Published online Jun 28, 2016. doi: 10.4329/wjr.v8.i6.588
Peer-review started: August 4, 2015
First decision: September 28, 2015
Revised: March 2, 2016
Accepted: March 17, 2016
Article in press: March 18, 2016
Published online: June 28, 2016
Processing time: 324 Days and 14.9 Hours
AIM: To characterize the effects of iodinated contrast material (ICM) on magnetic resonance imaging (MRI) comparing different sequences and magnetic fields, with emphasis to similarities/differences with well-known signal characteristics of hemorrhage in the brain.
METHODS: Aliquots of iopamidol and iodixanol mixed with normal saline were scanned at 1.5T and 3T. Signal intensity (SI) was measured using similar spin-echo (SE)-T1, SE-T2, gradient-echo (GRE) and fluid-attenuation-inversion-recovery (FLAIR) sequences at both magnets. Contrast to noise ratio (CNR) (SI contrast-SI saline/SD noise) for each aliquot were calculated and Kruskall-wallis test and graphic analysis was used to compare different pulse sequences and ICMs.
RESULTS: Both ICM showed increased SI on SE-T1 and decreased SI on SE-T2, GRE and FLAIR at both 1.5T and 3T, as the concentration was increased. By CNR measurements, SE-T2 had the greatest conspicuity at 3T with undiluted iopamidol (92.6 ± 0.3, P < 0.00) followed by iodixanol (77.5 ± 0.9, P < 0.00) as compared with other sequences (CNR range: 15-40). While SE-T2 had greatest conspicuity at 1.5T with iopamidol (49.3 ± 1, P < 0.01), SE-T1 showed similar or slightly better conspicuity (20.8 ± 4) than SE-T2 with iodixanol (23 ± 1.7). In all cases, hypo-intensity on GRE was less conspicuous than on SE-T2.
CONCLUSION: Iodixanol and iopamidol shorten T1 and T2 relaxation times at both 1.5T and 3T. Hypo-intensity due to shortened T2 relaxation time is significantly more conspicuous than signal changes on T1-WI, FLAIR or GRE. Variations in signal conspicuity according to pulse sequence and to type of ICM are exaggerated at 3T. We postulate T2 hypointensity with less GRE conspicuity differentiates ICM from hemorrhage; given the well-known GRE hypointensity of hemorrhage. Described signal changes may be relevant in the setting of recent intra-arterial or intravenous ICM administration in translational research and/or human stroke therapy.
Core tip: After recent groundbreaking stroke clinical trials have shown positive outcomes with endovascular therapy, the use of imaging, particularly magnetic resonance imaging (MRI) is expected to increase in this setting. Iodinated contrast material (ICM) is inherent to this scenario and can be deposited in the brain after intra-arterial or intra-venous injection. Differentiation of ICM from hemorrhagic changes is of upmost clinical importance. This paper demonstrates the signal characteristics of in vitro ICM with routine MR sequences [including not previously reported changes on gradient-echo (GRE)]. Changes at high magnetic field (3T) are to the best of our knowledge described for the first time, with T2 hypo intensity as the signal change with greatest conspicuity as compared with other routine brain sequences. Furthermore, no significant conspicuity/hypo intensity on GRE is demonstrate and postulated as a way to differentiate contrast deposition (T2 hypo intensity or T1 hyper intensity) from hemorrhagic changes.
- Citation: Morales H, Lemen L, Samaratunga R, Nguyen P, Tomsick T. Effects of iodinated contrast on various magnetic resonance imaging sequences and field strength: Implications for characterization of hemorrhagic transformation in acute stroke therapy. World J Radiol 2016; 8(6): 588-593
- URL: https://www.wjgnet.com/1949-8470/full/v8/i6/588.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i6.588
To the best of our knowledge, only two reports of the effects of iodinated contrast material (ICM) on magnetic resonance imaging (MRI) have been published[1,2]; both describing shortening of T1 and T2 relaxation times on routine spin-echo sequences. Reported relaxation times/signal changes of ICM can potentially overlap with known relaxation times/signal changes of blood[1]. Relaxation times for iodinated contrast, blood and physiologic saline are shown in Table 1[2-5]. Thus, unusual hyper-intense T1 or hypo-intense T2 areas could be present with both ICM extravasation-deposition and/or hemorrhagic changes in the brain. This overlap is relevant in the setting of endovascular-therapy for stroke, where use of iodinated contrast is inherent and where new evidence regarding efficacy of endovascular therapy is expected to increase the use of not only computed tomography but also MRI[6,7].
We compared MRI signal intensity (SI) effects of not only multiple spin-echo but also gradient-echo sequences of isosmolal and low-osmolal ICM in vitro. We also compared the effects of various sequences used on routine brain imaging on 1.5T and 3T clinical magnets.
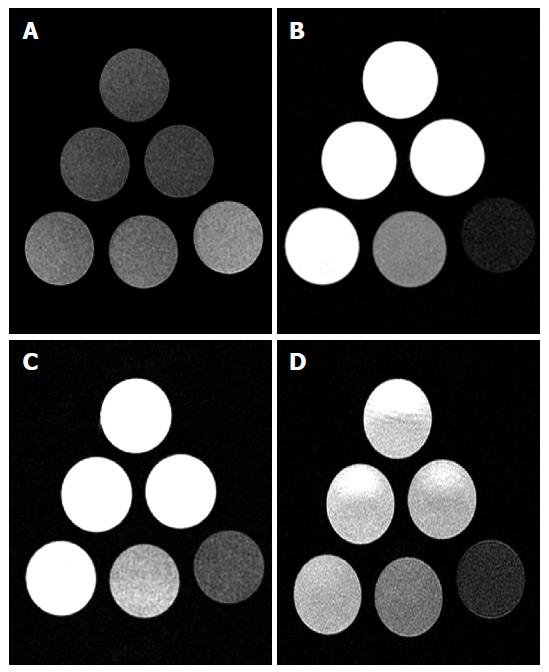
Aliquots of iopamidol (300 mgI/mL) and iodixanol (320 mgI/mL) mixed with normal saline were scanned at 1.5 T (Espree Singo MR-B50, Siemens) and 3T (Signa Excite, GE Healthcare). Six vials were prepared with dilutions of contrast and saline as follow: 100% (full strength contrast), 50%, 25%, 12.5%, 6.25% and 0% (normal saline) (Figure 1). SI was measured using similar sequences at both magnets, as follow: Spin-echo (SE) T2 (TR/TE: 2500/92, Matrix: 256 × 256, NEX: 1); SE T1 (TR/TE: 867/20, Matrix: 256 × 256, NEX: 1); T2 fluid-attenuation-inversion-recovery (FLAIR) (TR/TE/TI: 12827/120/2250, Matrix: 352 × 224, NEX: 1) and gradient-echo (GRE) (TR/TE/Flip Angle: 550/20/20, Matrix: 224 × 224, NEX: 2). The sequences were tailored to match a concomitant experiment of ICM infusion in a rat model of temporary ischemia performed on the same 3T magnet[8].
SI of each aliquot was measured and normalized to saline (SI contrast/SI saline). Contrast to noise ratio (CNR) (SI contrast-SI saline/SD noise) were also calculated for each aliquot. Multiple SI values (5-10) were recorded per aliquot of contrast and subsequently mean CNR were compared between groups using Kruskall-Wallis test. Error-bar graphic analysis was also performed to compare CNR between groups and determine the sequence with greatest lesion conspicuity and highest potential detection rate.
Statistical analysis was performed with SPSS for Windows, version 16.0 (SPSS Inc.). A P value less than 0.05 was considered statistically significant.
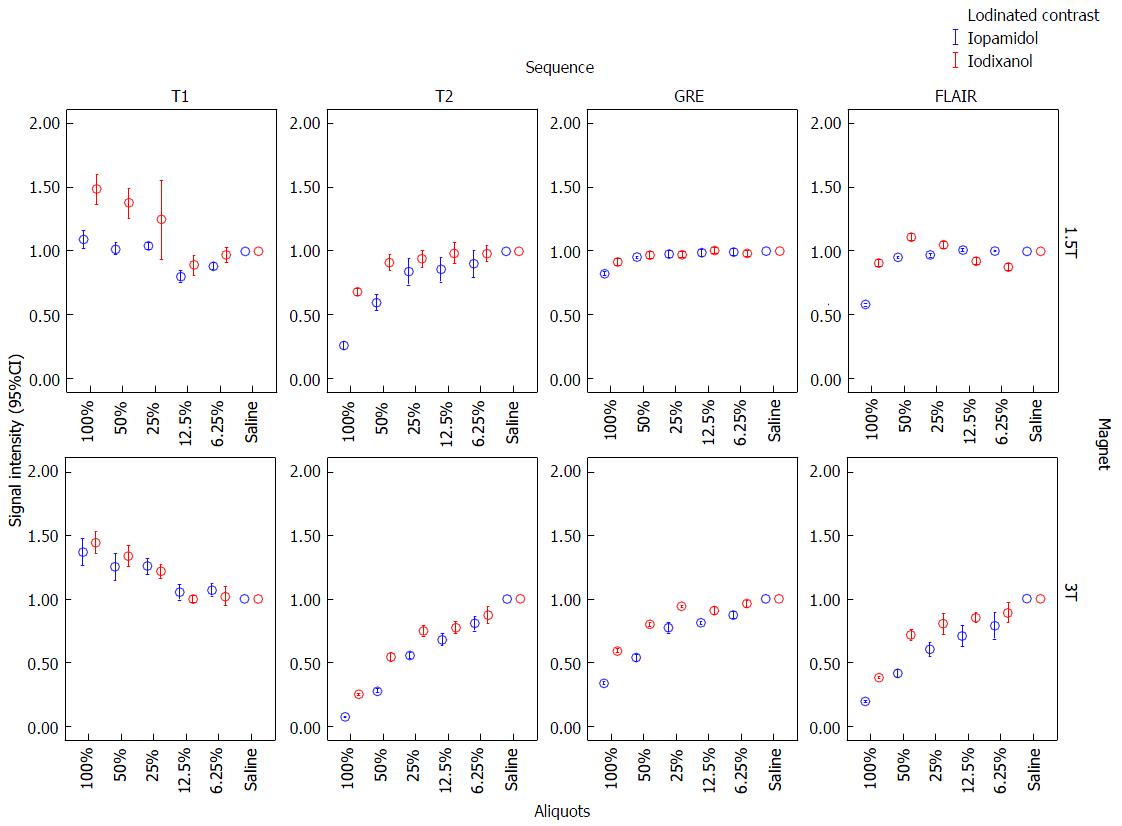
Both iopamidol and iodixanol demonstrated increasing SI on T1 and decreasing SI on SE-T2, GRE and FLAIR at 1.5T and 3T, as the concentration of ICM mixed with saline was increased (Figures 1 and 2).
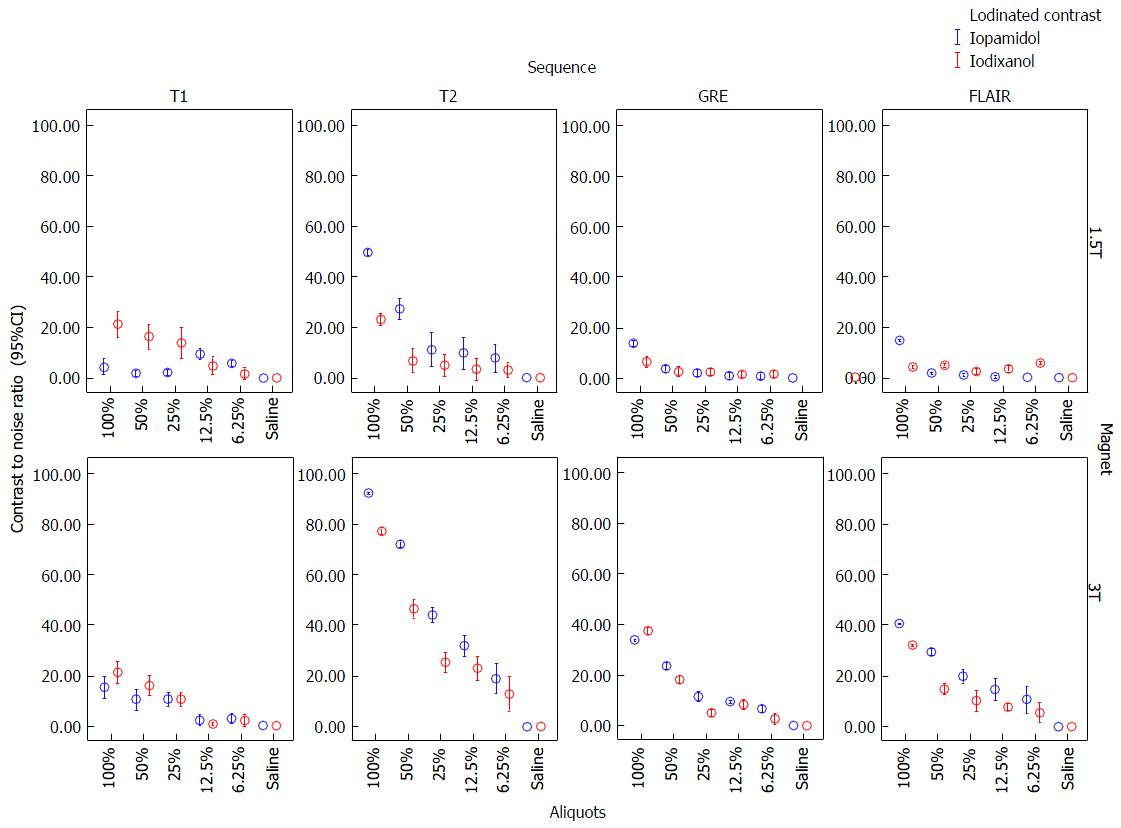
CNR of 100% and 50% aliquots of iopamidol and iodixanol at 1.5T and 3T are shown in Table 2. Error-bar graphic analysis of CNR (Figure 3) comparing various sequences and magnetic fields is also shown. By CNR measurements, SE-T2 had the greatest conspicuity at 3T with undiluted iopamidol (92.6 ± 0.3, P < 0.00) followed by iodixanol (77.5 ± 0.9, P < 0.00) as compared with other sequences (CNR range: 15-40). While SE-T2 had greatest conspicuity at 1.5T with iopamidol (49.3 ± 1, P < 0.01); SE-T1 showed similar or slightly better conspicuity (20.8 ± 4) than SE-T2 with iodixanol (23 ± 1.7). In all cases, hypo-intensity on GRE was less conspicuous than on SE-T2.
| Iodinated contrast/sequences | Iopamidol magnetic field and concentration | Iodixanol magnetic field and concentration | ||||||
| 1.5T | 3T | 1.5T | 3T | |||||
| 100% | 50% | 100% | 50% | 100% | 50% | 100% | 50% | |
| T1-SE | 4.2 ± 2 | 1.6 ± 0.7 | 15.2 ± 3.5 | 10.4 ± 3.5 | 20.8 ± 4 | 16 ± 4 | 21.2 ± 3.4 | 16 ± 3 |
| T2-SE | 49.3 ± 1 | 27 ± 3 | 92.6 ± 0.3 | 72 ± 1 | 23 ± 1.7 | 6.5 ± 3.8 | 77.5 ± 0.9 | 47 ± 3 |
| Gradient-echo | 13.6 ± 0.8 | 3.7 ± 0.6 | 34 ± 0.4 | 23.7 ± 1.4 | 6.3 ± 1.5 | 2.4 ± 1.6 | 37.7 ± 1 | 18.3 ± 0.9 |
| Fluid-attenuation-inversion-recovery | 14.5 ± 0.3 | 1.8 ± 0.2 | 40.8 ± 0.3 | 29.6 ± 1 | 4.2 ± 0.2 | 4.9 ± 0.5 | 32.3 ± 0.4 | 14.8 ± 1.5 |
| Statistics: Kruskall-Wallis test | P < 0.001 | P < 0.001 | P < 0.00 | P < 0.00 | P < 0.001 | P < 0.004 | P < 0.00 | P < 0.002 |
MRI effects after IV or intrathecal administration of ICMs have been reported in cases of in vivo imaging of the central nervous system (CNS)[1,2]. The effects included increased T1 SI within the thecal sac or decreased T2 SI in CNS tumors such as meningioma. The same reports included in vitro analysis of various types of ICMs, where the predominant effect is shortening of the T1 and T2 relaxation times as compared with saline or cerebrospinal fluid (CSF). However, to our knowledge, characterization of effects on GRE or at high magnetic field strength (3.0 T) has not been performed.
Jinkins et al[1] and Hergan et al[2] evaluated the SI effect of multiple types of ICMs, including iopamidol. All but iohexol demonstrated T1 and T2 shortening. We report increased SI on T1-WI and decreased SI on T2-WI of iopamidol and iodixanol as compared with saline. In addition, we found decreased SI on GRE and FLAIR. The magnitude of SI alteration increased with higher ICM concentration. The structure of the side chains of the ICM has been postulated as responsible for the signal alterations on MRI. Iodixanol has increased number of hydroxyl as compared with iopamidol, which probably explain its slightly more conspicuous increased T1 SI (T1-shortening) on 1.5T. However, T1 and T2 relaxation times are basically independent phenomena.
In an attempt to confirm optimal pulse sequence for MRI ICM identification, we observed CNRs were the highest on T2-WI, and lowest on T1-WI, FLAIR and GRE, particularly at 3T (Figure 2). Iopamidol had higher CNRs than iodixanol on T2-WI, translating into greater T2 hypointensity on test tube MR analysis. We failed to confirm higher CNRs on T1-WI, likely due to the application of higher magnetic fields, where there is a reduction of T1-shortening as magnetic field strength increases. We postulate that T1-WI would probably not be useful in identification of ICM deposition.
In cases where hemorrhage after recent administration of ICM is questioned, our in vitro analysis indicates MRI might better characterize the SI alteration of contrast enhancement/extravasation as areas of hypointense T2 signal with corresponding less-conspicuous GRE hypointensity. The signal characteristic of hemorrhage is well known. Although areas of hypointense T2 and hyperintense T1 signal might be seen at different stages of blood clotting, there is known increased conspicuity on GRE (blooming) as compared with T2-WI[9]. Specifically, during the acute stage of parenchymal hematomas in the brain there is peripheral hypointensity on GRE due to early de-oxihemoglobina formation. Later, in the subacute stage there is blooming due to meta-hemoglobin; although this effect is expected to be less prominent than the typical T1-shortening[10]. During the chronic stages, hemosiderin predominates and is the cause of hypointentisty on both T2 and GRE images[11].
Iodine itself has no paramagnetic or T2-shortening effect. In theory, ICM should not demonstrate paramagnetic effects[2], however other forms of susceptibility artifact on GRE might be possible. Hence, we have shown the absence of susceptibility artifact of ICM with its potential implication in clinical practice.
On FLAIR, hemorrhagic changes can produce hypo or hyperintensity (usually similar than T2-WI), according to the stages of blood products in the brain. Areas of subarachnoid hemorrhage typically show hyperintensity on FLAIR; an effect caused by both incomplete CSF suppression and T1-shortening (FLAIR has an intrinsic T1 contrast in addition to the well known T2 contrast effect)[12]. CNR values of ICM on FLAIR images were not the highest in our experiment. Nevertheless, we found hypointensity on higher concentrations of ICM, which might contribute to the differentiation of hemorrhagic changes in the subarachnoid spaces.
The MR effects of ICM appear to persist as long as 2 h or 8 h after IV or intrathecal administration respectively[1,2]. Thus, signal alteration would be expected to persist during the first hours after IA or IV administration of ICM in the setting of stroke.
Our main goal was to compare the visual effects on various sequences to include GRE, so we did not characterize T1 and T2 relaxation times per se as this had been performed previously[2]. Similarly, we did not compare side-by-side signal changes of blood and ICM (T1 and T2 relaxation times for blood are well known in the literature - Table 1). One potential limitation is that our T1-WI sequence was similar in both 1.5T and 3T magnets, with an optimized TR for high magnetic fields[8]. Even though this could lead to changes in SI not usually seen at 1.5T, we believe the characterization on T2-WI was significantly more conspicuous, particularly with iopamidol. The iodine concentration slightly differs in between iopamidol (300 mgI/mL) and iodixanol (320 mgI/mL) in our phantom study. Although this might have an effect on T1-WI, the difference between signal intensities of different iodine concentrations appears hardily detectable on T2-WI as demonstrate by Hergan et al[2]. Overall, under the parameters used in this experiment, it seems the small differences in signal intensities are most likely secondary to the type of ICM used. Additional in vitro or in vivo observations with multiple type of ICM may be helpful, particularly to evaluate changes on T1-WI.
In the setting of acute stroke evaluation and endovascular therapy in humans, ICM is known to be deposited in the brain, as either enhancement or extravasation, and may be difficult to distinguish from hemorrhage[13]. Defining the range of MR signal changes of ICM following infusion in computed tomography and/or digital angiography promises to have value in distinction. With high-field 3.0 T scanners more widely available, the potential for providing a comprehensive diagnostic assessment for stroke has become reality. Furthermore, understanding the MR signal effects of ICM may open a translational research window on ICM’s potential clinical effects, beneficial or harmful, in stroke management[8].
In conclusion, iopamidol and iodixanol are most conspicuous at high concentration on T2-WI, with limited conspicuity on GRE. Detection of deposition of ICM in the setting of acute stroke might be possible, particularly at 3T as areas of hypointensity on T2-WI. Less conspicuous hypointensity of ICM on GRE images may allow distinction from hemorrhage. Understanding the described imaging characteristics of ICM on MRI promises to be useful not only in translational stroke research, but also in acute stroke diagnosis and intervention in humans.
After recent groundbreaking stroke clinical trials have shown positive outcomes with endovascular therapy, the use of imaging, particularly magnetic resonance imaging (MRI) is expected to increase in this setting. Iodinated contrast material (ICM) is known to be deposited in the brain, as either enhancement or extravasation, and may be difficult to distinguish from hemorrhage. Defining the range of magnetic resonance signal changes of ICM following infusion in computed tomography and/or digital angiography promises to have value in distinction.
Only two researchers evaluated the signal intensity (SI) effect of multiple types of ICMs, including iopamidol. All but iohexol demonstrated T1 and T2 shortening.
The authors compared MRI SI effects of not only multiple spin-echo but also gradient-echo sequences of isosmolal and low-osmolal ICM in vitro. They also compared the effects of various sequences used on routine brain imaging on 1.5T and 3T clinical magnets.
Understanding the described imaging characteristics of ICM on MRI promises to be useful not only in translational stroke research, but also in acute stroke diagnosis and intervention in humans.
This is a well-designed and written study about the ICM effect on various MRI sequences.
P- Reviewer: Battal B, Kilickesmez O S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Jinkins JR, Robinson JW, Sisk L, Fullerton GD, Williams RF. Proton relaxation enhancement associated with iodinated contrast agents in MR imaging of the CNS. AJNR Am J Neuroradiol. 1992;13:19-27. [PubMed] |
| 2. | Hergan K, Doringer W, Längle M, Oser W. Effects of iodinated contrast agents in MR imaging. Eur J Radiol. 1995;21:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Greenman RL, Shirosky JE, Mulkern RV, Rofsky NM. Double inversion black-blood fast spin-echo imaging of the human heart: a comparison between 1.5T and 3.0T. J Magn Reson Imaging. 2003;17:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Stanisz GJ, Li JG, Wright GA, Henkelman RM. Water dynamics in human blood via combined measurements of T2 relaxation and diffusion in the presence of gadolinium. Magn Reson Med. 1998;39:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Janne d’Othée B, Rachmuth G, Munasinghe J, Lang EV. The effect of hyperoxygenation on T1 relaxation time in vitro. Acad Radiol. 2003;10:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Menon BK, Campbell BC, Levi C, Goyal M. Role of imaging in current acute ischemic stroke workflow for endovascular therapy. Stroke. 2015;46:1453-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Fisher M, Wakhloo A. Dawning of a new era for acute stroke therapy. Stroke. 2015;46:1438-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Morales H, Lu A, Kurosawa Y, Clark JF, Leach J, Weiss K, Tomsick T. Decreased infarct volume and intracranial hemorrhage associated with intra-arterial nonionic iso-osmolar contrast material in an MCA occlusion/reperfusion model. AJNR Am J Neuroradiol. 2014;35:1885-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Unger EC, Cohen MS, Brown TR. Gradient-echo imaging of hemorrhage at 1.5 Tesla. Magn Reson Imaging. 1989;7:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Liu C, Li W, Tong KA, Yeom KW, Kuzminski S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging. 2015;42:23-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 402] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 11. | Atlas SW, KR T. Chapter 13 - Intracranial Hemorrhage, in Magnetic Resonance Imaging of the Brain and Spine, A. SW, Editor 2009, Lippincott - Williams & Wilkins. . |
| 12. | Verma RK, Kottke R, Andereggen L, Weisstanner C, Zubler C, Gralla J, Kiefer C, Slotboom J, Wiest R, Schroth G. Detecting subarachnoid hemorrhage: comparison of combined FLAIR/SWI versus CT. Eur J Radiol. 2013;82:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |