordon L, Williams S, Winter J, Krishnamurthy J, Duffey S, Singh V, Meagher R, Grinblatt D, Kaminer L, Singhal S. Optimizing the CD34 + cell dose for reduced-intensity allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2009;50:1434-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Kanate AS, Craig M, Cumpston A, Saad A, Hobbs G, Leadmon S, Bunner P, Watkins K, Bulian D, Gibson L, Abraham J, Remick SC, Hamadani M. Higher infused CD34+ cell dose and overall survival in patients undergoing in vivo T-cell depleted, but not t-cell repleted, allogeneic peripheral blood hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2011;4:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kałwak K, Porwolik J, Mielcarek M, Gorczyńska E, Owoc-Lempach J, Ussowicz M, Dyla A, Musiał J, Paździor D, Turkiewicz D, Chybicka A. Higher CD34(+) and CD3(+) cell doses in the graft promote long-term survival, and have no impact on the incidence of severe acute or chronic graft-versus-host disease after in vivo T cell-depleted unrelated donor hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2010;16:1388-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Nakamura R, Auayporn N, Smith DD, Palmer J, Sun JY, Schriber J, Pullarkat V, Parker P, Rodriguez R, Stein A, Rosenthal J, Wang S, Karanas C, Gaal K, Senitzer D, Forman SJ. Impact of graft cell dose on transplant outcomes following unrelated donor allogeneic peripheral blood stem cell transplantation: higher CD34+ cell doses are associated with decreased relapse rates. Biol Blood Marrow Transplant. 2008;14:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Gorin NC, Labopin M, Boiron JM, Theorin N, Littlewood T, Slavin S, Greinix H, Cahn JY, Alessandrino EP, Rambaldi A, Nagler A, Polge E, Rocha V; Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. Results of genoidentical hemopoietic stem cell transplantation with reduced intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond--the Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24:3959-3966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Kim DH, Won DI, Lee NY, Sohn SK, Suh JS, Lee KB. Non-CD34+ cells, especially CD8+ cytotoxic T cells and CD56+ natural killer cells, rather than CD34 cells, predict early engraftment and better transplantation outcomes in patients with hematologic malignancies after allogeneic peripheral stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Cao TM, Wong RM, Sheehan K, Laport GG, Stockerl-Goldstein KE, Johnston LJ, Shizuru JA, Negrin RS, Lowsky R. CD34, CD4, and CD8 cell doses do not influence engraftment, graft-versus-host disease, or survival following myeloablative human leukocyte antigen-identical peripheral blood allografting for hematologic malignancies. Exp Hematol. 2005;33:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Reshef R, Huffman AP, Gao A, Luskin MR, Frey NV, Gill SI, Hexner EO, Kambayashi T, Loren AW, Luger SM, Mangan JK, Nasta SD, Richman LP, Sell M, Stadtmauer EA, Vonderheide RH, Mick R, Porter DL. High Graft CD8 Cell Dose Predicts Improved Survival and Enables Better Donor Selection in Allogeneic Stem-Cell Transplantation With Reduced-Intensity Conditioning. J Clin Oncol. 2015;33:2392-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;34:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Tario JD, Wallace PK. Reagents and Cell Staining for Immunophenotyping by Flow Cytometry. Pathobiology of Human Disease, Elsevier. 2014;3678-3701. |
| 22. | Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2745] [Cited by in RCA: 2891] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 23. | Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, Huh YO, Giralt S, Braunschweig I, van Besien K, Champlin R. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Martin PS, Li S, Nikiforow S, Alyea EP, Antin JH, Armand P, Cutler CS, Ho VT, Kekre N, Koreth J, Luckey CJ, Ritz J, Soiffer RJ. Infused total nucleated cell dose is a better predictor of transplant outcomes than CD34+ cell number in reduced-intensity mobilized peripheral blood allogeneic hematopoietic cell transplantation. Haematologica. 2016;101:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Singh AK, Savani BN, Albert PS, Barrett AJ. Efficacy of CD34+ stem cell dose in patients undergoing allogeneic peripheral blood stem cell transplantation after total body irradiation. Biol Blood Marrow Transplant. 2007;13:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013;122:3116-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Barrett AJ, Le Blanc K. Immunotherapy prospects for acute myeloid leukaemia. Clin ExP Immunol. 2010;161:223-232. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Zarcone D, Tilden AB, Lane VG, Grossi CE. Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood. 1989;73:1615-1621. [PubMed] |
| 29. | Noval Rivas M, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 2010;184:6790-6798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113:4458-4467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |