Published online Aug 15, 2016. doi: 10.4291/wjgp.v7.i3.242
Peer-review started: April 28, 2016
First decision: June 16, 2016
Revised: July 1, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: August 15, 2016
Processing time: 105 Days and 22 Hours
Hepatocellular carcinoma is on the rise and occurs in the setting of chronic liver disease and cirrhosis. Though treatment modalities are available, mortality from this cancer remains high. Medical therapy with the utilization of biologic compounds such as the Food and Drug Administration approved sorafenib might be the only option that can increase survival. Immunotherapy, with modern pharmacologic developments, is a new frontier in cancer therapy and therefore the immunobiology of hepatocarcinogenesis is under investigation. This review will discuss current concepts of immunobiology in hepatocarcinogenesis along with current treatment modalities employing immunotherapy. The tumor microenvironment along with a variety of immune cells coexists and interplays to lead to tumorigenesis. Tumor infiltrating lymphocytes including CD8+ T cells, CD4+ T cells along with regulatory T cells, tumor associated macrophages, tumor associated neutrophils, myeloid derived suppressor cells, and natural killer cells interact to actively provide anti-tumor or pro-tumor effects. Furthermore, oncogenic pathways such as Raf/mitogen-activated protein kinase/extracellular-signal-regulated kinase pathway, phosphatidyl-3-kinase/AKT/mammalian target or rapamycin, Wnt/β-catenin, nuclear factor-κB and signal transducers and activators of transcription 3 may lead to activation and proliferation of tumor cells and are also considered cornerstones in tumorigenesis. Immunotherapy directed at this complex milieu of cells has been showned to be successful in cancer treatment. The use of vaccines, adoptive cell therapy and immune checkpoint inhibitor modulation are current options for therapy. Further translational research will shed light to concepts such as anti-tumor immunity which can add another alternative in the therapeutic armamentarium.
Core tip: Hepatocellular carcinoma is on the rise and is associated with high mortality. Cancer immunology is an expanding field with promise. This review will summarize the current concepts in the immunobiology of hepatocarcinogenesis including the interplay of a variety of immune cells involved in anti-tumor and pro-tumor effects. Oncogenic pathways currently known to effect hepatocarcinogenesis will also be discussed. Finally, currently tested and developed treatment modalities employing immunotherapy will be discussed with an outlook on future therapies.
- Citation: Patel P, Schutzer SE, Pyrsopoulos N. Immunobiology of hepatocarcinogenesis: Ways to go or almost there? World J Gastrointest Pathophysiol 2016; 7(3): 242-255
- URL: https://www.wjgnet.com/2150-5330/full/v7/i3/242.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v7.i3.242
Hepatocellular carcinoma (HCC) is a tumor of the hepatocytes that often occurs in the setting of chronic liver disease and cirrhosis[1]. In men, it is the fifth most commonly diagnosed cancer worldwide and the second leading cause of cancer mortality in the world with rising incidence[2,3]. The incidence of HCC varies throughout the world. The rates of liver cancer are highest in both East and South-East Asia as well as Northern and Western Africa[2]. The higher incidence of HCC in these areas could reflect the elevated prevalence of chronic hepatitis B virus (HBV) infection[4]. In contrast, in North America, Europe, and Japan, infection with hepatitis C virus (HCV) and use of alcohol are the main risk factors[5]. HBV and HCV account for one-third of infection-related cancer cases such as gastric, liver and cervical. Most of these cancers were liver in origin[6,7]. These chronic infections induce inflammation and over time can lead to liver fibrosis and subsequently cirrhosis. Cirrhosis is the formation of dysplastic nodules that predispose patients to hepatocarcinogenesis. It has been reported that cirrhosis is a major factor in hepatocarcinogenesis in patients with HCV. Though for hepatitis B and apparently for non-alcoholic steatohepatitis (NASH), cirrhosis is not a prerequisite[8].
The diagnosis of HCC is based on strict screening and surveillance protocols published by various liver societies such as the American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), and the Asian Pacific Association for the Study of the Liver (APASL)[9-11]. If these practices are not followed patients typically present at the late stages of the disease and the therapeutic options available are rather limited. Grading and prognosis criteria such as the Barcelona Clinic Liver Cancer (BCLC) staging system help determine appropriate treatment options[12]. Resection and transplantation are cornerstones for curative management. Locoregional therapies including percutaneous ablation, radiofrequency ablation (RFA), trans-arterial chemo-embolization (TACE) and radiation therapy are available for those patients who aren’t suitable for resection or transplantation[13,14].
HCC diagnosis has progressed but it remains a major cause of cancer mortality with median survival beyond 5 years only if using better selection criteria and optimum treatment delivery[15]. Drug-based therapies that target tumor signaling pathways are being developed. Of these, sorafenib, a multi-targeted tyrosine kinase inhibitor is the only drug that has been Food and Drug Administration (FDA) approved that prolongs survival in patients with HCC[16].
Immunotherapy is an evolving frontier in cancer therapy and further research into the immunobiology of HCC might help develop targeted novel therapies in anticipation of improving mortality.
Tumors acquire mutations in oncogenes and tumor suppressor genes which help promote tumorigenesis[17]. However, it is now becoming evident that the nonmalignant cells in the microenvironment of the tumor can aid and provide support to its malignant expression as well[17]. This tumor microenvironment is essential in cancer development and behavior. Chronic inflammation and a variety of host components including stromal cells, angiogenesis, and the inflammatory infiltrate produce an environment favorable for tumor growth[18-21].
Both the local and systemic environment are essential in tumorigenesis as they are involved in the production of a persistent inflammatory response to a myriad of stimuli[22]. This persistent inflammation might be induced by a chronic infection with viruses such as hepatitis B and C in the liver and fatty infiltration. This inflammation has been associated with cancer initiation[23].
Chronic viral hepatitis induces changes in the liver that can lead to hepatocarcinogenesis and ultimately HCC[24]. Chronic HCV for example can induce changes in lipid metabolism and gene expression and HBV is believed to alter the transcription of several genes that may lead to the development of HCC[25,26]. Chronic inflammation alone can lead to induction of hepatocarcinogenesis even if active viral hepatitis is not detected[27].
A variety of immune cells coexist and interplay in a complex cascade of pathways that ultimately lead to tumor carcinogenesis and proliferation. Below we discuss a few of those cells and their role in the formation of cancer and in particular hepatocarcinogenesis.
Tumor infiltrating lymphocytes (TIL) are a class of cells that shape the tumor microenvironment and therefore effect carcinogenesis. Most cancers that express a high amount of CD8+ T cells usually portend a better prognosis[28,29] as opposed to tumors with increased expression of protumor cells such as regulatory T cells (Treg) which are usually associated with a worse prognosis[30-32]. Therefore, the balance between these counter-regulatory TILs is crucial to the determination of antitumor response. TILs interact with a variety of tumor-associated antigens (TAAs) which are responsible for producing an immune response. A variety of TAAs are found in HCC. The major ones include oncofetal antigens such as α-fetoprotein (AFP) and glypican-3 (GPC-3) along with cancer antigens such as melanoma-associated antigen 1 (MAGEA), synovial sarcoma X breakpoint 2 (SSX2), NY-ESO-1, and human telomerase-reverse transcriptase (hTERT)[33]. The breakdown in response to the above TAAs is responsible for hepatocarcinogenesis. The following cells are responsible for an effective anti-tumor response but complex interplay amongst a variety of molecules can lead to pro-tumoral effects.
CD8+ T cells are integral to antitumor immunity via direct antigen-specific cytotoxic targeting of tumors. Most tumors or their antigens are ingested by the host antigen presenting cell and are processed to produce peptides. These peptides are then displayed bound to class I MHC molecules in order to be recognized by CD8+ T cells[34]. Studies have shown that an increased number of CD8+ T cells infiltrating cancer tissue is connected to a favorable prognosis in ovarian[29] and colorectal cancers[35].
In HCC, a similar association has been found with tumor penetration of predominantly CD8+ T cells[36]. These patients have a lower recurrence of cancer, better recurrence-free survival after liver resection and better overall prognosis[37,38]. These T cells contributed to an inflammatory microenvironment that significantly improved patient survival and therefore served an anti-tumoral role in HCC. One mechanism of the cytotoxic effect on tumor cells was described in mice models of HCC in which IL-12 mediated activation of CD8+ T cells caused IFN-γ production and apoptosis of hepatoma cells[39].
Recent work by Flecken et al[40] has further elucidated CD8+ T cells that respond to specific TAAs in HCC that were mentioned earlier. Important findings from the study show that TAA-specific CD8+ T cell activity was detectable in more than 50% of HCC patients and was seen with even early stage disease. Furthermore, the presence of these TAA-specific CD8+ T cell responses was associated with an improved progression-free survival, once again confirming that the cytotoxic activity of these cells is important to anti-tumor immunity. Lastly, responses to multiple TAA’s showed a trend toward better progression-free survival, though a study with a larger cohort may be necessary to confirm this finding[40].
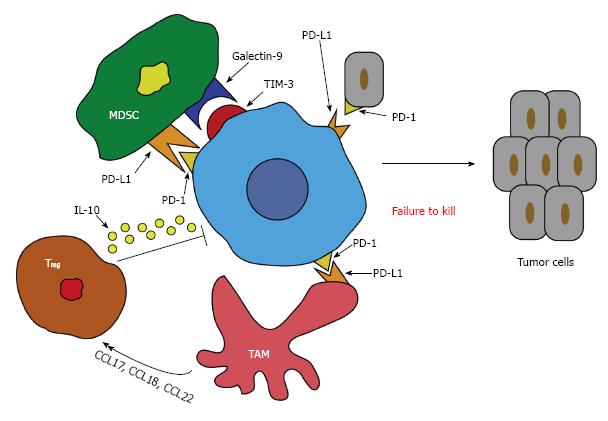
In contrast, dysfunction of CD8+ T cells in patients with HCC has also been seen[41]. Programmed death 1 (PD-1) is a co-inhibitory molecule that is seen on activated T and B cells and is a pivotal molecule for T cell activity[42]. The ligand for PD-1 (PD-L1) is expressed on a variety of tumor cells and is responsible for delivering a signal for inhibition to PD-1 expressing T cells leading to suppression of the cytotoxic T cell response[43]. This inhibition leads to apoptosis and unresponsiveness of these T cells[44]. Studies have shown that the interaction of PD-1 and PD-L1 negatively regulate T cell function in tumors and ultimately may affect the aggressiveness of the tumor (Figure 1). In a cohort of HCC patients, it was demonstrated that there was a significant increase in peripheral and intratumor PD-1 expression on CD8+ T cells. The tumor cells were also rich in PD-L1 expression and therefore predicted a poorer outcome and early recurrence of HCC after liver resection due to the promotion of CD8+ T cell apoptosis[45].
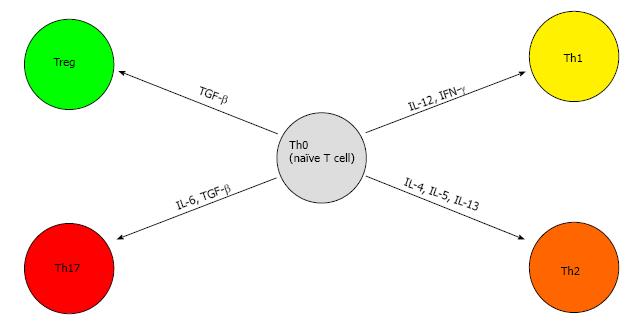
Better known as helper T cells, CD4+ T cells can differentiate to subsets of cells via the expression of a variety of cytokines and transcription factors, namely TH1, TH2, TH17 and Treg cells[46].
TH1 is responsible for antitumor response and differentiates from naive CD4+ T cells via signaling from IL-12 and IFN-γ (mentioned above). IFN-γ exerts its antiviral, proinflammatory, and antitumor effects on cells by regulation of numerous genes and via specific interaction with the IFN-γ receptor on cell membranes[47]. This receptor has been shown to up-regulated in acute and chronic liver disease but diminished in cases of HCC[48]. Therefore, down-regulation or loss of the IFN-γ receptor on the surface of HCC cells may be another escape mechanism of host immune surveillance ultimately leading to HCC progression and metastasis[49].
TH2 polarization, on the other hand, has been shown to promote tumor formation and progression[50]. This process is coordinated by IL-4 signaling through STAT-6. These polarized cells secrete IL-4, IL-5 and IL-13 and are associated with potent humoral immunity[50]. This polarization of CD4+ T cells to TH2 blocks the differentiation towards TH1 and therefore inhibits the antitumor response. These cells have also been shown to suppress the CD8+ T cell responses mentioned above[51].
TH17 differentiation is induced by the presence of IL-6 and transforming growth factor-β (TGF-β). The presence of these cytokines leads to expression of the transcription factor ROR-γt via activation of the signal transducers and activators of transcription 3 (STAT-3) signaling pathway[52]. These TH17 cells have potent pro-inflammatory properties via the secretion of IL-17[53,54]. In mouse models, IL-17 has been found to promote tumor growth by amplifying angiogenesis and increasing the intra-tumoral burden of phagocytes[55,56]. TH17 cells have been seen in a myriad of cancers including HCC and have been shown to promote HCC growth[57,58] via the activation of the STAT-3 signaling pathway[59]. Increased levels of IL-17 producing cells in HCC patients was correlated with lower overall and disease-free survival[58]. Interestingly TH17 cells have been shown to increase in certain infections as well[60].
Treg are a subset of CD4+ T cells that suppress T-cell immunity[61]. A major Treg is CD4+CD25+ and it expresses the transcription factor FOXP3. It is activated by exposure to TGF-β in the periphery[62-64]. Another molecule commonly found on Treg is cytotoxic T-lymphocyte protein 4 (CTLA-4). This molecule binds its ligands CD80 and CD86 on the antigen-presenting cell (APC) membrane and thereby blocks any stimulatory effects from the CD28 protein[65]. CTLA-4 conveys inhibitory signals on T cells and is involved in inducing Treg cell activity[66]. These cells are capable of suppressing CD8+ T cells similar to the TH2 class of cells and have been found in high numbers in HCC[67]. Studies have also shown an increase proportion of Treg correlated adversely with clinical outcome amongst patients with HCC[68]. A recent study investigating the roles of Treg and CD8+ T cells in hepatocarcinogenesis showed that Treg increased in a stepwise fashion from patients with chronic viral hepatitis, pre-cirrhosis, and liver cirrhosis to precursor lesions of adenomatous hyperplasia and atypical adenomatous hyperplasia, early HCC and advanced HCC. Therefore, we can conclude that the number of Treg cells increases proportionally with progressive hepatocarcinogenesis[69]. The differentiation of naïve T cells can be visualized in Figure 2.
Tumor associated macrophages (TAM) are a well-known entity of the inflammatory infiltrate in tumors and are key producers of chemokines and other mediators of inflammation. These chemokines participate in triggering and maintaining the inflammatory process in tumor cells[70]. Similar to T cells, TAMs have plasticity and are therefore able to polarize to opposite phenotypes giving rise to M1 macrophages or M2 macrophages. The M1 variety is classically involved in the Th1 response with signals from IFN-γ and produce effector molecules (reactive oxygen species) and cytokines [e.g., IL-1β, IL-6 and tumor necrosis factor alpha (TNF-α)] to help provide anti-tumor immunity[71]. They also produce chemokines that attract Th1 lymphocytes in order to mount a more robust response[72]. However, once tumors are produced, the macrophages are transitioned to become protumoral and this is when the tumor microenvironment fosters change toward the opposite macrophage phenotype, M2, causing a Th2 response[73].
These M2 macrophages are activated by IL-4 and IL-13 along with growth factors such as colony stimulating factor-1 (CSF-1) which inhibit the classical activation of the M1 macrophage. M2 macrophages suppress Th1 adaptive immunity by averting anti-tumor immunity and therefore promote tumor growth and progression[74]. This tumor environment is represented by TGF-β1 and Arginase 1[75]. The current thinking is that tumors acquire mutations that subsequently lead to the production of these factors that help promote polarization of macrophages to stimulate tumor-producing cells.
HCC TAMs are identified by immunohistochemistry as human leukocyte antigen (HLA)-DR+, CD163+, CD206+ and with the presence of elevated arginase activity. The number of TAMs found in these patients is positively correlated with poor prognosis[76].
The link between TAMs and HCC can be traced back to murine models[77]. In a Md2-knockout model, the mice automatically developed hepatitis and subsequent HCC. The TAMs produce TNF-α, activating nuclear factor kappa B (NF-κB) which has a protective role in hepatocytes by preventing apoptosis and in turn promoting tumor growth[78]. In another mouse model using the chemical carcinogen, diethylnitrosamine (DEN), hepatocyte proliferation was driven by TAM-derived TNF-α and IL-6 leading to hepatocarcinogenesis[77]. In this same model, it was found that there is a gender disparity in the formation of HCC, with DEN-induced HCC of 100% in male mice and only 10% in female mice. It is believed that the estrogen levels in females play a critical role in inhibiting IL-6 production and therefore decreasing the activation of transcription factor, NF-κB[79]. This finding supports the possible use of estrogen therapy in decreasing the risk of hepatocarcinogenesis.
TAMs have also been implicated in angiogenesis as their density in microvessels has been shown to be elevated. They secrete growth factors such as TGF-β, vascular endothelial growth factor (VEGF), fibroblast growth factor, platelet-derived growth factor (PDGF), angiogenic factor thymidine phosphorylase, angiogenesis-modulating enzyme cyclooxygenase-2 and matrix metalloproteinases (MMPs), particularly MMP-9 and 12, which all promote angiogenesis[22]. MMP-9 overexpression is associated with increased invasiveness of HCC[80].
Furthermore, TAMs move to parts of the tumor that are hypoxic[81]. The hypoxia-induced factor 1α in TAMs is necessary for its activation and migration in vivo studies. The hypoxia stimulates TAM chemokine production (CCL2, CCL5, IL-8, CXCL10, CXCL12 and CXCL13) which aids angiogenesis and tumor progression[82].
In addition, it has been published that TAMs produce a variety of chemokines such as CCL17, CCL18, and CCL22, which all attract Treg and Th2 cells to the cancer site and therefore impede cytotoxic T cell activation (Figure 1)[83,84]. This might lead to the conclusion that a positive feedback loop exists between TAMs and Treg, which provides an added layer to the immunosuppressive effects of HCC.
Finally, in cases of HBV-associated HCC, TAMs have been shown to express high levels of galectin-9 especially on Kuppfer cells (KC)[85]. The ligand for galectin-9 is T-cell immunoglobulin and mucin-domain containing-3 (TIM-3)[86]. High levels of TIM-3 have also been seen in HBV-associated HCC and are colocalized with galectin-9. When galectin-9 binds to TIM-3, it induces dormancy of these T cells and therefore effector T cell function. More importantly, blocking this pathway can recover the T cell function[85].
Similarly, TAMs decrease T cell function through the expression of the co-inhibitory molecule, PD-L1 (also known as B7-H1), which binds to PD-1 on T cells. This process reduces T cell effector function analogous to the galectin-9/Tim-3 pathway as mentioned above. Both IL-10 and TNF-α play a role in the induction of this PD-L1/PD-1 pathway in HCC (Figure 1)[76]. Likewise, blocking this pathway can help recover T cell function and its antitumor efficacy[87].
Therapy aimed at TAM reduction has been documented. In particular, zoledronic acid or clodronate-encapsulated liposomes (clodrolip), in combination with tyrosine kinase inhibitors such as sorafenib, has been shown to reduce tumor growth and angiogenesis further in mice compared to just sorafenib alone[88]. Therefore, additional therapies implementing depletion of TAMs may be a worthwhile avenue to explore.
Analogous to TAM, tumor associated neutrophils (TAN) have recently been described to contribute to carcinogenesis as well. They can produce both pro-tumoral as well as anti-tumoral effects on cancer and can be manipulated toward distinct phenotypes via tumor signaling[89,90].
In a similar fashion to TAMs, TANs can be divided into two main subtypes, N1 (anti-tumoral) and N2 (pro-tumoral). The plasticity of these subtypes depends on the presence of TGF-β. Neutrophils can be polarized by TGF-β to become the N2 phenotype while the inhibition of TGF-β along with increased IFN-β induces the N1 phenotype[89,91].
The pro-tumoral functions of both TAM and TAN include extracellular matrix remodeling, cancer cell invasion, angiogenesis, lymphangiogenesis, metastasis and most importantly, the inhibition of anti-tumoral immune surveillance[92]. The anti-tumoral effects of TAM and TAN include direct cytotoxic activity against the tumor cells and the production of a variety of mediators to recruit and activate the immune system. These mediators include cytokines, chemokines and growth factors[74,93].
In HCC murine models, TANs have been shown to mediate intratumoral infiltration of macrophages and Treg cells by secreting CCL2 and CCL17. This pathway stimulated neovascularization, enhanced HCC growth and metastasis and also contributed to sorafenib resistance. It has been suggested that the extent of TAN infiltration could be used as a biomarker in HCC and can predict responsiveness to sorafenib therapy and that TAN depletion can enhance sorafenib’s efficacy[94].
In addition to the above cells, cancer growth can also be attributed to myeloid derived suppressor cells (MDSC)[95]. These cells, though not extensively studied, have shared relationships with TAM and TAN in carcinogenesis. They represent a diverse mix of immature cells of myeloid origin that are able to lessen immune responses[96].
In HCC, MDSC (specifically the CD14+HLA-DR-/low phenotype) were seen in high numbers in the peripheral blood and were able to help the host evade the immune response against cancer by directly increasing arginase activity and directly suppressing the response of tumor-specific CD4+ T cells. They were also seen to indirectly suppress T-cell function by inducing CD4+CD25+Foxp3+ Treg mentioned earlier[97]. Interestingly, in the presence of hypoxia or tumor-derived factors, MDSCs can differentiate toward immunosuppressive TAMs as well[98].
In a similar fashion to TAMs, MDSCs also express galectin-9 which binds to Tim-3 on T cells, thereby inducing senescence[99]. MDSCs also respond to liver macrophages and increase the levels of PD-L1, which as mentioned earlier reduces T cell effector function (Figure 1)[96]. MDSC have also been implicating in angiogenesis by producing high levels of MMP-9[100].
Natural killer (NK) cells are innate lymphoid cells that are the immune system’s first line of defense against infections and tumors[101]. They are essential in the
liver’s immune function where they account for close to 50% of liver lymphocytes[102]. Their anti-tumor response is by direct lysis of malignant cells[101]. They express a number of immune receptors (NKRs) that are able to recognize ligands on hepatocytes, stellate cells and Kupffer cells to maintain proper immune function[103,104].
MHC class I-related chain A (MICA) is a ligand for NK cell stimulatory receptor NKG2D and is expressed in HCC[105]. The interaction of MICA and NKG2D serves as a pathway for immune surveillance in HCC. However, a soluble form of MICA (sMICA) has been shown to isolate NKG2D and inhibit its expression. These soluble forms are increased in a variety of tumors and may serve as a tumor evasion mechanism[106].
According to the frequency of CD56 expression (dim or bright) and the presence or absence of CD16, NK cells can be divided into two subsets: CD56brightCD16neg (produces cytokines) and CD56dimCD16pos (cytotoxic)[107].
The density of NK cells in the peripheral blood of patients with HCC positively correlated with survival and prognosis similar to many of the other cell lines already mentioned[108]. Specifically, peripheral CD56dimCD16pos NK subsets were dramatically reduced, which resulted in an increased ratio of CD56bright to CD56dim NK cells in HCC and therefore decreased cytotoxic capability. These cells exhibited poorer capacity to produce IFN-γ and kill target cells. This phenomenon was found to be associated with the increased CD4+CD25+ Treg in the tumor environment as mentioned earlier. Decreased functionality of these cells was shown to correlate with early development and recurrence of HCC as well[109]. Murine models of hepatoma revealed that activation of NK cells can lead to clearance of the hepatoma which suggests a possible immune target in patients with liver disease[110].
Sorafenib, as mentioned earlier, is the only molecularly-targeted drug shown to have survival benefit in patients with HCC[16]. Recently, it was found to initiate liver NK cell activation and induce the anti-tumor response of these cells by triggering TAMs and increasing IFN-γ secretion[111]. The proteasome inhibitor, Bortezomib, has also been shown to stimulate cytotoxicity and IFN-γ production of NK cells by increasing the expression of MICA/B on the cell surface of hepatoma cells.
A new class of agents that have shown positive results in a variety of malignancies including HCC are the histone deacetylase inhibitors (HDACi). HDACis, such as sodium valproate, promote MICA expression on hepatoma cells and coordinate NK cell-mediated lysis. This mechanism supports this type of therapy for future treatment of HCC[112].
The mechanisms of pro-tumoral and anti-tumoral effects in hepatocarcinogenesis of the above cell types are summarized in Table 1.
| Cell type | Mechanism | |
| Pro-tumor | Anti-tumor | |
| CD8+ T cells | Exhaustion of CD8+ T cells | Increased CD8+ T cells |
| Upregulation of PD-1 | ||
| CD4+ T cells | TH2 response | TH1 response |
| TH17 cells | IL-17 production via STAT-3 | |
| Treg cells | Increased Treg | |
| Impaired CD8+ T cells via CTLA-4 | ||
| TAM | M2 (Th2 response) | M1 (Th1 response) |
| Increased galectin-9 via TIM-3 | ||
| Expression of B7-H1 | ||
| Induction of TH17 | ||
| TAN | N2 phenotype | N1 phenotype |
| Angiogenesis via Treg | ||
| MDSC | Induction of Treg and TAM | |
| Suppress CD4+ T cells | ||
| Suppress NK activity | ||
| NK cells | Increased CD56dimCD16pos | Increased CD56brightCD16neg |
| Increased Treg | ||
Multiple growth factor signaling cascades are deregulated in hepatocarcinogenesis. Activation of oncogenes seems to be a late event in human HCC[113]. The tumor microenvironment in concert with the presence of cirrhosis induces a selection to eliminate certain checkpoint genes. This may be an early event which leads to the activation of oncogenes and tumor growth[113]. Amongst the most frequently seen pathways, the following will be discussed: Raf/mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase pathway (ERK), phosphatidyl-3-kinase/AKT/mammalian target or rapamycin (PI3K/AKT/MTOR), Wnt/β-catenin, NK-κB, and STAT-3.
The Raf/MAPK/ERK plays an important role in tumor cell signaling and is involved in cell growth and differentiation. The pathway translates extracellular signals from tyrosine kinase receptors, such as epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), insulin-like growth factor receptor (IGFR), c-Met, and platelet-derived growth factor receptor (PDGFR), and hepatocyte growth factor receptor (MET) to the nucleus via a series of phosphorylation events[114]. The GTPase, RAS, and the serine/threonine kinase (Raf) are key regulators in signal transduction in this pathway[115]. Increased activation of this pathway is well known in HCC and also positively correlates with disease severity[116,117]. The increased activity of this pathway in HCC can be explained by the down-regulation of the Raf kinase inhibitor which is a suppressor of the Raf/MAPK/ERK pathway[118]. Therefore, Raf kinase targeting for treatment of HCC is a promising approach. Sorafenib is one example of a drug with inhibitory effects on Raf-1 kinase and can halt tumor progression via its downstream effects using this mechanism[119].
Similar to the Raf/MAPK/ERK pathway, the PI3K/AKT/mTOR pathway is a major intracellular signaling cascade that controls cell growth, proliferation, motility, survival, and apoptosis[120]. Activation of the AKT signaling pathway has been reported in over 40% of human HCC. This activation has prosurvival and growth-stimulatory effects by decreasing TGF-β-dependent apoptosis[121]. Evidence has demonstrated that the abnormal activation of this pathway occurs in patients with HCC. Furthermore, the expression of the mTOR downstream effector, p70s6k, is up-regulated in 45% of patients with HCC[122]. Therefore, this pathway is another important target for therapy.
Some of the inhibitors that have been studied targeting this pathway include sorafenib and PI-103 (a dual PI3K/mTOR inhibitor) alone or in combination. This was found to significantly inhibit EGF-stimulated proliferation of HCC via the blockage of both the PI3K/AKT/mTOR and Ras/Raf/MAPK pathways[123]. Certain mTOR inhibitors such as everolimus have been tested against placebo as second-line agents in sorafenib-refractory or intolerant HCC patients. However, there was no difference in overall survival compared to the placebo group. In subgroup analysis, patients with HBV infection derived a significant benefit [hazard ratio (HR) = 0.64; 95%CI: 0.45-0.93] as compared to those with HCV infection (HR = 0.93; 95%CI: 0.75-1.15)[124].
Wnt/β-catenin
The canonical Wnt (Wnt/β-catenin) pathway controls many processes, including cell fate determination, proliferation and stem cell maintenance. The pathway is activated by the binding of growth factors to the Frizzled (Fzd) receptors on the surface of target cells. This leads to activation of different signal transduction pathways further downstream[125]. Physiologically, β-catenin is involved in intercellular adhesion via interactions with E-cadherin and transmission of the proliferative signals of the Wnt pathway[126,127]. In the absence of the above, the β-catenin protein is rapidly phosphorylated by the destruction complex which consists of AXIN1, adenomatous polyposis coli (APC), glycogen synthase kinase-3β (GSK-3β), and casein kinase 1 (CK1) proteins[126,127]. This phosphorylation then leads to β-catenin becoming ready for proteolysis through the ubiquitin/proteasome system. Mechanisms that bypass this phosphorylation or mutations in the destruction complex lead to the translocation of the β-catenin to the cytoplasm and eventually the nucleus to interact with transcription factors. This translocation triggers activation of multiple genes involved in cell proliferation, survival and migration[128]. Aberrant activation of this pathway is observed in both murine and human studies of HCC[129,130].
In addition, the protein regulator of cytokinesis 1 (PRC1) is involved in cytokinesis and microtubule organization[131-133]. PRC1 has been shown to be upregulated in HCC and has an oncogenic function in promoting cancer, tumorigenesis, and metastasis. It is a direct Wnt signaling target and is necessary for Wnt signaling to mediate oncogenesis. PRC1 also potentiates Wnt signaling by promoting membrane sequestration of the destruction complex, thereby reducing APC stability and promoting stabilization of β-catenin[134].
Therefore, there is mounting evidence that β-catenin is essential for tumor cell proliferation and survival. It is obvious that targeting the Wnt/β-catenin pathway may be an attractive option for therapy and is already being investigated. Therapeutic monoclonal antibodies are useful for targeting ligands or receptors. Wnt and Fzd proteins are prime targets for these antibodies. An anti-Wnt-1 antibody has been shown to inhibit β-catenin signaling and induce apoptosis and decrease tumor growth in HCC[135]. Furthermore, the Fzd receptor is another interesting target. A soluble Fzd peptide (sFZD-7) was shown to inhibit Wnt signaling and also sensitized the HCC to the traditional chemotherapeutic agent, doxorubicin[136].
Axin, one of the components of the destruction complex mentioned earlier is another molecular target utilizing this pathway[137]. Adenovirus-mediated gene transfer of wild-type Axin1 induced apoptosis in hepatocellular and colorectal cancer cells, once again confirming the importance of the destruction complex on the senescence of β-catenin[138]. Disruption of the actual β-catenin and transcription factor complex is a promising avenue of immunotherapeutics. However, the complexity of the transcriptional regulation of this pathway makes drug targeting a future endeavor[139].
NF-κB
Cancer, as mentioned previously, has been linked to both infection and inflammation and a variety of signaling pathways can lead to an inflammatory tumor microenvironment. Toll-like receptors (TLR) are present on the cells of the innate immune system and are activated via their interaction with foreign cells such as pathogens[140]. This interaction triggers the production of a variety of cytokines and chemokines that begin the inflammatory cascade[141]. The pathway controlled by the transcription factor NF-κB is essential for this to occur[142]. NF-κB is activated downstream in inflammatory cells via signals from TLR using the adaptor molecule myeloid differentiation primary response gene 88 (MyD88)[140] or other inflammatory cytokines such as TNF-α and IL-1β[142].
NF-κB’s ties to tumorigenesis has been reported using murine models. Ablation of the gene encoding for IκB kinase β-subunit (IKK-β) which is a kinase necessary for the activation of NF-κB in myeloid cells, resulted in a reduction of tumor size in mice with colitis-associated cancer[143]. Furthermore, deletion of the gene for this kinase in Kupffer cells and hepatocytes in models of HCC resulted in reduced tumor burden and resulted in less production of inflammatory cytokines such as TNF-α and IL-6 with DEN administration. However, deletion of the kinase gene in only the hepatocytes and not in Kupffer cells resulted in a marked increase in hepatocarcinogenesis[77]. These conflicting findings underscore the complex nature of the NF-κB signaling pathway and once again the importance of immune cells in tumorigenesis.
Lastly, another emerging pathway in hepatocarcinogenesis is the STAT-3 signaling pathway, which has been mentioned previously and will be briefly discussed here. The STAT-3 is part of the transcription factor family and is involved in signal transduction via cytokines, growth factors, and oncoproteins. Once activated, STAT-3 translocates to the nucleus and binds to target genes that regulate a variety of cell activities including growth, differentiation, apoptosis, and angiogenesis[144-146]. Mostly, activated STAT-3 participates in carcinogenesis by either promoting angiogenesis or stimulating cellular proliferation[147]. Furthermore, the expression of activated STAT-3 was correlated with histological grading and intratumor microvessel density in more than 50% of patients with HCC in one study[148]. This highlights the potential importance of this pathway in tumorigenesis and progression.
It is clear that the inhibition of STAT-3 signaling could be vital in halting tumor growth[149]. This was proven in an experiment in which over-activated STAT-3 was blocked in human HCC cells. Results showed that the proliferation of HCC cells was suppressed dramatically and was associated with increased apoptosis and cell arrest[149]. Therefore, STAT-3 is an interesting target for HCC treatment and there is a current phase 1 clinical trial evaluating an inhibitor of STAT-3 in patients with HCC (NCT01839604).
A better understanding of tumor immunobiology has led to unique avenues of targeted therapy. Below are some of the classes of therapies available in addition to ones mentioned earlier.
Vaccination to target TAAs such as AFP, GPC-3, NY-ESO-1, MAGEA, SSX2, and hTERT is an evolving strategy in immunotherapy. Two peptide-derived vaccines have been developed and tested. One was a nonrandomized, open-label phase 1 clinical trial against GPC3 peptides, which proved to be safe and showed a measurable immune response with higher CTL activity. However, only one of the 33 patients treated had objective tumor response[150]. The second vaccine was with an hTERT-derived peptide that revealed no signs of clinical or CTL activity[151].
Adoptive cell therapy (ACT) involves the isolation and expansion of tumor-specific T cells to gain a greater number of cells than would be expected otherwise in an ex vivo fashion. These T cells are then infused into the patient in an attempt to boost the immune response against the tumor[152].
Autologous or allogeneic ex vivo expanded NK cells is an example of ACT. These NK cells can be expanded 1600-fold using new technology and can be used for their cytotoxic effects against tumor targets[153]. A Phase II clinical trial (NCT02008929) is underway to evaluate the efficacy and safety of MG4101 (ex vivo expanded allogeneic NK cells) as an adjuvant treatment of advanced HCC after curative liver resection and high risk of recurrence.
Cytokine-induced killer cells (CIK) therapy using ACT has seen great results in Asia. In two randomized trials using this technique in the adjuvant setting after liver resection, showed an increase in recurrence-free survival but no difference in overall survival[154,155].
A study that compared CIK ACT with best supportive care after combined TACE and RFA showed a dramatic reduction in the 1-year recurrence rate in the CIK-treated group (9% vs 30%)[156]. Furthermore, another randomized study of patients with a wide range of HCC tumor grades was treated with standard therapy or CIK in addition to standard therapy. There was an increase in overall survival and progression-free survival in the CIK-treated group but no significant improvement in patients that had undergone surgery[157]. This may indicate that CIK therapy may be more useful for those with earlier stage disease.
Immune checkpoints are coinhibitory molecules that block the immune response to tumor cells and decrease the overactivation of T cells. Members of this group have been mentioned earlier and include CTLA-4, PD-1, and TIM-3. Two others that will not be discussed are LAG-3 (lymphocyte activation gene-3 protein) and BTLA (B and T lymphocyte attenuator)[158,159].
Targeting these immune checkpoints has gained recent interest after three different checkpoint inhibitors were approved by the United States FDA to treat melanoma. These drugs include ipilimumab (anti-CTLA-4), pembrolizumab (anti-PD-1) and nivolumab (anti-PD-1) along with some others[160].
Two anti-CTLA-4 monoclonal antibodies (mAbs) have been developed thus far for advanced cancer such as melanoma. Ipilimumab is an IgG1 anti-CTLA-4 human mAb with a half-life of 2 wk and has shown great efficacy in metastatic melanoma[161].
Tremelimumab is an anti-CTLA-4 mAb and is from the IgG2 subclass with a longer half-life of 22 d. In a phase III trial, the primary end-point of progression-free survival in metastatic melanoma was not met[162]. However, its use in patients with HCC and chronic HCV infection yielded partial response rate in 17.6% of patients and control of their disease in 76.4% of patients, with a median time to cancer progression of 6.48 mo. Furthermore, a significant drop in HCV viral load was seen with treatment[163].
PD-1 is a strong inhibitor of T cell responses as mentioned earlier. Therefore, its blockade is an important target for therapy. Another mAb that has been tested is nivolumab, which is a human IgG4 anti-PD-1 mAb that has been investigated in a variety of solid tumors with positive results[164].
A multicenter phase I/II trial that studied nivolumab in advanced HCC patients with intolerance to sorafenib was started in 2012. Preliminary results show that the safety profile of the treatment was acceptable and durable responses were seen and overall survival at 6 mo was 72%[165]. These results are promising and further assessment of immune checkpoint inhibitors as potential immunotherapy are being undertaken.
HCC is of increasing importance due to its rising incidence and mortality. Current therapeutic options are limited and therefore, other avenues are being pursued. The immunobiology of the tumor environment is tremendously complicated but plays a pivotal role in the development of inflammation and hepatocarcinogenesis. The multitude of ways that cancer cells can evade the immune response makes it a very difficult disease to control. The interplay amongst immune cells and tumor microenvironment determines the ultimate outcomes of patients with cancer.
Many of these cells have been well known for many years but their complex interactions are being discovered on a daily basis. Targeted therapy towards these important cells has become a fascinating topic that has fostered many studies on murine models and clinical trials on humans. The most critical aspect of immunotherapy will be to find the most appropriate patient population for treatment and testing. The advances thus far have proven to provide desirable results for hepatocarcinogenesis and will further solidify as active translational research improves.
Not all tumors are identical and individualized treatment modalities along with the current standard of care may be the future of immunotherapy in patients with HCC. These newer treatment modalities include vaccines, monoclonal antibodies against immune checkpoint inhibitors as well as ACT amplifying TIL. Enhancing the natural anti-tumor response of the body along with breaking the barriers of immune tumor evasion may be among the keys to the future of cancer treatment.
In this review, we have highlighted the role of the immune system and immunomodulatory therapy against tumors, particularly hepatocarcinomas. It is probable that we will need to develop non-immune associated therapies that together will have the most impact in treating and repressing recurrence of even the seemingly cancer-free state. This will likely be refined within the scope of precision medicine.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fava G, Hung LY, Ooi LLPJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Davis GL, Dempster J, Meler JD, Orr DW, Walberg MW, Brown B, Berger BD, O’Connor JK, Goldstein RM. Hepatocellular carcinoma: management of an increasingly common problem. Proc (Bayl Univ Med Cent). 2008;21:266-280. [PubMed] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21372] [Article Influence: 2137.2] [Reference Citation Analysis (3)] |
| 3. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 666] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 4. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Articles Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;6736:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2000] [Article Influence: 200.0] [Reference Citation Analysis (4)] |
| 5. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 6. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1720] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 7. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 10. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 11. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 12. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 13. | Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK, Are C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg. 2016;263:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 14. | Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M, Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF, Carrillo-Pérez DL. Diagnosis and treatment of hepatocellular carcinoma: An update. World J Hepatol. 2015;7:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 17. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2949] [Cited by in RCA: 2767] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 18. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8184] [Article Influence: 545.6] [Reference Citation Analysis (0)] |
| 19. | Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 779] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 20. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11283] [Article Influence: 490.6] [Reference Citation Analysis (2)] |
| 21. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47161] [Article Influence: 3368.6] [Reference Citation Analysis (5)] |
| 22. | Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 23. | Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1320] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 24. | Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010;134:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Moradpour D, Blum HE. Pathogenesis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2005;17:477-483. [PubMed] |
| 26. | Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol. 2009;51:810-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 258] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4911] [Article Influence: 258.5] [Reference Citation Analysis (0)] |
| 29. | Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2457] [Cited by in RCA: 2647] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 30. | Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127:748-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295-307. [PubMed] [DOI] [Full Text] |
| 32. | Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;54:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. Häftad: Engelska 2014; . |
| 35. | Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491-3494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 892] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 37. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Komita H, Homma S, Saotome H, Zeniya M, Ohno T, Toda G. Interferon-gamma produced by interleukin-12-activated tumor infiltrating CD8+T cells directly induces apoptosis of mouse hepatocellular carcinoma. J Hepatol. 2006;45:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 41. | Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, Lim SG, Bertoletti A. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2009;137:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1190] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 43. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3572] [Cited by in RCA: 4059] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 44. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3543] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 45. | Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 46. | Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 434] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 47. | Shuai K. Interferon-activated signal transduction to the nucleus. Curr Opin Cell Biol. 1994;6:253-259. [PubMed] |
| 48. | Volpes R, van den Oord JJ, De Vos R, Depla E, De Ley M, Desmet VJ. Expression of interferon-gamma receptor in normal and pathological human liver tissue. J Hepatol. 1991;12:195-202. [PubMed] |
| 49. | Nagao M, Nakajima Y, Kanehiro H, Hisanaga M, Aomatsu Y, Ko S, Tatekawa Y, Ikeda N, Kanokogi H, Urizono Y. The impact of interferon gamma receptor expression on the mechanism of escape from host immune surveillance in hepatocellular carcinoma. Hepatology. 2000;32:491-500. [PubMed] |
| 50. | Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167:6497-6502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 53. | Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1817] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 54. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3074] [Cited by in RCA: 3361] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 55. | Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 635] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 56. | Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698-3704. [PubMed] |
| 57. | Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 58. | Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 59. | Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 670] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 60. | Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 2010;78:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3907] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 62. | Rudensky AY, Gavin M, Zheng Y. FOXP3 and NFAT: partners in tolerance. Cell. 2006;126:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2532] [Cited by in RCA: 2559] [Article Influence: 121.9] [Reference Citation Analysis (0)] |
| 64. | Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 1842] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 65. | Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 735] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 67. | Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Hirano S, Iwashita Y, Sasaki A, Kai S, Ohta M, Kitano S. Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes. J Gastroenterol Hepatol. 2007;22:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 70. | Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2889] [Cited by in RCA: 3075] [Article Influence: 279.5] [Reference Citation Analysis (0)] |
| 71. | Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3696] [Cited by in RCA: 4740] [Article Influence: 364.6] [Reference Citation Analysis (1)] |
| 72. | Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3531] [Cited by in RCA: 3827] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 73. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2566] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 74. | Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1108] [Cited by in RCA: 1057] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 76. | Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067-8075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 77. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 954] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 78. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [PubMed] [DOI] [Full Text] |
| 79. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1490] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 80. | Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, Mizumoto M, Fukumoto M, Imamura M. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Hypoxia and tumor-associated macrophages: A deadly alliance in support of tumor progression. Oncoimmunology. 2014;3:e27561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1528] [Cited by in RCA: 1623] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 83. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3882] [Article Influence: 184.9] [Reference Citation Analysis (0)] |
| 84. | Schutyser E, Struyf S, Proost P, Opdenakker G, Laureys G, Verhasselt B, Peperstraete L, Van de Putte I, Saccani A, Allavena P. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem. 2002;277:24584-24593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 86. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1590] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 87. | Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 724] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 88. | Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420-3430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 89. | Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2035] [Cited by in RCA: 2541] [Article Influence: 158.8] [Reference Citation Analysis (0)] |
| 90. | Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519-531. [PubMed] [DOI] [Full Text] |
| 91. | Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 92. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8327] [Article Influence: 489.8] [Reference Citation Analysis (0)] |
| 93. | Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. 2013;23:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 94. | Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646-1658.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 618] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 95. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5331] [Article Influence: 333.2] [Reference Citation Analysis (0)] |
| 96. | Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514-5521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 97. | Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 637] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 98. | Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439-2453. [PubMed] |
| 99. | Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 100. | Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 910] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 101. | Caligiuri MA. Human natural killer cells. Blood. 2008;112:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1409] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 102. | Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 722] [Article Influence: 42.5] [Reference Citation Analysis (1)] |
| 103. | Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell Mol Immunol. 2013;10:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 106. | Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1225] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 107. | Cooper MA, Caligiuri MA. Isolation and characterization of human natural killer cell subsets. Curr Protoc Immunol. 2004;Chapter 7:Unit 7.34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 108. | Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer. 1998;83:58-63. [PubMed] |
| 109. | Chuang WL, Liu HW, Chang WY. Natural killer cell activity in patients with hepatocellular carcinoma relative to early development and tumor invasion. Cancer. 1990;65:926-930. [PubMed] |
| 110. | Miyagi T, Takehara T, Tatsumi T, Kanto T, Suzuki T, Jinushi M, Sugimoto Y, Sasaki Y, Hori M, Hayashi N. CD1d-mediated stimulation of natural killer T cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver. Int J Cancer. 2003;106:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 112. | Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321-6329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 113. | El-Serag HB, Lechel A, Rudolph KL. Epidemiology and Molecular Mechanisms of Hepatocarcinogenesis. UK: Elsevier Inc 2012; . [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 114. | Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866-3884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 115. | Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351 Pt 2:289-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 116. | Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 291] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 117. | Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 118. | Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208-1217. [PubMed] |
| 119. | Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1149] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 120. | Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2125] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 121. | Chen RH, Su YH, Chuang RL, Chang TY. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene. 1998;17:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 122. | Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421-8425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 123. | Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, Evers BM. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951-4958. [PubMed] |
| 124. | Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 125. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3831] [Cited by in RCA: 4055] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 126. | Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7:634-640. [PubMed] |
| 127. | Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 520] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 128. | Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 129. | de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847-8851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 130. | Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 131. | Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol. 2007;17:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 132. | Jiang W, Jimenez G, Wells NJ, Hope TJ, Wahl GM, Hunter T, Fukunaga R. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol Cell. 1998;2:877-885. [PubMed] |
| 133. | Subramanian R, Wilson-Kubalek EM, Arthur CP, Bick MJ, Campbell EA, Darst SA, Milligan RA, Kapoor TM. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell. 2010;142:433-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 134. | Chen J, Rajasekaran M, Xia H, Zhang X, Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut. 2016;pii:gutjnl-2015-310625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 135. | Wei W, Chua MS, Grepper S, So SK. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer. 2009;8:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 136. | Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 137. | Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 816] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 138. | Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 723] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 139. | Dahmani R, Just PA, Perret C. The Wnt/β-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 140. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8061] [Cited by in RCA: 8812] [Article Influence: 463.8] [Reference Citation Analysis (0)] |
| 141. | Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 688] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 142. | Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2790] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 143. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1961] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 144. | Bromberg J, Darnell JE. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 969] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 145. | Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1813] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 146. | Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1418] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 147. | Calò V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 463] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 148. | Yang SF, Wang SN, Wu CF, Yeh YT, Chai CY, Chunag SC, Sheen MC, Lee KT. Altered p-STAT3 (tyr705) expression is associated with histological grading and intratumour microvessel density in hepatocellular carcinoma. J Clin Pathol. 2007;60:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 149. | Sun X, Zhang J, Wang L, Tian Z. Growth inhibition of human hepatocellular carcinoma cells by blocking STAT3 activation with decoy-ODN. Cancer Lett. 2008;262:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 150. | Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-3696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 151. | Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. [PubMed] |
| 152. | Humphries C. Adoptive cell therapy: Honing that killer instinct. Nature. 2013;504:S13-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Alici E, Sutlu T, Björkstrand B, Gilljam M, Stellan B, Nahi H, Quezada HC, Gahrton G, Ljunggren HG, Dilber MS. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 154. | Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 155. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 156. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 157. | Yu X, Zhao H, Liu L, Cao S, Ren B, Zhang N, An X, Yu J, Li H, Ren X. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol. 2014;34:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 158. | Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 159. | Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nat Rev Immunol. 2015;15:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 496] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 160. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10353] [Article Influence: 796.4] [Reference Citation Analysis (34)] |
| 161. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 162. | Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 614] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 163. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 751] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 164. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9912] [Article Influence: 762.5] [Reference Citation Analysis (0)] |
| 165. | Elkhoueiry AB, Melero I, Crocenzi TS, Welling TH, Yau TC, Yeo W, Chopra A, Grosso J, Lang L, Anderson J. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol (Meeting Abstracts) May. 2015;33:no. 15_supplLBA101 Available from: http://meetinglibrary.asco.org/content/146146-156. |