Peer-review started: August 26, 2016
First decision: October 8, 2016
Revised: December 6, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: February 28, 2017
Processing time: 187 Days and 0.3 Hours
To present the typical and atypical magnetic resonance (MR) imaging findings of alcoholic and non-alcoholic Wernicke’s encephalopathy.
This study included 7 patients with Wernicke’s encephalopathy (2 men, 5 women; mean age, 52.3 years) that underwent brain MR examination between January 2012 and March 2016 in a single institution. Three patients were alcoholics and 4 patients were non-alcoholics. MR protocol included a T2-weighted sequence, a fluid attenuation inversion recovery (FLAIR) sequence, a diffusion-weighted sequence (b = 0 and 1000 s/mm2), and a contrast-enhanced MR sequence. All MR images were retrospectively reviewed at baseline and follow-up by two radiologists.
All patients with Wernicke’s encephalopathy had bilateral areas showing high signal intensity on both T2-weighted and FLAIR MR images in the typical sites (i.e., the periaqueductal region and the tectal plate). Signal intensity abnormalities in the atypical sites (i.e., the cerebellum and the cerebellar vermis) were seen in 4 patients, all of which had no history of alcohol abuse. Six patients had areas with restricted diffusion in the typical and atypical sites. Four patients had areas showing contrast-enhancement in the typical and atypical sites. Follow-up MR imaging within 6 mo after therapy (intravenous administration of thiamine) was performed in 4 patients, and demonstrated a complete resolution of all the signal intensities abnormalities previously seen in all patients.
MR imaging is valuable in the diagnosis of Wernicke’s encephalopathy particularly in patients presenting with atypical clinical symptoms, or with no history of alcohol abuse.
Core tip: The purpose of this study was to describe the typical and atypical magnetic resonance (MR) imaging findings of alcoholic and nonalcoholic Wernicke’s encephalopathy. Bilateral increased T2-weighted and fluid attenuation inversion recovery MR signal intensity in the typical areas were seen in all patients. Signal-intensity alterations in atypical sites were seen only in nonalcoholic patients. This study demonstrated that MR imaging was useful in supporting the diagnosis of Wernicke’s encephalopathy.
- Citation: Sparacia G, Anastasi A, Speciale C, Agnello F, Banco A. Magnetic resonance imaging in the assessment of brain involvement in alcoholic and nonalcoholic Wernicke’s encephalopathy. World J Radiol 2017; 9(2): 72-78
- URL: https://www.wjgnet.com/1949-8470/full/v9/i2/72.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i2.72
Wernicke’s encephalopathy (WE) is a neurologic disease caused by thiamine (vitamin B1) deficiency[1]. Thiamine is involved in sustainment of the osmotic gradients of cell membranes and in the maintenance of membrane integrity. A thiamine deficiency could lead intra and extra cellular edema, as due to an inability of cell membranes to maintain osmotic gradient.
Thiamine deficiency is a consequence of inadequate dietary intake and of impaired absorption of the vitamin. Alcohol abuse is the most common cause of WE, other causes could be gastrointestinal surgery, hyperemesis gravidarum, anorexia nervosa and protracted parenteral therapy. Usually nonalcoholic WE is more difficult to diagnose. In previous study only 20% or less of patients with nonalcoholic WE were diagnosed premortem[1].
However, in industrialized countries, 90% of the cases of thiamine deficiency are associated with alcohol abuse[2]. Alcohol chronic abuse can cause a thiamine deficiency directly and indirectly with inadequate dietary intake, impaired intestinal absorption and poor intracellular use of thiamine.
The typical clinical presentation of WE is characterized by a triad of symptoms: Ocular manifestations (like nystagmus, bilateral lateral rectus palsies, ophthalmoplegia and conjugate gaze palsies), ataxia and global confusion. In the clinical practice, this typical triad occurs in only 13%-18% of all patients with WE[3-5].
The prognosis of WE patients depends on prompt and early intravenous administration of thiamine supplementation[3]. Sometimes WE become irreversible because the diagnosis is delayed or is misdiagnosed based on ambiguous symptoms such as dizziness, weakness, anorexia, and memory disturbance[4]. Magnetic resonance (MR) imaging may be valuable for the diagnosis especially when the clinical diagnosis is difficult.
In the periventricular regions, there is a high rate of thiamine-related glucose and oxidative metabolism, which has been considered the cause of blood-brain barrier deficiency of these regions in WE[6].
The typical MR imaging findings in acute WE are represented by a symmetrical increased T2-weighted and fluid-attenuated inversion recovery (FLAIR) signal intensity of periaqueductal area, medial thalami, mammillary bodies, tectal plate and periventricular region of third and fourth ventricles[5]. In these areas, the maintenance of cellular osmotic gradients is considered to be strictly related to thiamine levels.
The atypical MR imaging findings are represented by abnormal signal-intensity involving the cerebellum, the cerebellar vermis, the cranial nerve nuclei, the red nuclei, the dentate nuclei, the caudate nuclei, the corpus callosum, and the cerebral cortex[5,7-21].
In this article the typical and atypical MRI findings of alcoholic and nonalcoholic Wernicke’s encephalopathy are presented and the value of MR imaging in the diagnosis and follow-up of the disorder is discussed.
The institutional review board of our institution approved this retrospective study and written informed consent was obtained from all patients. All patients were referenced with the suspect of WE. There were a total of 7 patients (2 men, 5 women; age 28-75 years; mean age, 52.3 years) that underwent MR examination between January 2012 and March 2016 in a single institution. Four out of 7 patients underwent to MR imaging follow-up within 2 mo after intravenous administration of thiamine. Demographic and epidemiologic data are summarized in Table 1.
| Patient | Age (yr) | Gender | Predisposing causes |
| 1 | 50 | M | Alcoholic |
| 2 | 55 | F | Alcoholic |
| 3 | 67 | F | Alcoholic |
| 4 | 45 | F | Parental nutrition |
| 5 | 75 | F | Leukemia in chemotherapy treatment |
| 6 | 28 | F | Pregnancy |
| 7 | 53 | M | Stomach cancer |
Three patients had a history of chronic alcohol abuse: (1) patient #1 (male, 50 years) was hospitalized for fatigue, malnutrition, and stupor; clinical examination showed a bilateral paralysis of the abducens, lateral nystagmus in both eyes, mental confusion and disorientation; (2) patient #2 (female, 55 years) was hospitalized for loss of consciousness, nystagmus, bilateral lateral rectus palsies and conjugate gaze palsies; and (3) patient #3 (female, 67 years) was admitted with ataxia and global confusion.
The remaining 4 patients hadn’t history of alcohol abuse: (1) patient #4 (female, of 45 years) was in parental nutrition from some weeks for acute pancreatitis, she showed sudden onset of nystagmus, disorientation, and ataxia; (2) patient #5 (female, 75 years), affected by leukemia, was treated with chemotherapy and parenteral nutrition, presented with vomiting, nausea, hallucinations, behavioral disturbances; (3) patient #6 (female, 28 years), at 32nd weeks of pregnancy, had hyperemesis gravidarum with neurological disorders; and (4) patient #7 (male, 53 years) had a stomach cancer with pyloric stenosis demonstrated at gastroscopy and was hospitalized for walking disorders, nausea, and vomiting.
All MR examinations were performed on a 1.5T MR scanner (Signa Excite, GE Medical Systems, Milwaukee, United States). MR imaging protocol included axial and sagittal fast spin-echo (FSE) T2W [5100/110 (TR/TE)] images, axial FLAIR [8000/140/2400 (TR/TE/TI)] images, along with axial, sagittal, and coronal non-enhanced and contrast-enhanced (0.1 mmol/Kg gadobutrol - Gadovist, Bayer, Germany) FSE T1W [650/15 (TR/TE)] images with a field of view (FOV) of 22 cm, matrix 512 × 512, slice thickness 5 mm, intersection gap 1 mm, number of excitations 2. Diffusion-weighted imaging (DWI) with echo-planar (TR 6500/TE 125) technique was obtained in all patients.
Typical and atypical MRI findings in WE patients are summarized in Table 2.
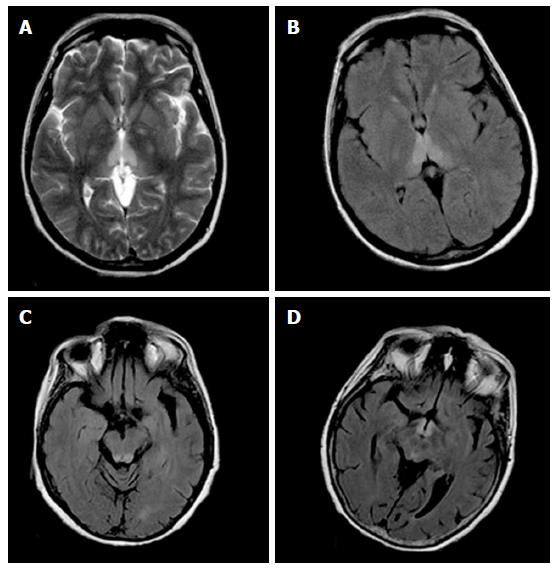
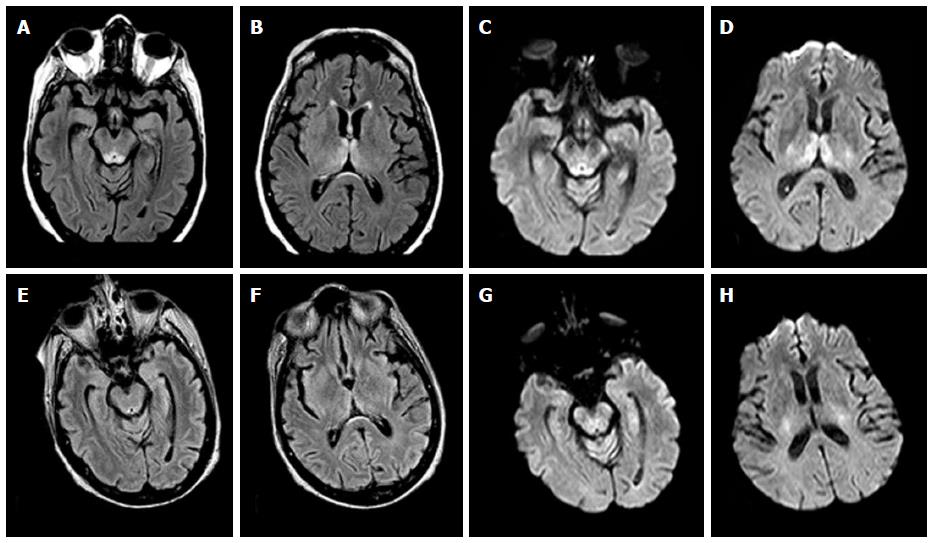
In all patients, high signal intensity on T2W and FLAIR sequences was demonstrated in the periaqueductal area and in the tectal plate in association with signal abnormalities around the mammillary bodies in 1 alcoholic WE (Figure 1) patient and in 2 nonalcoholics WE patients. Concomitant involvement of the medial thalami was seen in 2 alcoholics WE patients and in 3 nonalcoholics WE patients.
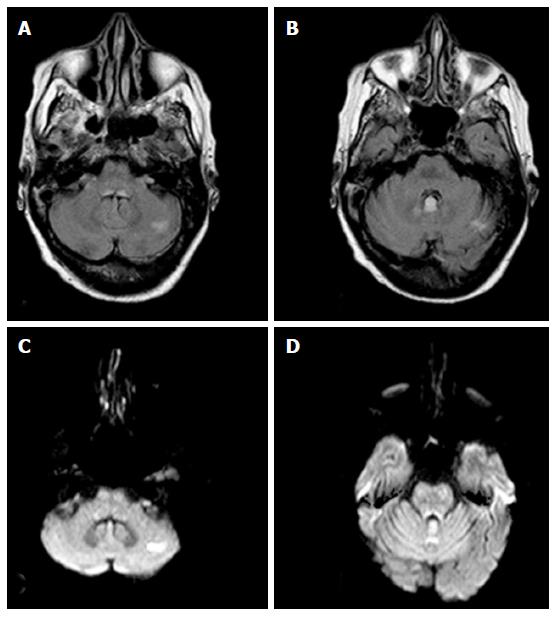
In the 4 nonalcoholics WE patients, in adjunction to the signal abnormalities in the periaqueductal area and in the tectal plate, high signal intensity on T2W and FLAIR images was seen in the cerebellar vermis and in the cerebellar hemispheres with restricted diffusion in these areas (Figure 2).
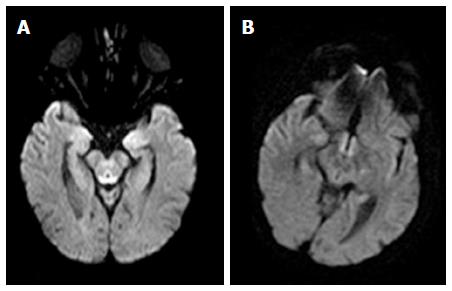
Restricted diffusion was demonstrated in the periaqueductal grey matter and around the mammillary bodies in 2 alcoholics WE patients in the acute phase of the disease (Figure 3).
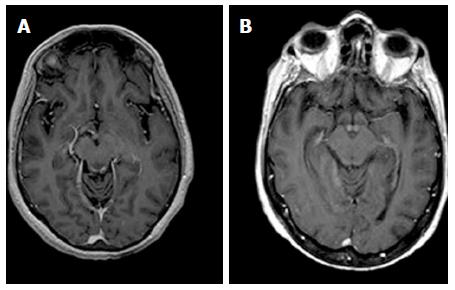
Three alcoholics and 1 nonalcoholic WE patients presented contrast-enhancement of the periaqueductal grey matter. One of the 3 alcoholic WE patients presented a concomitant contrast-enhancement of the mammillary bodies in the acute phase of the disease (Figure 4).
MR imaging follow-up, performed within 6 mo after intravenous administration of thiamine in 4 (2 alcoholic and 2 nonalcoholics WE) patients, demonstrated disappearance of the signal abnormalities seen on T2-weighted, FLAIR, and diffusion-weighted images (Figure 5) previously observed concomitant with clinical recovery.
Our results confirm that MR imaging can assist the diagnosis of WE, particularly in patients presenting with atypical clinical symptoms, or with no history of alcohol abuse, and in the acute phase of the disease by using DWI sequences and contrast-enhanced MR imaging.
The pathology of the alterations detected in Wernicke’s encephalopathy was not completely understood. Thiamine deficiency could lead an inability in sustainment of osmotic gradients of cell membranes and in the maintenance of membrane integrity with following intra and extra cellular edema.
In the periventricular regions, there is a high rate of thiamine-related glucose and oxidative metabolism and it can account as the cause of blood-brain barrier deficiency of these regions[11,12].
Cytotoxic edema is considered the most distinctive lesion of acute WE, and it is easily shown on DWI images as it detects changes in the diffusion of water molecules.
However, the presence of high signal intensities on DWI and decreased apparent diffusion coefficient values must be carefully evaluated as it could result from T2 shine-through effects[10]. Signal alterations on DWI most likely represent tissue at risk, similar to the cells in the ischemic penumbra, related to the cytotoxic edema and can be reversible[21]. In this study restricted diffusion was seen in 4 nonalcoholics and 2 alcoholics WE patients. At follow-up MR imaging obtained in 4 patients within 6 mo after thiamine therapy, resolution of these alterations was demonstrated confirming that restricted diffusion can be reversible (Figure 5).
According to previous reports[13,22], contrast enhancement was observed more frequently in the mammillary bodies in alcoholic patients (Figure 4), suggesting that mammillary bodies may be particularly susceptible to the toxic effects of alcohol.
Mammillary bodies enhancement, without mammillary body atrophy, is a finding that was associated with the acute phase of the disease, thus can allow an earlier diagnosis and treatment[22].
If not treated or inappropriately treated, Wernicke’s encephalopathy can lead to irreversible brain damage and, in 20% of cases, to death. Initial improvements in acute symptoms can be observed within the first week after thiamine administration, but usually, one to 3 mo are needed to resolve[15,22].
Our result confirms that MR signal abnormalities in WE can be reversible if a prompt therapy is established as demonstrated at follow-up MR imaging in 4 (2 alcoholic and 2 nonalcoholics WE) patients in our series (Figure 5).
The main limitation of this study is that it is a retrospective study without predefined criteria for diagnosis, which may have lead to a selection bias. Specifically, some patients with WE may not have been identified due to missed clinical diagnosis, particularly if they had atypical clinical manifestations and no history of alcohol abuse.
Despite these limitations, the diagnosis was confirmed in all patients as they clinically recovered after thiamine therapy and in 4 out of 7 patients resolution of the signal intensities abnormalities previously seen was demonstrated at follow-up MR imaging.
In conclusion, MR imaging is valuable in supporting the diagnosis of WE in patients with or without a history of alcohol abuse, especially in patients without the classic clinical presentation. Bilateral and symmetric increased T2-weighted and FLAIR MR signal intensity in the typical areas (periaqueductal area, medial thalami, mammillary bodies, tectal plate) are more often seen in alcoholic WE patients. Signal-intensity alterations in atypical sites (cerebellum, cranial nerve nuclei, and cerebral cortex) are found more frequently in nonalcoholic patients. Contrast-enhanced MR imaging and DWI MR imaging provides additional information to help diagnosis, especially in the acute phase of the disease, and should be included in the standard MR protocol in patients suspected to have WE. Knowledge of these MR imaging findings may be useful in the early diagnosis of WE and in reducing the complications associated with the disease.
Wernicke’s encephalopathy (WE) is a serious neurologic disease, with acute onset, caused by thiamine deficiency. Alcohol abuse is the most common cause. Typical clinical presentation is characterized by a triad of symptoms: Ocular manifestations, ataxia and global confusion. However, this triad occurs in only 13%-18% of patients. Thus, magnetic resonance imaging (MRI) results are of utmost importance for the diagnosis of WE.
This study demonstrated that MR imaging can assist the diagnosis of WE, especially in patients with atypical clinical symptoms, or with no history of alcohol abuse.
MR imaging is useful in supporting the diagnosis of WE. Knowledge of MR imaging findings can help radiologist in the early diagnosis, thus reducing the risk of complications.
Wernicke’s encephalopathy: Acute neurologic disease caused by thiamine (vitamin B1) deficiency.
Well written article. Nicely summarises brain imaging findings in WE including typical and atypical cases.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kumar A, Prakash N S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Gui QP, Zhao WQ, Wang LN. Wernicke’s encephalopathy in nonalcoholic patients: clinical and pathologic features of three cases and literature reviewed. Neuropathology. 2006;26:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke’s Encephalopathy and Korsakoff’s Psychosis. Alcohol Alcohol. 2006;41:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B, Giadás TC, Bottari W, Mandrioli J, Bertolini M. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. 2007;28:1328-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Fei GQ, Zhong C, Jin L, Wang J, Zhang Y, Zheng X, Zhang Y, Hong Z. Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am J Neuroradiol. 2008;29:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Nolli M, Barbieri A, Pinna C, Pasetto A, Nicosia F. Wernicke’s encephalopathy in a malnourished surgical patient: clinical features and magnetic resonance imaging. Acta Anaesthesiol Scand. 2005;49:1566-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Bonucchi J, Hassan I, Policeni B, Kaboli P. Thyrotoxicosis associated Wernicke’s encephalopathy. J Gen Intern Med. 2008;23:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Blanco-Múñez O, Suárez-Gauthier A, Martín-García H, Díaz-Konrad V, San Antonio-Román V, Cabello A. Unusual cortical compromise in a case of Wernicke’s encephalopathy. Rev Neurol. 2006;42:596-599. [PubMed] |
| 8. | Lindboe CF, Løberg EM. Wernicke’s encephalopathy in non-alcoholics. An autopsy study. J Neurol Sci. 1989;90:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Asato R, Okumura R, Konishi J. “Fogging effect” in MR of cerebral infarct. J Comput Assist Tomogr. 1991;15:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Bae SJ, Lee HK, Lee JH, Choi CG, Suh DC. Wernicke’s encephalopathy: atypical manifestation at MR imaging. AJNR Am J Neuroradiol. 2001;22:1480-1482. [PubMed] |
| 12. | Gallucci M, Bozzao A, Splendiani A, Masciocchi C, Passariello R. Wernicke encephalopathy: MR findings in five patients. AJNR Am J Neuroradiol. 1990;11:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Sun GH, Yang YS, Liu QS, Cheng LF, Huang XS. Pancreatic encephalopathy and Wernicke encephalopathy in association with acute pancreatitis: a clinical study. World J Gastroenterol. 2006;12:4224-4227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR Am J Roentgenol. 1998;171:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Zhong C, Jin L, Fei G. MR Imaging of nonalcoholic Wernicke encephalopathy: a follow-up study. AJNR Am J Neuroradiol. 2005;26:2301-2305. [PubMed] |
| 16. | Park SH, Kim M, Na DL, Jeon BS. Magnetic resonance reflects the pathological evolution of Wernicke encephalopathy. J Neuroimaging. 2001;11:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Liu YT, Fuh JL, Lirng JF, Li AF, Ho DM, Wang SJ. Correlation of magnetic resonance images with neuropathology in acute Wernicke’s encephalopathy. Clin Neurol Neurosurg. 2006;108:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Shogry ME, Curnes JT. Mamillary body enhancement on MR as the only sign of acute Wernicke encephalopathy. AJNR Am J Neuroradiol. 1994;15:172-174. [PubMed] |
| 19. | D’Aprile P, Gentile MA, Carella A. Enhanced MR in the acute phase of Wernicke encephalopathy. AJNR Am J Neuroradiol. 1994;15:591-593. [PubMed] |
| 20. | Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, Pipitone N. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. Biomed Res Int. 2014;2014:503596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Sparacia G, Banco A, Lagalla R. Reversible MRI abnormalities in an unusual paediatric presentation of Wernicke’s encephalopathy. Pediatr Radiol. 1999;29:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |