Peer-review started: October 10, 2016
First decision: December 13, 2016
Revised: December 17, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 28, 2017
Processing time: 142 Days and 13.4 Hours
To demonstrate feasibility of vessel wall imaging of the superficial palmar arch using high frequency micro-ultrasound, 7T and 3T magnetic resonance imaging (MRI).
Four subjects (ages 22-50 years) were scanned on a micro-ultrasound system with a 45-MHz transducer (Vevo 2100, VisualSonics). Subjects’ hands were then imaged on a 3T clinical MR scanner (Siemens Biograph MMR) using an 8-channel special purpose phased array carotid coil. Lastly, subjects’ hands were imaged on a 7T clinical MR scanner (Siemens Magnetom 7T Whole Body Scanner) using a custom built 8-channel transmit receive carotid coil. All three imaging modalities were subjectively analyzed for image quality and visualization of the vessel wall.
Results of this very preliminary study indicated that vessel wall imaging of the superficial palmar arch was feasible with a whole body 7T and 3T MRI in comparison with micro-ultrasound. Subjective analysis of image quality (1-5 scale, 1: poorest, 5: best) from B mode, ultrasound, 3T SPACE MRI and 7T SPACE MRI indicated that the image quality obtained at 7T was superior to both 3T MRI and micro-ultrasound. The 3D SPACE sequence at both 7T and 3T MRI with isotropic voxels allowed for multi-planar reformatting of images and allowed for less operator dependent results as compared to high frequency micro-ultrasound imaging. Although quantitative analysis revealed that there was no significant difference between the three methods, the 7T Tesla trended to have better visibility of the vessel and its wall.
Imaging of smaller arteries at the 7T is feasible for evaluating atherosclerosis burden and may be of clinical relevance in multiple diseases.
Core tip: The evaluation of smaller arteries in the hand may be clinically useful in a variety of vascular diseases. Imaging the vessel wall of such small caliber arteries (2.5 mm to 3.1 mm) requires very high spatial resolution and the use of either high frequency micro-ultrasound or 7 Tesla magnetic resonance imaging (MRI) would be the ideal tool to acquire these images. We sought to demonstrate feasibility of vessel wall imaging of the superficial palmar arch using 7T and 3T MRI in comparison with very high frequency micro-ultrasound.
- Citation: Pruzan AN, Kaufman AE, Calcagno C, Zhou Y, Fayad ZA, Mani V. Feasibility of imaging superficial palmar arch using micro-ultrasound, 7T and 3T magnetic resonance imaging. World J Radiol 2017; 9(2): 79-84
- URL: https://www.wjgnet.com/1949-8470/full/v9/i2/79.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i2.79
A variety of pathological processes can affect the hand, including atherosclerosis, scleroderma, thromboangiitis obliterans (TO), hypothenar hammer syndrome and acute arterial thrombosis related to intraarterial injection[1].
Atherosclerosis is a chronic complex process involving multiple factors including inflammation, oxidative stress, endothelial dysfunction, smooth muscle cell proliferation, platelet activation, and thrombosis, all leading to pathologic changes in the arterial wall with plaque formation[2]. The plaque is primarily composed of inflammatory cells, lipids, and calcium[3]. Chronic atherosclerosis can cause peripheral vascular occlusive disease in the extremities. In the upper extremity, atherosclerosis is more prevalent in the proximal subclavian artery but can occur in smaller distal vessels with resultant morbidity[4]. For example, young diabetics who have diffuse distal atherosclerosis, end-stage renal disease and are on renal dialysis are at high risk for developing finger gangrene[5]. In the autoimmune disorder scleroderma, ultrasound evaluation has shown that the ulnar artery proximal to the wrist is specifically targeted[6]. The vasculopathy of scleroderma can cause secondary Raynaud’s phenomenon, as well as digital ulcers. Doppler sonography has proven useful in assessing palmar and digital arteries in these cases[7,8]. TO also known as Buerger’s disease, is a panarteritis of unknown origin, which demonstrates a strong concurrence with tobacco use. It affects both small and medium-sized vessels of the extremities. The thrombotic and inflammatory changes associated with TO cause vascular changes with associated arteriographic findings[9-11]. Hypothenar hammer syndrome, a post-traumatic pathology arising from repetitive hitting of the hypothenar region of the hand, can cause ulnar artery occlusive disease with thromboembolism and resulting ischemia to the digits[12,13]. The thrombosis secondary to TO and hypothenar hammer syndrome may be relatively localized, whereas diffuse arterial thrombosis of the hand may occur secondary to intraarterial injection of medications or illegal substances[14]. Visualizing atherosclerotic changes in small arteries such as the superficial palmar arch may provide valuable clinical insights into the progression of these disease states.
Imaging the vessel wall of smaller caliber arteries requires higher resolution imaging when compared to imaging larger vessels such as the aorta and carotid arteries. For this purpose we conducted MR evaluation of the superficial palmar arch using 7T and 3T whole body MR scanners and compared the images acquired with high frequency micro-ultrasound imaging. The superficial palmar arch is the continuation of the ulnar artery as it passes distal to the flexor retinaculum in the hypothenar region of the palm. We chose to focus on the superficial palmar arch because it is easily and consistently visualized using the modalities employed. We hypothesize that 7T MRI will provide better delineation of the vessel wall as compared to 3T and may be a suitable method to visualize small arteries in the hand. We also believe that ultrasound may be a convenient alternative compared to 3T and 7T due to its image acquisition speed and wide availability. But, conventional clinical ultrasound (about 3-7 MHz) may lack resolution, so the use of a high frequency (45-MHz) micro-ultrasound typically used in animal models may be better suited for superficial structures such as the palmar arch.
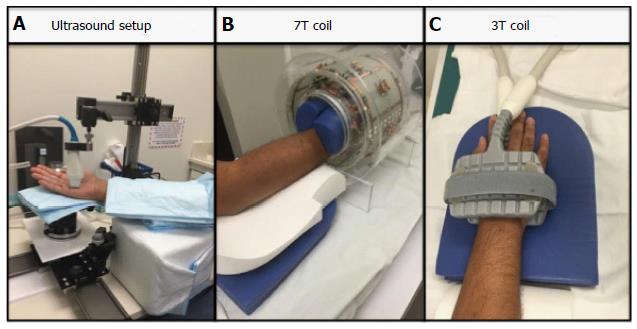
Four subjects (ages 22-50 years) were scanned on a micro-ultrasound system with a 45-MHz transducer (Vevo 2100, VisualSonics, Toronto, Canada) (Figure 1A). While this system is primarily used in animal studies, it has been successfully and safely used in clinical studies, which include infants and children.[15,16]. Subjects’ hands were then imaged on a 3T clinical MR scanner (Siemens Biograph MMR) using an 8-channel special purpose phased array carotid coil (Figure 1C). Lastly, subjects’ hands were imaged on a 7T clinical MR scanner (Siemens Magnetom 7T Whole Body Scanner) using a custom built 8-channel transmit receive carotid coil (Figure 1B).
The Vevo Imaging Station (Vevo 2100, VisualSonics, Toronoto, Canada) was used for mounting the transducer and for stabilizing the position of the hand. The subject was seated during the scan with the hand in supine position with slight rotation toward neutral. Padding was also used under the hand and arm for stabilization and comfort purposes (Figure 1A). With this positioning, images in B mode, Power mode, Doppler mode and M mode of the superficial palmar arch were obtained. Images were then subjectively analyzed for image quality. Three readers rated the images on a score: (1) is a non-visible vessel with non-visible walls and poor image quality; (2) represents a vessel with indistinct vessel walls and poor image quality; (3) is a vessel with adequate image quality with walls moderately well seen; (4) represents a vessel with distinct vessel walls and good image quality; and (5) is excellent visualization of the vessel and image quality. Results from the three readers were averaged. Criteria used for subjective evaluation were the overall image quality, visualization of the vessel wall, adequate flow suppression and absence of artifacts. We also obtained measures of average peak Doppler velocity, intima media thickness, wall thickness, lumen diameter, and total vessel diameter.
Subjects’ hands were imaged on a 3T clinical MR scanner (Siemens Biograph MMR) using an 8- channel special purpose phased array carotid coil (Figure 1C). Subjects’ hands were imaged on a 7T clinical MR scanner (Siemens Magnetom 7T Whole Body Scanner) using a custom built 8-channel transmit receive carotid coil (Figure 1B). Subjects were imaged in a head first prone position with hand extended above the head. The imaging protocols between 3T and 7T were matched as closely as possible. Total scan time was approximately 20 min each. We acquired a localizer, a 3D time-of-flight (TOF) MR angiography sequence followed by a 3D T2 weighted Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) sequence[1,17,18] and a 0.6 mm isotropic resolution in all dimensions (Table 1). MR images were also subjectively analyzed for image quality and visualization of the vessel wall. The imaging criteria used were similar to that for the ultrasound. Furthermore, we also measured wall thickness, lumen and outer diameters from the MR images. The order of the imaging tests was randomized.
| Parameter | 7T T2 SPACE | 3T T2 SPACE | 7T TOF | 3T TOF |
| TE | 101 | 77 | 2.81 | 3.23 |
| TR | 1500 | 1600 | 60 | 21 |
| Slice thickness | 625.00 μm | 630.00 μm | 160.00 mm | 1.00 mm |
| Pixel size | 0.600/0.600 | 0.625/0.625 | 0.3125/0.3125 | 0.234/0.234 |
| Number of averages | 2 | 4 | 1 | 1 |
| Field of view | 136 × 160 | 100 × 160 | 160 × 160 | 107 × 120 |
The data were expressed as the mean ± SD. For the statistics, a RM one-way ANOVA and Tukey’s multiple comparisons test were used. P < 0.05 was considered as statistically significant.
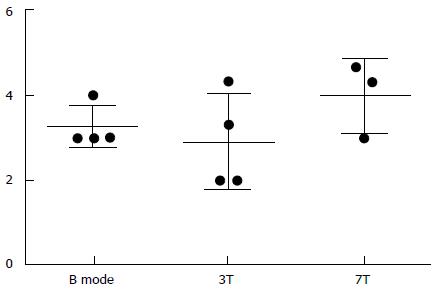
Subjective analysis of image quality was performed (1-5 scale, 1: Vessel non-visible, 5: Clearly visible vessel wall) from B mode, ultrasound, 3T SPACE MRI and 7T SPACE MRI. These results are shown in Table 2 and Figure 2.
| Subject | B mode | 3T | 7T |
| 1 | 3.00 | 3.33 | 3.00 |
| 2 | 3.00 | 2.00 | Not available |
| 3 | 3.00 | 4.33 | 4.67 |
| 4 | 4.00 | 2.00 | 4.33 |
| Average | 3.25 | 2.92 | 4.00 |
| Standard deviation | 0.50 | 1.13 | 0.882 |
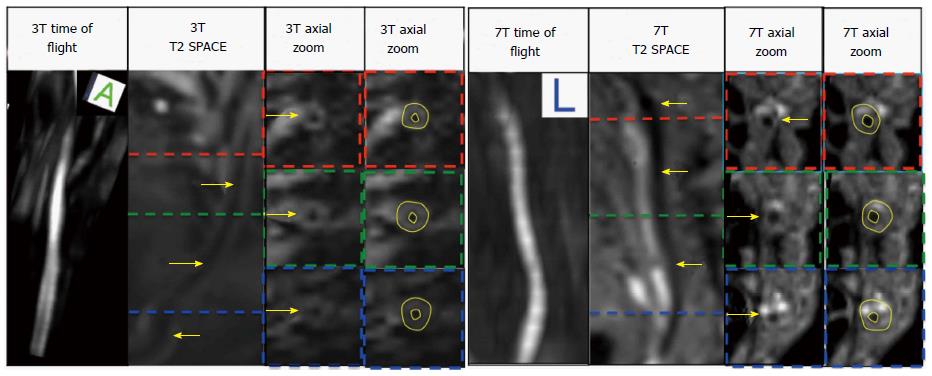
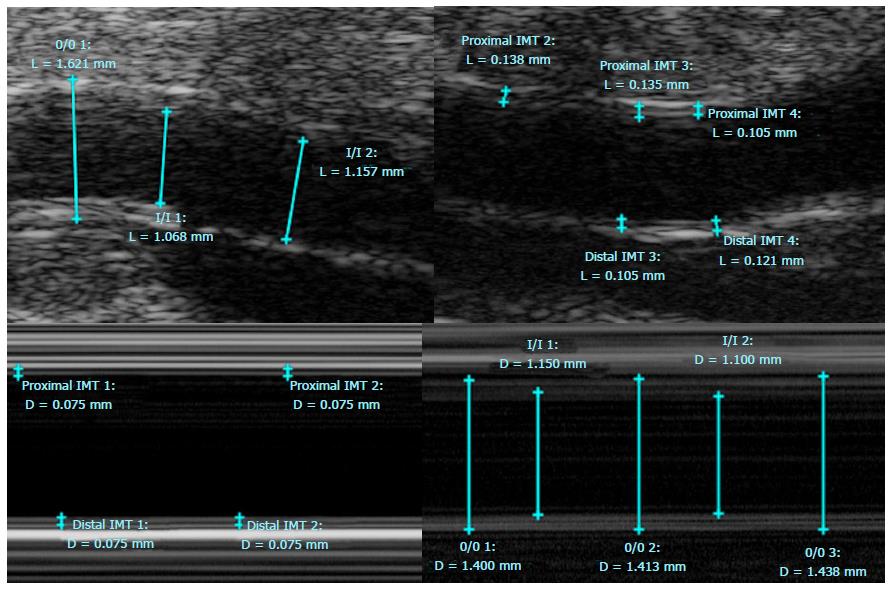
In addition, lumen area, wall area, total vessel area, and wall thickness were automatically calculated by a MatLab script (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States) for the manually drawn contours on the 3T and 7T images using a customized software program (Vessel Mass Software, Leiden University Medical Center, The Netherlands) (Figure 3). Sonographic images were analyzed on the micro-ultrasound machine using the software on the B and M Mode photos (Figure 4) and data was extracted and used to calculate the aforementioned measurements by hand in Microsoft Excel.
Qualitative analysis indicated that the image quality obtained at 7T trended to be superior to both 3T MRI and micro-ultrasound (Figure 2 and Table 2). Compared to the 3D Time of Flight, the 3D SPACE sequence with isotropic voxels allowed for multi-planar reformatting of images and allowed for less operator dependent results as compared to high frequency micro-ultrasound imaging. Although there was no significant difference between the three methods (P = 0.5647, One-Way ANOVA), the 7T Tesla trended to have better visibility of the vessel and its wall (Table 2, Figures 2 and 3).
The One-Way ANOVA between all 3 imaging modalities showed that 7T, 3T and micro-ultrasound imaging are all comparable imaging methods with no statistical difference between extracted values, (lumen area, wall area, total vessel area, wall thickness)[19,20] [P > 0.05 (Table 3)]. Post hoc test using Tukey’s correction however showed significant differences when comparing 3T and micro-ultrasound for wall thickness.
| Vessel wall measurements | P value |
| Lumen area | 0.3152 |
| Wall area | 0.0628 |
| Total vessel area | 0.0573 |
| Normalized wall index | 0.0362a |
| Wall thickness | 0.0519 |
Results of this very preliminary study indicated that vessel wall imaging of the superficial palmar arch was feasible with a whole body 7T MRI with subjective evaluations indicating that the image quality obtained at 7T is superior to both 3T MRI and micro-ultrasound. The 3D SPACE sequence with isotropic voxels allowed multi-planar reformatting of images and allows for less operator dependent results as compared to ultrasound imaging. Scanning time for the micro-ultrasound portion of the study approached that of the MR scan times, however with more operator experience the micro-ultrasound scan time could improve. The time spent on post-processing image analysis was similar across all modalities. Limitations of this study include a very small sample size and the fact that only healthy individuals without any atherosclerotic disease were examined. Future investigation should study individuals with diseased conditions in a larger sample size. Additionally, methods to optimize image quality could include warming the region of interest prior and during imaging to dilate the vessel and provide better contrast between the lumen and the vessel wall structures. This technique has been shown to optimize image quality in peripheral 2D TOF MRA evaluation[21].
In conclusion, imaging of the superficial palmar arch at the 7T is feasible with high frequency micro-ultrasound, 3T and 7T MRI and should be considered for evaluating arterial pathology. This may be of clinical relevance in specific disease conditions such as atherosclerosis, scleroderma, diabetes, TO, hypothenar hammer syndrome and acute arterial thrombosis related to intraarterial injection. Future studies need to be performed in diseased individuals and in a larger number of subjects to better establish clinical feasibility of the approach.
Imaging the vessel wall of smaller caliber arteries requires higher resolution imaging when compared to imaging larger vessels such as the aorta and carotid arteries. For this purpose the authors conducted MR evaluation of the superficial palmar arch using 7T and 3T whole body MR scanners and compared the images acquired with high frequency ultrasound imaging. The author chose to focus on the superficial palmar arch because it is easily and consistently visualized using the modalities employed.
A variety of pathological processes can affect the hand, including atherosclerosis, scleroderma, thromboangitiis obliterans, hypothenar hammer syndrome and acute arterial thrombosis related to intraarterial injection. These diseases are currently evaluated using sonography, arteriography, and standard 1.5 or 3T MRI. The intent of the investigation is to improve and potentially advance imaging techniques for evaluation of these diseases.
A thorough search of medical literature shows few if any prior investigations related to imaging the superficial palmar arch on a 7T MRI and therefore this is a novel application.
Future evaluation for atherosclerosis and other diseases may involve non-invasive 3T or 7T MR as well as micro-ultrasound imaging of the superficial palmar arch.
Imaging of smaller artery is feasible using micro-ultrasound and 3T and 7T MRI. Interesting and good study enough for publication.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chow J, Gumustas OG, Tang GH S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Wong SK, Mobolaji-Iawal M, Arama L, Cambe J, Biso S, Alie N, Fayad ZA, Mani V. Atherosclerosis imaging using 3D black blood TSE SPACE vs 2D TSE. World J Radiol. 2014;6:192-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004;109:2617-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Alie N, Eldib M, Fayad ZA, Mani V. Inflammation, Atherosclerosis, and Coronary Artery Disease: PET/CT for the Evaluation of Atherosclerosis and Inflammation. Clin Med Insights Cardiol. 2014;8:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Zimmerman NB. Occlusive vascular disorders of the upper extremity. Hand Clin. 1993;9:139-150. [PubMed] |
| 5. | Yeager RA, Moneta GL, Edwards JM, Landry GJ, Taylor LM, McConnell DB, Porter JM. Relationship of hemodialysis access to finger gangrene in patients with end-stage renal disease. J Vasc Surg. 2002;36:245-249; discussion 249. [PubMed] |
| 6. | Stafford L, Englert H, Gover J, Bertouch J. Distribution of macrovascular disease in scleroderma. Ann Rheum Dis. 1998;57:476-479. [PubMed] |
| 7. | Lescoat A, Coiffier G, Rouil A, Droitcourt C, Cazalets C, de Carlan M, Perdriger A, Jégo P. Vascular evaluation of the hand by Power Doppler Ultrasonography provides new predictive markers of ischemic digital ulcers in systemic sclerosis. Arthritis Care Res (Hoboken). 2016; Jul 7; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Chikui T, Izumi M, Eguchi K, Kawabe Y, Nakamura T. Doppler spectral waveform analysis of arteries of the hand in patients with Raynaud’s phenomenon as compared with healthy subjects. AJR Am J Roentgenol. 1999;172:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Rivera-Chavarría IJ, Brenes-Gutiérrez JD. Thromboangiitis obliterans (Buerger’s disease). Ann Med Surg (Lond). 2016;7:79-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Gallagher KA, Tracci MC, Scovell SD. Vascular arteritides in women. J Vasc Surg. 2013;57:27S-36S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Fazeli B, Rezaee SA. A review on thromboangiitis obliterans pathophysiology: thrombosis and angiitis, which is to blame? Vascular. 2011;19:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Marie I, Hervé F, Primard E, Cailleux N, Levesque H. Long-term follow-up of hypothenar hammer syndrome: a series of 47 patients. Medicine (Baltimore). 2007;86:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Carpentier PH, Biro C, Jiguet M, Maricq HR. Prevalence, risk factors, and clinical correlates of ulnar artery occlusion in the general population. J Vasc Surg. 2009;50:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Iannuzzi NP, Higgins JP. Acute Arterial Thrombosis of the Hand. J Hand Surg Am. 2015;40:2099-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Ymalay R, Sadanala U, Tinney JP, Kriss V, Keller BB. Initial Observations and Limitations of Vevo 2100 High Resolution Ultrasound Imaging in Neonates. J Invest Med. 2012;60:379. |
| 16. | Kaufman CL, Ouseph R, Blair B, Kutz JE, Tsai TM, Scheker LR, Tien HY, Moreno R, Ozyurekoglu T, Banegas R. Graft Vasculopathy in Clinical Hand Transplantation. Am J Transplant. 2012;12:1004-1016. [DOI] [Full Text] |
| 17. | Mihai G, Chung YC, Merchant A, Simonetti OP, Rajagopalan S. T1-weighted-SPACE dark blood whole body magnetic resonance angiography (DB-WBMRA): initial experience. J Magn Reson Imaging. 2010;31:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Zhang Z, Fan Z, Carroll TJ, Chung Y, Weale P, Jerecic R, Li D. Three-dimensional T2-weighted MRI of the human femoral arterial vessel wall at 3.0 Tesla. Invest Radiol. 2009;44:619-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 417] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 20. | Balu N, Chu B, Hatsukami TS, Yuan C, Yarnykh VL. Comparison between 2D and 3D high-resolution black-blood techniques for carotid artery wall imaging in clinically significant atherosclerosis. J Magn Reson Imaging. 2008;27:918-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Blackband SJ, Buckley DL, Knowles AJ, Gibbs P, Turnbull LW, Horsman A. Improved peripheral MR angiography with temperature regulation in healthy patients. Radiology. 1996;198:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |