INTRODUCTION
The intrahepatic biliary tree is network of interconnected ducts, which play a key role in determining the final composition of the bile reaching the duodenum by a series of secretive and absorptive events[1,2]. Cholangiocytes are the epithelial cells that line the biliary tract and regulate these secretive and absorptive events. These cells also possess marked proliferative capacity, which is evidenced during experimental conditions such as cholestasis induced by bile duct ligation (BDL), as well as in human cholangiopathies such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC) and extrahepatic biliary obstruction and biliary atresia[1,2]. These disorders are responsible for 20% of the liver transplantations among adults and close to 50% in pediatrics in the United States[3]. The ductal reactions during these diseases range from hyperplastic cholangiocyte proliferation to severe ductopenia[1,4]. What regulates cholangiocyte proliferation and death and how these mechanisms fail is still undefined.
BIOGENIC AMINES
Serotonin, norepinephrine, epinephrine, dopamine, and histamine are often collectively referred to as “biogenic amines”[5-7]. These agents play key roles in neurotransmission and other signaling functions[5-7]. They are relatively small in size, can act as neurotransmitters to elicit various physiological responses and all have various other sites of action throughout the body[5-7]. Generally, they can be synthesized at various sites throughout the body and are released from intracellular vesicles into the surrounding tissue where they can then bind to cell membrane-located receptors on the neighboring cells to elicit their responses[8]. These molecules are capable of affecting mental functions such as mood and appetite as well as regulating blood pressure, body temperature and other bodily processes[8]. This review will focus on the effects of serotonin and dopamine on the hyperplastic and neoplastic proliferation of cholangiocytes.
SEROTONIN
Serotonin, or 5-hydroxytryptamine (5-HT), is a neuroendocrine hormone that is synthesized in serotonergic neurons in the central nervous system[9] and in enterochromaffin cells throughout the gastrointestinal tract[10]. It is synthesized by the systematic hydroxylation and decarboxylation of the amino acid tryptophan by the enzymes tryptophan hydroxylase (TPH1) and amino acid decarboxylase, respectively[9]. There are 16 serotonin receptors through which serotonin exerts its multiple effects. With the exception of the 5-HT3 receptor, a ligand-gated ion channel, all other 5-HT receptors are G protein-coupled, seven transmembrane receptors that activate intracellular secondary messenger systems[11]. Once serotonin has activated the receptor it is cleared from the extracellular space by specific re-uptake transporters whereupon it undergoes catabolism[12]. Degradation of serotonin is carried out primarily by the enzyme monoamine oxidase (MAO), which occurs as two molecular subtypes called MAO A and MAO B, which have some differences in their tissue and cellular distributions[13]. MAO A is more selective for serotonin oxidation (and has a higher affinity for the substrate) than MAO B, as it is able to metabolize serotonin with a much lower Km value[14].
Serotonin in liver function
Serotonergic nerve fibers are part of the autonomic nervous system and nerve endings have been found on the branches of the hepatic artery, portal vein, bile ducts and connective tissue of the interlobular septa in humans[15], as well as in portal tracts and the fibrous septa within rat hepatic lobules[16]. The function of serotonergic fibers in liver function appears to be related to the regulation of blood flow through the portal vein as well as sinusoidal blood flow[17].
Serotonin in cell proliferation
In the liver, inhibition of the 5-HT2 receptors by ketanserin arrested liver regeneration only when administered late (16 h) after partial hepatectomy, suggesting that serotonin may have a role in the G1/S transition check point through 5-HT2 receptors[18]. Studies have shown that liver regeneration after partial hepatectomy was completely dependent upon platelet-derived serotonin, as a mouse model of thrombocytopenia inhibited normal liver regeneration in a 5-HT2 receptor-dependent manner[19].
Serotonin is involved in the pathogenesis of certain clinical features of cholangiopathies: pruritus and fatigue in particular[20,21]. In animal models of chronic cholestasis, this may be due to an enhanced release of serotonin in the central nervous system and its subsequent interactions with subtype 1 serotonin receptors[21].
Recently, it was demonstrated that cholangiocytes have the capacity to synthesize and secrete serotonin, both of which are increased in proliferating rat cholangiocytes after BDL[22]. In addition, the 5-HT 1A and 1B receptors are found predominantly on the basolateral membrane of cholangiocytes in the liver[22]. It was postulated that this autocrine loop is integral in limiting the growth of the biliary tree as a result of chronic cholestasis. This was based on the observation that chronic treatment of rats with the 5-HT 1A and 1B receptor agonists inhibited cholangiocyte proliferation in BDL rats[22]. Furthermore, this effect is more than likely due to a direct effect of the receptor agonists on cholangiocytes, as the treatment of cholangiocytes with serotonin had a similar inhibitory effect. By immunoneutralizing the endogenous serotonin secreted from cholangiocytes as a result of BDL, using an anti-serotonin antibody, we were able to enhance the growth of the biliary tree in the course of chronic cholestasis, suggesting that the autocrine secretion of serotonin does, indeed, play an important role in the control of cholangiocyte growth[22].
Certain physiological aspects of cholangiocyte function were also inhibited by 5-HT 1A and 1B receptor agonists in proliferating cholangiocytes after BDL, but not in mitotically dormant cholangiocytes[22]. Both secretin-stimulated bile and bicarbonate secretion were inhibited by chronic in vivo administration of the serotonin receptor agonists[22]. In freshly isolated cultures of cholangiocytes, serotonin receptor agonists inhibited both secretin-stimulated cAMP synthesis and protein kinase A (PKA) activity[22]. This suggests that activation of both 5-HT 1A and 1B receptors can modulate not only cholangiocyte proliferation and survival, but also physiological functions of cholangiocytes.
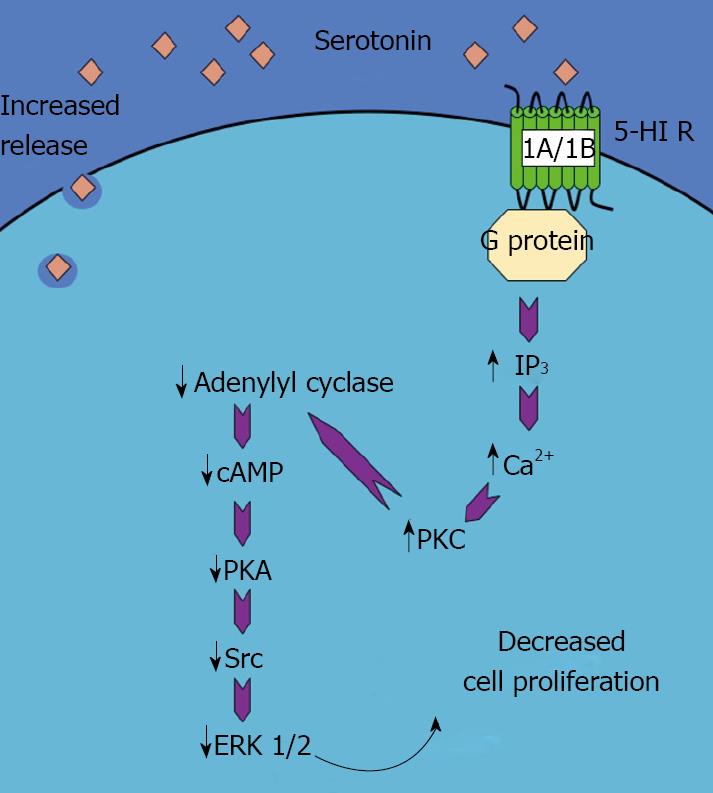
The intracellular signaling pathways that may be responsible for the antiproliferative effects of serotonin are associated with enhanced IP3 levels, increased Ca2+-dependent PKC activity and a reduction in the cAMP/PKA pathway[22]. Downstream of these events is a reduction in the activation of the Src/ERK1/2 cascade, which directly effects cholangiocyte proliferation[22]. A schematic representation of this pathway can be seen in Figure 1 (adapted from[3]).
Figure 1 Schematic representation of the mechanism of the serotonin-induced decrease in cholangiocyte proliferation.
Activation of 5-HT 1A/1B receptors results in an increase in IP3/Ca2+/PKC pathway, which in turn decreases the adenylyl cyclase/cAMP/PKA/ERK1/2 pathway. This ultimately leads to a decrease in cholangiocyte proliferation. (This figure was adapted from DeMorrow et al[3] and reproduced with permission from the Society for Experimental Biology and Medicine).
A role for serotonin in biliary choleresis is also supported by the observation that selective serotonin reuptake inhibitors such as citalopram and paroxetine have been linked with cholestasis as well as severe acute and chronic hepatitis in humans[23-25]. However, the mechanisms by which selective serotonin reuptake inhibitors contribute to cholestasis are unknown[17].
Serotonin control of neoplastic growth in the biliary epithelium
Several opposing effects of serotonin on tumor growth have been reported[26]. On one hand, serotonin is known as a growth factor for several types of non-tumoral cells[27,28], and it has been proposed to take part in the autocrine loops of growth factors contributing to cell proliferation in aggressive tumors such as small cell lung carcinoma[29], prostate cancer[30], breast cancer[31] and bladder cancer[32]. In contrast, several studies have reported that serotonin can inhibit tumor growth, mainly via the specific vasoconstrictive effects of serotonin on the vessels irrigating the tumors[26,32-34]. In addition, the synthesis and secretion of serotonin have previously been shown to be dysregulated in neuroendocrine tumors, with these cells possessing a higher biogenic amine content than normal cells[35-37].
We have recently shown that serotonin is overproduced in cholangiocarcinoma. It can be detected in the supernatant from cholangiocarcinoma cell lines as well as in the bile taken from cholangiocarcinoma patients at higher levels, compared to those with non-malignant biliary diseases[38]. Further analysis revealed disequilibrium between the synthesis and degradation pathways of serotonin[38]. Specifically, the rate-limiting synthesis enzyme TPH1 is overexpressed in cholangiocarcinoma cell lines and tumor tissue, whereas the expression of MAO A is dramatically downregulated[38]. This increase in serotonin production has growth-promoting effects on cholangiocarcinoma and blocking serotonin synthesis by treating animals with a specific TPH1 inhibitor effectively reduces tumor growth[38].
Precisely why serotonin goes from having growth-suppressing activities in non-malignant cholangiocytes to promoting growth in neoplastic cholangiocarcinoma cells is unknown and is a topic of ongoing research in our laboratory.
DOPAMINE
Dopamine is synthesized mainly by nervous tissue and adrenal glands, first by the enzymatic conversion of tyrosine to DOPA (3,4-dihydroxyphenilalanine) by tyrosine hydroxylase and then by the decarboxylation of DOPA by aromatic-L-amino-acid decarboxylase. As a member of the catecholamine family, dopamine is also a precursor to epinephrine and norepinephrine. The dopamine receptors are a class of metabotropic G protein-coupled receptors and, to date, there are 5 types: D1-D5[39]. Activation of these receptors has differing effects on signal transduction pathways. For example, the D1 receptor interacts with the Gs complex to activate adenylyl cyclase, whereas the D2 receptor interacts with Gi to inhibit cAMP production[39]. As with serotonin, dopamine is rapidly cleared from the extracellular space by a dopamine-specific re-uptake transporter whereupon it is degraded, predominantly by MAO A[40].
In the brain dopamine acts as a neurotransmitter where it activates dopamine receptors, but it can also act as a neurohormone which is released by the hypothalamus and exerts various effects on the pituitary[41]. Dopamine has been implicated in the etiology of Parkinson’s disease[42] and schizophrenia[43] and plays a major role in the reward system of behavior[44].
Dopamine in hyperplastic cell proliferation in the liver
The studies into the effects of dopaminergic innervation on cholangiocytes have focused on the D2 dopamine receptor[45]. Expression of the other dopamine receptors was absent from cholangiocytes under all conditions studied (mitotically dormant and proliferating cholangiocytes), whereas the D2 dopamine receptor was expressed in normal cholangiocytes and markedly upregulated after BDL[45]. In similar experiments to those described above for serotonin, the effects of D2 dopamine receptor activation on other aspects of cholangiocyte physiology have been determined[45]. Infusion of quinelorane had no effect on basal bile flow and bicarbonate concentration and secretion. However, co-infusion of quinelorane with secretin resulted in a decrease in secretin-stimulated bile flow and bicarbonate secretion, an effect that could be abolished with the D2 receptor antagonist eticlopride[45]. It has been repeatedly demonstrated that agents that inhibit secretin-stimulated bile flow also exhibit growth-suppressive actions on cholangiocytes[22,46-48]. This further supports a tentative role for D2 dopamine receptor activation in the suppression of cholangiocyte proliferation after BDL.
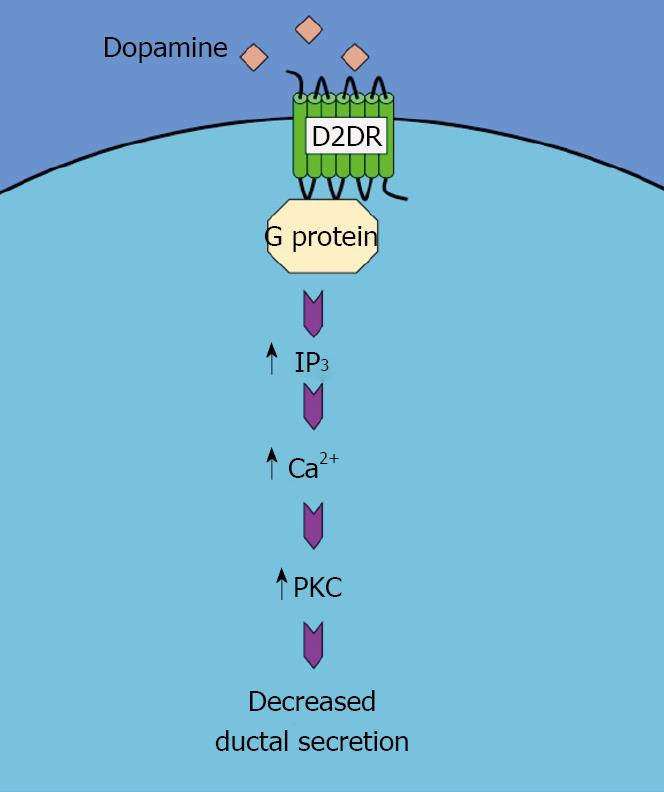
The mechanism by which quinelorane inhibits secretin-induced ductal secretion and, by extension, cholangiocyte growth, is similar to that observed after serotonin receptor activation. That is, quinelorane activates the Ca2+-dependent Protein kinase C (PKC)-γ but not any other PKC isoform. Once again, blocking PKC-γ activity effectively inhibits the effects of D2 dopamine receptor activation on ductal secretion[45]. This pathway is summarized in Figure 2.
Figure 2 Schematic representation of the mechanism of the dopamine-induced decrease in ductal secretion.
Activation of D2DR results in an increase in IP3/Ca2+/PKC pathway, which in turn decreases the ductal secretion.
Information regarding the ability of cholangiocytes to synthesize and secrete dopamine is lacking, so it is not possible to say whether these dopamine-induced effects on cholangiocytes are through an autocrine mechanism and/or are a direct result of dopaminergic innervation of the liver.
Dopamine control of neoplastic growth in the biliary epithelium
Similar to serotonin, several opposing effects of dopamine on tumor growth have been reported. There is an increase in the circulating level of dopamine in lung tumors, which seems to play a protective role by inhibiting cytotoxic t-cell proliferation, thereby preventing their ability to mount an adequate attack on the tumor cells[49,50]. Furthermore, dopamine secretion is increased in some cases of the rare malignancy pheochromocytoma[51,52] and in carcinoid tumors[53], although the consequences of this secretion on tumor growth or progression are unclear. However, agents such as dexamethasone that increase pheochromocytoma dopamine content also increase cell proliferation[54], suggesting that there may be a causal link between increased dopamine content and cell proliferation in these tumor cells. Conversely, in malignant colon tissue[55] and gastric cancer tissue[56] where dopamine levels are depleted, dopamine treatment slows tumor growth, presumably by decreasing the expression of vascular epithelial growth factor and subsequent angiogenesis[56,57]. In addition, modulation of dopamine receptors is being proposed as a possible treatment for pituitary tumors due to the suppressive effects of dopamine on prolactin secretion[58]. It may also have a role in the treatment of neuroblastoma cells, where D1DR agonists have a toxic effect on cell proliferation, which appears to be neuronal specific.
We have recently shown that dopamine, similar to serotonin, is overproduced in cholangiocarcinoma and can be detected in both the supernatant from cholangiocarcinoma cell lines and in the bile from cholangiocarcinoma patients[59]. The two enzymes responsible for dopamine synthesis, tyrosine hydroxylase and dopa decarboxylase are both overexpressed in cholangiocarcinoma cell lines and in cholangiocarcinoma tumor tissue[59]. Once again, the increased dopamine production increased cell proliferation and tumor growth, and inhibiting dopamine synthesis by using specific inhibitors of dopa decarboxylase suppresses tumor growth in vitro and in vivo[59].
As is the case with serotonin, the explanation of why dopamine has growth-inhibitory actions in cholangiocytes but has growth-promoting effects in cholangiocarcinoma is unclear and is the topic of ongoing research in our laboratory.
CONCLUSIONS AND FUTURE DIRECTIONS
Biogenic amines such as serotonin and dopamine regulate a plethora of biological responses. We, and others, have strived to dissect the precise effects of these important biological molecules on cholangiocyte growth and physiology as well as on the malignant growth and progression of cholangiocarcinoma. Specifically, we suggest that both serotonin and dopamine have a buffering role in limiting the cholangiocyte hyperplastic proliferation seen after bile duct ligation. Conversely, somewhere during the course of the malignant transformation of cholangiocytes, serotonin and dopamine effects become growth promoting rather than growth suppressive and may contribute to tumor growth and the progression of cholangiocarcinoma. Studies have highlighted the potential importance of these molecules and their receptors in the pathological processes associated with chronic cholestatic liver diseases and cholangiocarcinoma. Further research into the molecular events associated with the actions of the various biogenic amines on hyperplastic and neoplastic cholangiocyte proliferation is ongoing in our laboratory. Regulation of cholangiocyte growth and cell death by therapeutic agents aimed at activating or blocking these receptor systems may prove beneficial in the treatment of various cholangiopathies such as primary biliary cirrhosis and sclerosing cholangitis, as well as for blocking or slowing the progression of cholangiocarcinoma.