Copyright
©The Author(s) 2020.
World J Clin Oncol. Nov 24, 2020; 11(11): 854-867
Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.854
Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.854
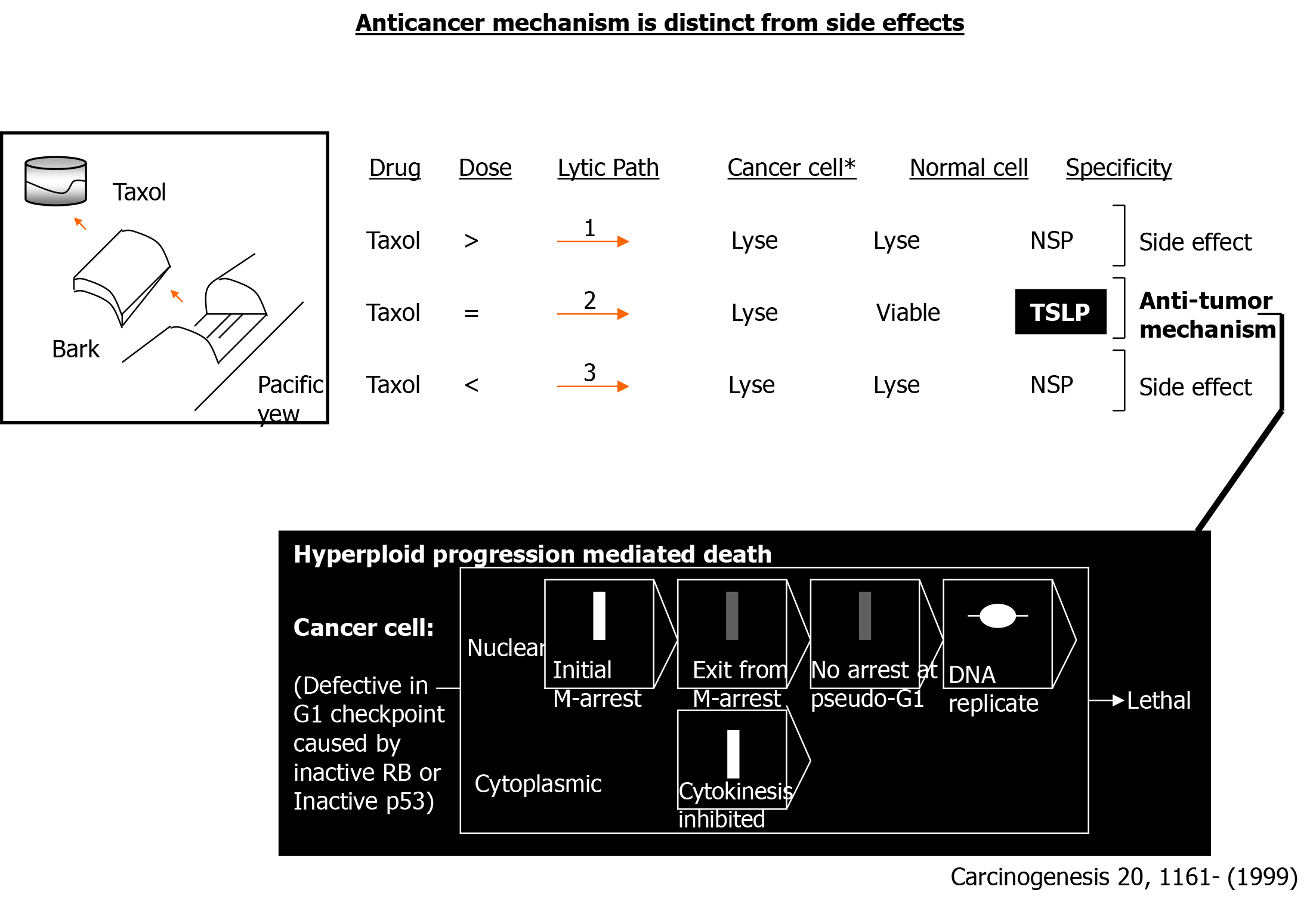
Figure 1 Side effects are distinct from the anticancer mechanism.
An exemplary case concerning taxol is depicted. TSLP: Tumor-specific lytic path; >: Greater than microbutule-disruping dose; =: Equivalent to microtubule-disrupting dose; <: Lesser than microtubule-disrupting dose; NSP: Nonspecific.
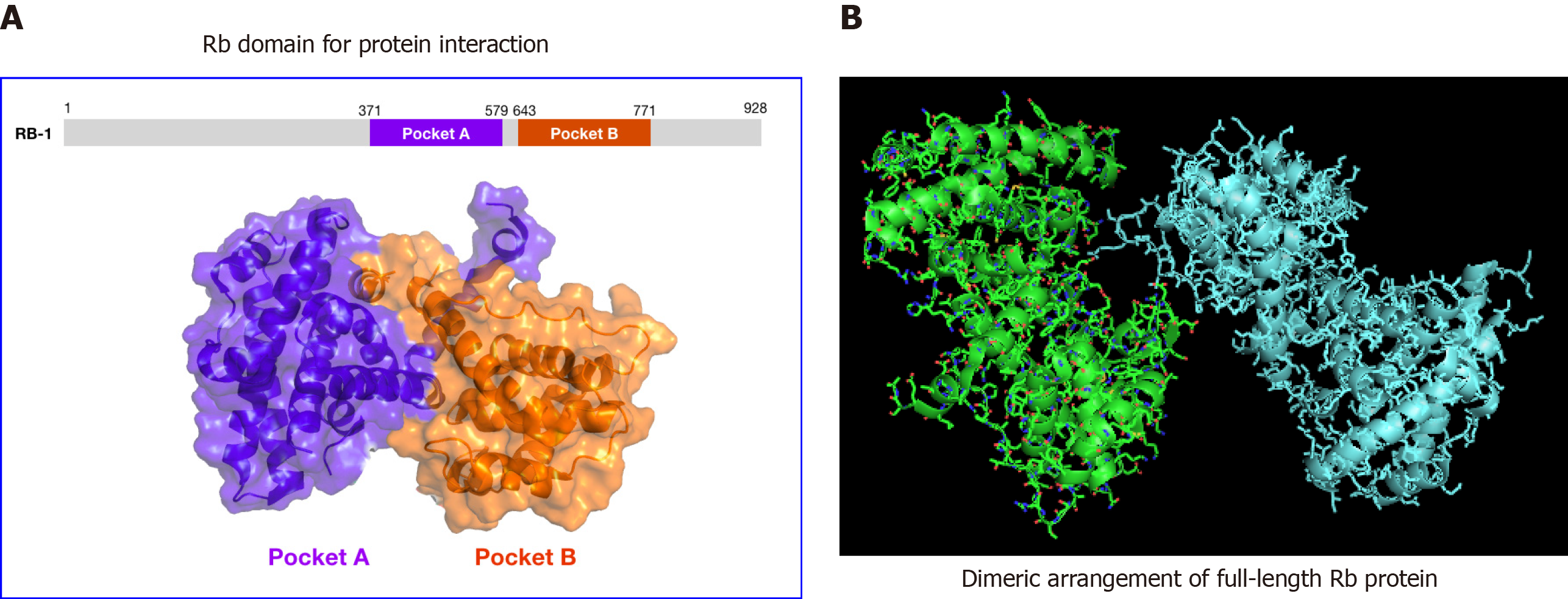
Figure 2 Structural analysis of human retinoblastoma protein.
A: 3D structure of the inactive retinoblastoma protein pocket domain of retinoblastoma-1 (PDB ID: 4ELL). The model was generated using PyMol molecular graphics system, version 1.2r3pre, Schrödinger, LLC. B: Structures (or structural model) of phosphorylated retinoblastoma. The model shows the protein dimer as observed in the asymmetric crystal unit for retinoblastomaPL–P. The structural coordinates for PDB ID 4ELL were downloaded from the NIH protein database. PyMol was used to re-enter the image of the model as shown. Rb: Retinoblastoma.
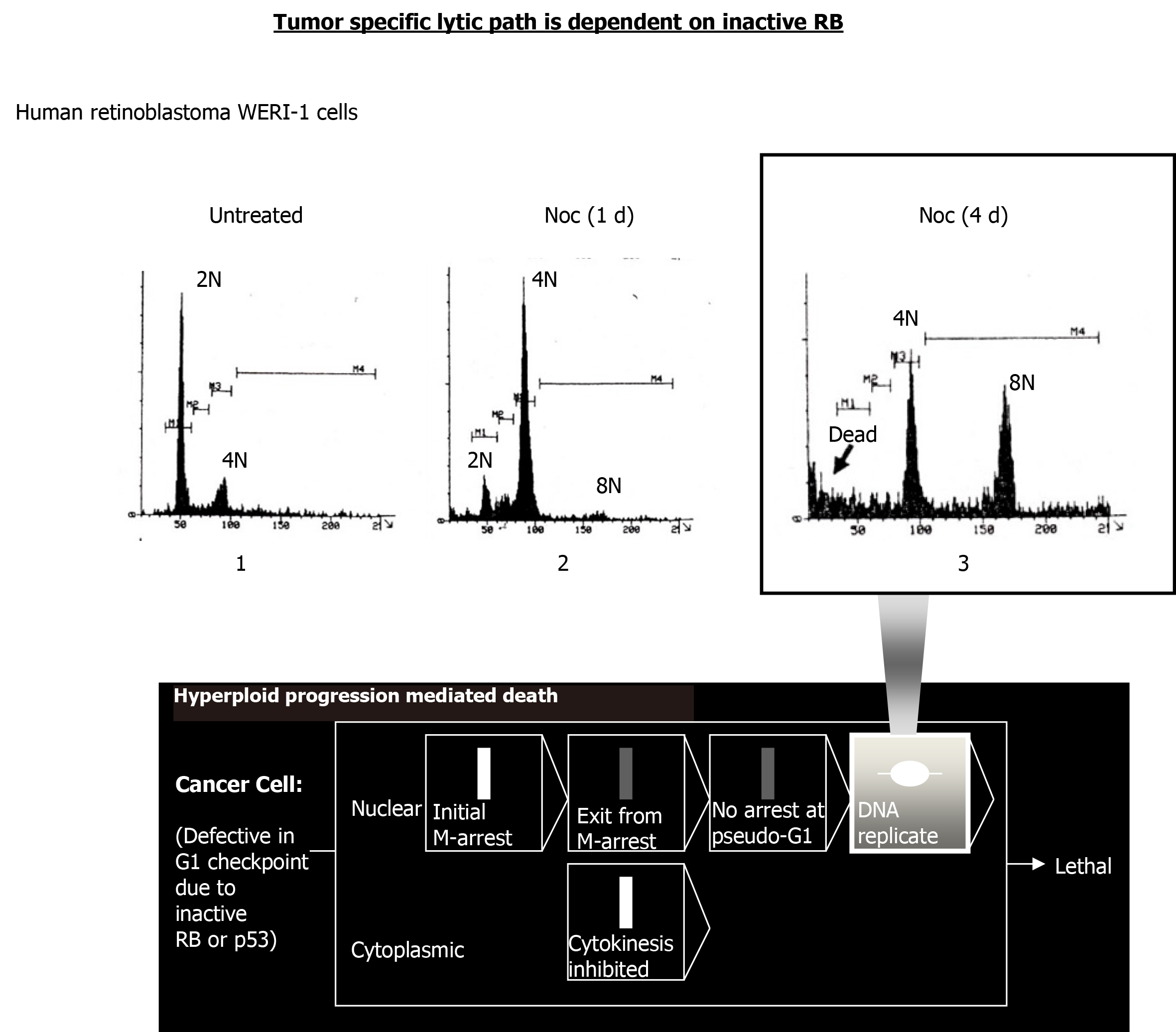
Figure 3 Nocodazole triggers hyperploid progression mediated death in retinoblastoma-mutant WERI-1 retinoblastoma cells.
A DNA content flow cytometric histogram of WERI-1 cells continuously treated with nocodazole (Noc; 0.415 µmol/L) for the duration (1 d, 24 h; 4 d, 96 h) is shown. Cells (5 × 105) were treated as indicated, harvested using trypsin and fixed in 70% ethanol. To determine DNA content per cell, propidium (100 Kunitz units/mL) and RNase A (50 µg/mL) were added and assayed using Beckton Dickinson flow cytometry. Persistent treatment with nocodazole led to the death of hyperploid cells induced. RB: Retinoblastoma.
- Citation: Hong F, Castro M, Linse K. Tumor-specific lytic path “hyperploid progression mediated death”: Resolving side effects through targeting retinoblastoma or p53 mutant. World J Clin Oncol 2020; 11(11): 854-867
- URL: https://www.wjgnet.com/2218-4333/full/v11/i11/854.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i11.854