Published online May 28, 2016. doi: 10.4329/wjr.v8.i5.460
Peer-review started: July 19, 2015
First decision: September 30, 2015
Revised: January 25, 2016
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: May 28, 2016
Processing time: 304 Days and 8.1 Hours
Emergency physicians are required to care for unstable patients with life-threatening conditions, and thus must make decisions that are both quick and precise about unclear clinical situations. There is increasing consensus in favor of using ultrasound as a real-time bedside clinical tool for clinicians in emergency settings alongside the irreplaceable use of historical and physical examinations. B-mode sonography is an old technology that was first proposed for medical applications more than 50 years ago. Its application in the diagnosis of thoracic diseases has always been considered limited, due to the presence of air in the lung and the presence of the bones of the thoracic cage, which prevent the progression of the ultrasound beam. However, the close relationship between air and water in the lungs causes a variety of artifacts on ultrasounds. At the bedside, thoracic ultrasound is based primarily on the analysis of these artifacts, with the aim of improving accuracy and safety in the diagnosis and therapy of the various varieties of pulmonary pathologic diseases which are predominantly “water-rich” or “air-rich”. The indications, contraindications, advantages, disadvantages, and techniques of thoracic ultrasound and its related procedures are analyzed in the present review.
Core tip: The close relationship between air and water in the lungs causes a variety of artifacts on ultrasounds. At the bedside, thoracic ultrasound is based primarily on the analysis of these artifacts, with the aim of improving accuracy and safety in the diagnosis and therapy of the various varieties of pulmonary pathologic diseases which are predominantly “water-rich” or “air-rich”. The indications, contraindications, advantages, disadvantages, and techniques of thoracic ultrasound and its related procedures are analyzed in the present review.
- Citation: Liccardo B, Martone F, Trambaiolo P, Severino S, Cibinel GA, D’Andrea A. Incremental value of thoracic ultrasound in intensive care units: Indications, uses, and applications. World J Radiol 2016; 8(5): 460-471
- URL: https://www.wjgnet.com/1949-8470/full/v8/i5/460.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i5.460
Emergency clinicians are required to take care of critically patients with life-threatening settings, thus must make decisions that are both quick and precise about unclear clinical situations[1-3]. As a result, there is increasing consensus in favor of using ultrasound as a real-time bedside clinical tool for clinicians in emergency settings alongside the irreplaceable use of historical and physical examinations.
Despite bedside portable chest radiography (CR) being relatively inexpensive, available in most hospital, and able to provide useful information, it has been shown to be inaccurate in many situations and has a few limitations[4]. The technical limitations of the tool (e.g., movement during X-ray exposure, breath holding during X-ray exposure, and cassette placed posteriorly in the thorax) may lead to an incorrect assessment of the most frequent pulmonary diseases (e.g., alveolar interstitial syndrome, pulmonary consolidation, and pleural effusion)[5]. In addition, the time required to achieve bedside ordinary CR and compile a report may retard the diagnosis and extend the patient’s stay in the emergency department, thereby contributing to overcrowding of the department. CR is never helpful for the diagnosis of pneumothorax in patients staying in the intensive care unit (ICU) during non-invasive ventilation.
Chest computer tomography (CCT) could be viewed as the best tool for imaging diagnosis in most thoracic settings, but it is costly, not usable in the ICU, and exposes at-risk patients to a potentially unsafe journey to the radiology unit[6]. Moreover, CCT exposes patients to high dose of radiation, which obviously restricts the number of times the procedure can be repeated and makes it unsuitable for pregnant or pediatric patients[7,8].
Ultrasound is an old technology, with its use in the diagnosis of chest illness being considered restricted by the presence of air contained inside the lungs, and by the presence of bone structure, which prevent the progression of the ultrasound[9-12].
As matter of fact, in the textbook “Harrison, Principles of Internal Medicine” concerning thoracic ultrasound (TUS), it is stated[13]: “Because ultrasound energy is rapidly dissipated in air, ultrasound imaging is not useful for evaluation of the pulmonary parenchyma. However, it is helpful in the detection and localization of pleural abnormalities and is often used as a guide to placement of a needle for sampling of pleural liquid (i.e., for thoracentesis)”.
However, thanks to the pioneering work of French intensivist Daniel Lichtenstein and others after him, it became understood that ultrasound applied to the chest and lungs generated sonographic artifacts, and that those artifacts patterns correlate with clinical and radiologic diagnosis in ICU.
It was Lichtenstein who proposed the basis and utility of TUS, which was formulated in a few basic principles. Firstly, the intimate relationship between air and water in the lungs causes a variety of artifacts visible via ultrasound. Since air, and consequently the lung, cannot be visualized by sonography, TUS is based primarily on the evaluations of these artifacts. Secondly, due to the fact that air and water have opposing gravitational dynamics, a variety of pathologic conditions (e.g., pleural effusions and consolidations) are predominantly “water-rich”. These pathologies are generally found in the posterior aspects of a supine patient. On the other hand, there are several “air-rich” conditions (e.g., pneumothorax), which are predominantly found in the anterior aspects of a supine patient (Table 1)[14]. The current review will focus on the potential clinical applications of TUS.
| Clinical setting | Artifacts |
| Normal lung | Some air |
| Pneumothorax | Full of air |
| Interstitial syndrome | Air and minimal fluid |
| Pleural effusion | Full of fluid |
| Lung consolidation | Fluid and air (more fluid, tissue-like) |
In order to permit bedside evaluation of patients, ultrasound machines should be lightweight, battery-powered, able to be carried by hand, compact, and easy to transport. Ultrasound instruments should also provide a hard disk and USB, for saving and unloading images and clips, as well as a paper recorder[15-17].
Since ultrasound machines are used for many patients, it should be kept in mind that probes can be a carrier for resistant germs that could be present in the ICU. Ultrasound instruments and probes should therefore be frequently decontaminated[18].
Ultrasound equipment suitable for TUS imaging must be provided with 3.5-MHz, 5-MHz, 7.5-MHz, or 10-MHz convex, linear, and sector transducers; each probe has its own inherent advantages and disadvantages.
Convex and linear transducers have a larger visual range than sector scanners, and are thus preferred for initial evaluations and screenings. The curved array convex probe (3.5-MHz) has the advantage of allowing for views of deeper lesions and rapid assessment of the lateral thoracic cavity for signs of pleural fluid in a supine patient. However, due to its large footprint, only a small portion of the intercostal space is accessible. The low frequency does not therefore allow for a detailed assessment of the more important zones in thoracic ultrasound, such as the pleural line. The high-frequency linear array probe (5-MHz or 7.5-MHz) provides the best resolution images of close structures, thereby allowing for detailed examination of the pleura and providing rapid assessment of superficial lesions in the chest wall and pleura, such as pneumothorax. However, its large footprint hinders access to larger areas of lung tissue because of interference from the ribs. Furthermore, the high-frequency provided sacrifices depth-of-penetration, thereby preventing the assessment of deeper structures such as atelectasis, consolidation, and large pleural effusions. For injuries with a short ultrasound view or very limited intercostal space, a sector probe is generally chosen, while a cardiac probe allows for simultaneous examination of the heart and lungs.
Each TUS study should begin with obtaining the patient’s history and a clinical evaluation.
TUS is commonly performed via two different approaches: A systematic approach, or a focused approach that starts at the area of chest distress[19].
During TUS systematic examination, patients are evaluated in a seated position that allows for anterior lateral, posterior, and supraclavicular approaches in order to scan both the posterior and anterior areas of the thorax. So as to facilitate this, the patient should keep both arms lifted over their head[20].
However, a methodical procedure is not always possible in an ICU, as it is a procedure that requires an amount of time that is simply impractical for patients with very severe clinical conditions. Current reviews have pointed out that there are simpler procedures that, independent of patient position and respiration, are more feasible when it comes to searching for sonographic signs in at-risk patients.
The thoracic cage can be divided into three different zones[10,11,21,22], namely the anterior, lateral, and posterior walls. Most of the diagnostic procedures of TUS concern the anterior wall, as well as lateral parts of the chest cage. The anterior wall is delimitated from the sternum to the anterior axillary row, the lateral wall is delimitated from the anterior to the posterior axillary row, and the posterior wall is delimitated from the paravertebral row, the scapular row, and the posterior axillary row[11,22-24]. Thoracic parts are obviously the same in both areas, but the presence of the heart on the left side reduces helpful scansion in that area.
The main limitation of ultrasound is when TUS needs to be applied to the posterior zone of the chest of a bedridden, immobilized, intubated, or unconscious patient. In these conditions, small probes may be useful, as they can be placed between the patient and bed. In order to obtain better images in infants and small children, they can be evaluated by being placed in a supine or oblique position. The transducer can slide behind the patient’s back and aimed roughly perpendicular to the chest wall[14,25].
Lower portions of the chest may be evaluated from an abdominal position. The right lung can be seen passing across the liver, while the left lung can be seen passing across the spleen; in both conditions, the diaphragm is crossed and visualized[19].
By moving the probe in an up or down motion, the pleura is represent under the ribs, moving during inspiration and expiration, and synchronized with breathing motions.
The probe is then shifted in a transverse or longitudinal way in order to better see the lung using intercostal spaces like ultrasound windows, thereby bypassing the ribs. Both longitudinal and convex probes allow for the visualization of a couple of ribs and the pleural row between them. This procedure is the first and most significant point of chest evaluation, as it permits easier and more accurate evaluation of the pleural row. When the pleural line has been located after placing and turning the probe in the intercostal space, a transverse scan can be obtained that provides the best visualization of the pleura and its artifacts[11,12].
A complete TUS evaluation includes the scansion of both hemidiaphragms (including normal zones) so as to compare normal and pathological areas.
Normal lung parenchyma is not visualized because it is composed primarily of air, which scatters and impedes the transmission of sound waves. The dramatic difference in the acoustic characteristics of smooth tissue and the lung makes the chest surface a particularly strong reflector of ultrasound waves, and is responsible for creating a number of reverberation artifacts that lend valuable information about the lung’s current pathophysiology.
Ultrasound pictures of the thoracic cage typically display smooth-tissue echogenicity with multiple layers of fascia, subcutaneous tissue, and muscle of varying thickness dependent on patient constitution.
The transducer can be positioned both perpendicular to the ribs and transverse over the intercostal spaces.
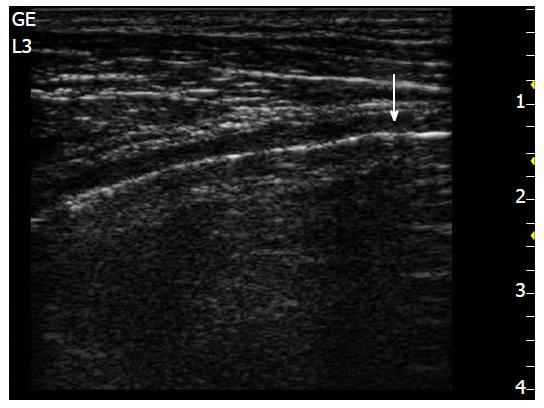
The longitudinal approach, perpendicular to the ribs, provides a view of the lower and the upper ribs and the pleural line (Figure 1), while the oblique approach, with the probe placed in the intercostal space, provides a larger view of the pleural line that is not hidden by rib shadows[25].
In the longitudinal approach, three fundamental structures can be highlighted after leaning the transducer on each intercostal space: The pleural line, the thoracic cage, and the pulmonary artifact.
Depth should be adjusted by patient size, patient habitus, and visible structures. In fact, in order to visualize the pleural line in obese or muscled patients, or in patients with very thick ribcages, higher depths are needed. On the other hand, in children and very thin patients, a shallower depth is required. Depth should also be correct by evaluation objective: If pneumothorax is the target, the depth should be shorter to facilitate better visualization of the pleural line and assess sliding; while in the case of pleural effusion, the depth should be higher. The focus should typically be placed at the pleural row level to allow for better visualization of artifacts.
Different portions of the thoracic cage may be highlight through TUS, but the most significant visualized structures are the margins of the ribs. They present as a line with uninterrupted echogenicity, much like the physiological pleural row, but without any movement; moreover, the margin of normal ribs produces an acoustic shadow that partially masks the structures above, with the exception of the cartilage zone of the ribs that permits the transmission of the ultrasound beam, showing the underside of the pleura and its sliding. TUS may be also used in the diagnosis of chest wall pathologies (such as fractures after cardiopulmonary resuscitation or trauma), but this is not in the scope of the current review.
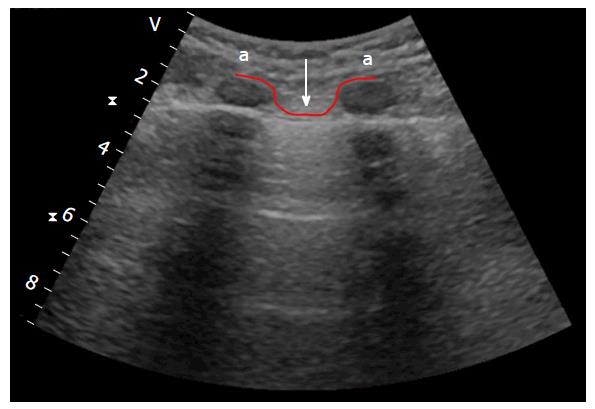
The second structure to highlight is the pleural row, which presents as a thin echogenic row under the ribs. This row represents the visceral and parietal layers that are seen together. Typically, the pleural lines are smooth and less than 2 mm thick, with the pleural space (less than 0.3/0.4 mm) between two rows. The near ribs (upper and lower), with the pleural line below them, delineate a typical ultrasound sign referred to as the “bat sign” (which is clearer when using a convex probe), due to its curvilinear shape (both near ribs are the “wings”, while the pleural line underneath them is the “body”). Recognition of this sign is important, since it clearly shows the pleural row (Figure 2).
The pleural row moves through respiration, as well as being synchronized with it[3]. These movements are named lung sliding or gliding[25], and its recognition is a significant part of chest evaluation, since is a sign of physiologic movement of the pleura.
Heartbeats cause another movement, which is referred to as “lung pulse”, and is the movement of the pleural row synchronous with the cardiac beam. Lung pulse is a vertical movement that is easier to see on the left hemithorax, and is produced by the transmission of heart rhythm.
The presence of intrapleural air (like in pneumothorax) prevents the transmission of any kind of movement (both vertical and horizontal) to the parietal pleura. As a matter of fact, visualizing the lung pulse makes it possible to exclude pneumothorax.
M-mode can be used to document lung sliding by recording a mark referred to as “seashore sign” (Figure 3)[26]. In this kind of picture, below the pleural line of the physiological lung, it is possible to see the pulmonary artifact made of a steady background pattern, finely sparkling, and with several linear artifacts. Sparkling artifacts are created when the ultrasound beam is not uniformly mirrored back to the transducer by the microspheric surfaces of air trapped in the alveoli[9,10].
Linear artifacts can be distinguished into two different types: Vertical and horizontal.
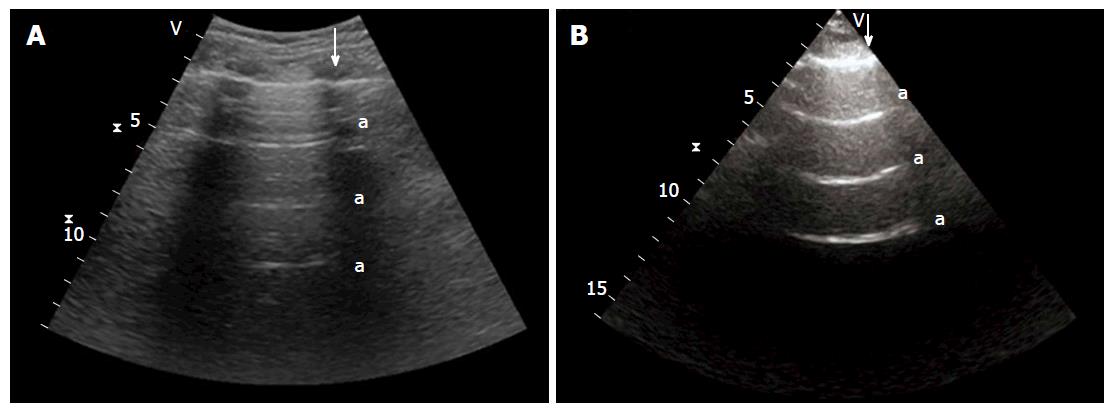
Horizontal artifacts are a hyperechogenic row that presents at standard intervals from the pleural row, and are referred to as A-lines (Figure 4). When matched with physiological lung sliding, these reverberation artifacts are a sign of the physiological presence of air in the alveoli[21,25].
A-lines occur when sound waves pass through superficial soft tissue and encounter air after crossing the pleural line, or in tissue that is almost completely composed of air, as is the case in a normal lung. These waves are strongly reflected by this tissue/air interface, bouncing back and forth between the transducer and lung surface; each volley of sound waves returns to the transducer after a longer period of time and is thus represented as a bright horizontal line that becomes deeper on the display screen. As this is a classic reverberation artifact, the distance from the skin to the pleural row equals the distance from the pleural row to the first A-line, the first A-line to the second A-line, and so forth.
Vertical artifacts are echogenic beams that originate from the pleural row, arriving at the opposite side of the screen in the absence of interruptions and with movements that are synchronous with lung sliding and respiratory frequency. They are well-defined, laser-like, hyperechoic, and erase A-lines[26].
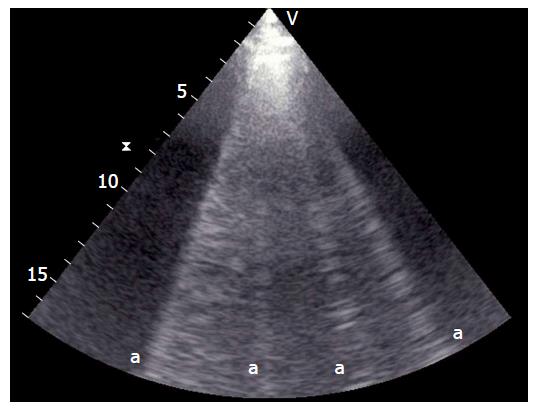
In the normal lung, there are generally no more than 2-3 vertical artifacts per chest part per hemithorax, and they are typically at the base of the lung[11,23,27]; these are named B-lines or comet tails (Figure 5)[12,28,29].
B-line images are connected via a small water-rich structure under the resolution of the sonographic beam enclosed by air, with artifacts being generated when said structure is struck by an ultrasound beam.
Under a clinical setting characterized by a damaged lung with enhanced free-water[30], vertical artifacts grow up from the pleura and continue to the opposite side of the display[31], which, as previously stated, are known as B-lines or comet tails. The quantity of these artifacts is related to increased free-water and lung aeration leakage[32].
It is important to remember, as previously stated, that one or two B-lines may be viewed ordinarily in bottom-dependent lung regions, such as normally-aerated lung bases[23].
It has been demonstrated that most B-lines nearer than 7 mm are determined by thickened interlobular septa with interstitial edema, while most B-lines that are 3 mm or less are produced by alveolar edema. As matter of fact, the amount of B-lines increases with the grade of aeration leakage.
TUS has proven itself to be useful in the assessment of lung reaeration after antimicrobial therapy[33] or non-invasive ventilation.
The cardiopulmonary system is so complex and interrelated, that an integrated method (lung ultrasound assessment combined with echocardiography) is fundamental to the assessment of pulmonary involvement in acute and chronic cardiac failure[34].
Evidence of many diffuse comet tails in both hemithorax correlated with left ventricular dysfunction or valvular disease, and is suggestive of a cardiac pulmonary cause of pulmonary edema[35-37].
On the other hand, evidence of B-lines related to normal systolic and diastolic function suggests a non-cardiogenic cause for congestion, although lung disease (e.g., pneumonia), acute respiratory distress syndrome, acute lung injury, or, particularly in a chronic setting, pulmonary fibrosis.
Focal multiplex comet tails can be present in physiologic lungs or other pathological conditions, including pleural distress, lung disease, focal pneumonia, pulmonary neoplasia lung contusion, or lung infarction.
This highlights the significance of integrated results obtained via TUS, echocardiography with patient anamnesis, clinical setting, and all instrumental information available[38].
Ultrasound imaging is the better tool for the diagnosis of pleural effusion, in that it is easy to use, specific, and very useful in differentiating the nature of pleural fluid[39,40].
Pleural effusion is very easy to locate via ultrasound as an echo-free zone, which shows up as a black area. As matter of fact, pleural effusion acts as an acoustic window, and when abundant enough to compress the lung, its inside will appear consolidated and moving (Figure 6).
Pleural effusion should be searched for in the declivous pulmonary zone, with the first objective being to determine whether the pleural effusion is transudate or exudate in nature.
Transudates appear anechoic with an echo-free pattern, although sometimes cured transudate pleural effusion can be characterized as congestive heart disease and echogenic[41].
Exudates, on the other hand, often appear echoic, with small moving dots that represent the presence of cells (e.g., macrophages, erythrocytes, or leukocytes) or little spots (e.g., protein or fibrin). Inflammatory pleural illnesses produce a characteristic effusion that holds fibrous strings and septations within encapsulated liquid that can be mobile or immobile (Figure 7)[42].
In the evaluation of pleural effusion, the second objective is to quantify its size. Various formulas have been used for the evaluation of pleural effusion volume, with the lung ultrasound method being suggested for its quantification.
In the supine setting, an inter-pleural space at the lung base of about 50 mm between the lung and the posterior chest cage is suggestive of a pleural effusion of about 500 mL[43].
Quantification of the inter-pleural space can be done at the end of either the expiration or inspiration phase, without any difference. All studies to date agree that ultrasound evaluation of the inter-pleural distance is not precise enough to quantify small (< 500 mL) or large (> 1000 mL) pleural effusions[44-46].
Evaluation of pleural effusion needs particular care when it comes to the left side of the spleen, right side of the liver, and both sides of the diaphragm, particularly when pleural puncture is considered. Thoracentesis and biopsy of the pleura have sometimes been necessary for the diagnosis of some diseases[47-49], with TUS being required in order to increase the safety of these procedures when performed at the bedside[50,51].
TUS permits the secure chest drainage of small and/or loculated pleural effusions, as well as allowing for the highlighting of pleural adherences that may complicate thoracentesis. TUS may also decrease the risk of intrafissural or intraparenchymal placing of pleural tubes[20,33].
Pneumothorax (PNT) is defined by the presence of air, or any other kind of gas, in the pleural space between the visceral and parietal pleural layers. Lung sliding is prevented due to this interposition, as ultrasound cannot pass across the air present in the pleural space due to lung disease. Moreover, B-lines are no longer visible, while the only visible A-lines are horizontal[23].
Several studies have recently confirmed that bedside TUS is more specific than CR in the diagnosis of PNT in critically ill patients[21,52-54]. In fact, bedside CR may actually underdiagnose up to 30% of conditions[55].
Radiographically “occult” PNT may quickly develop into tension PNT, particularly in patients who have received mechanical ventilation (both invasive and non-invasive), in which a missed or delayed diagnosis may be fatal.
Lung sliding failure is the first sign in the diagnosis of PNT.
In general, PNT should be searched for in the least gravitationally-dependent zone first.
The presence of lung sliding allows for the discounting of PNT; in fact, its negative predictive value is almost 100%[55].
Moreover, the absence of lung sliding in PNT can be also assessed by M-mode, which displays a characteristic setting called “stratosphere sign”, which is a picture that is opposite to the physiological seashore sign (Figure 8).
In any case, the absence of lung sliding never implies PNT. Other, different, conditions can cause the absence of lung sliding, such as severe pulmonary fibrosis, pleural adherences, massive atelectasis, bullous emphysema, advanced chronic obstructive pulmonary disease, presence of thoracic tube, and high-frequency ventilation. Moreover, the absence of B-lines is another state needed for a thoracic ultrasound diagnosis of PNT, with the presence of B-lines allowing for the discounting of a PNT diagnosis[31].
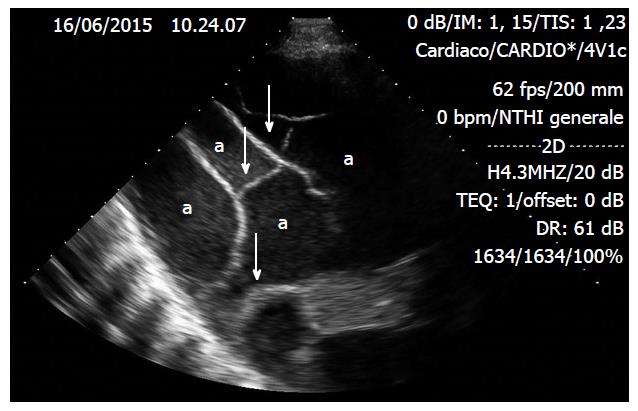
The only pathognomonic lung ultrasound sign of PNT is the called the “lung point”, which permits confirmation of a PNT diagnosis at a specificity of 100% and a sensitivity of 65%. Lung point is the exact zone of the thoracic wall, where normal lung sliding displaces the characteristic PNT setting. It represents the area where the visceral and parietal pleura layers recover alongside each other. Additionally, M-mode performed at the lung point demonstrates an evident shift from one setting to the other, with normal seashore sign changing to the characteristic PNT pattern (Figure 9)[7,38,56].
The diaphragm is the most important respiratory muscle[57,58]. Ultrasound assessment of the diaphragm has become a characteristic necessity for the evaluation of diaphragmatic role in different clinical settings in an ICU. Pathological diaphragmatic movement is seen in various situations, such as patients in critical condition who are using mechanical ventilation (invasive and non-invasive)[59] after cardiac or abdominal surgery[57], as well as in phrenic nerve injury or neuromuscular diseases.
Since diaphragmatic movement performs a fundamental role in spontaneous respiration, evaluation of the diaphragm motion seems necessary.
In ICU patients, ultrasound can assess physiological and pathological motion in different clinical settings. Study of the diaphragm using ultrasound is carried out using a 3.5-MHz, 5-MHz transducer, with the transducer placed under the left or right costal margin in the mid-clavicular row, or in the left or right anterior axillary row and directed medially, cranially, and caudally; as consequence the ultrasound beam will arrive perpendicularly at the rear third of the hemidiaphragm.
The 2-dimensional mode is employed first in order to get a better approach, while the M-mode is utilized to show the movement of the anatomical formations over the selected plane[60].
Preferentially, patients are examined over the long axis of the intercostal spaces, with the whole right hemidiaphragm observed via ultrasound; in fact, the liver allows for the perfect transmission of the beam, filling the dome entirely, while the left acoustic window is smaller because the spleen only fills half of the corresponding hemidiaphragm[20].
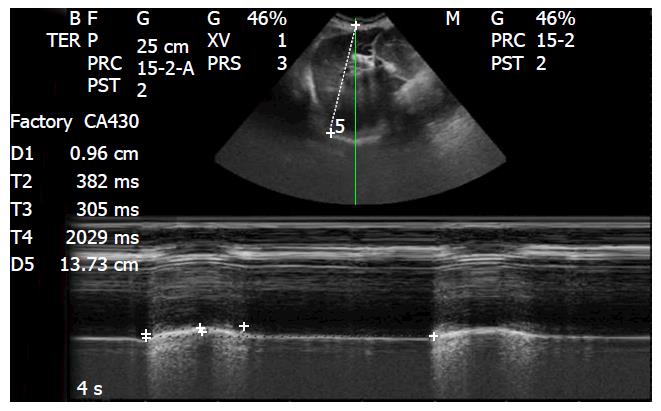
Left and right hemidiaphragm respiratory motion should always be evaluated; physiological inspiratory diaphragmatic motion is caudal, as the diaphragm moves toward the transducer, while physiological expiratory movement is cephalic, as the diaphragm shifts away from the transducer. As a consequence of these movements, in the M-mode we can measure: Diaphragmatic shift (displacement, cm), velocity of diaphragmatic contraction (slope, cm/s), inspiratory time (Tinsp, s), and length of cycle (Ttot, s) (Figure 10).
In mechanically ventilated patients, assessment of diaphragmatic movement could occasionally reveal the need to disengage the patient from the ventilator in order to better assess spontaneous breathing stresses. A lot of ICU patients may also be affected by pulmonary consolidation, atelectasis, or pleural effusions, which allow for better evaluation of the hemidiaphragms. The values of diaphragmatic movement in healthy subjects were indicate to be: 1.8 ± 0.3, 7.0 ± 0.6, and 2.9 ± 0.6 cm for men, and 1.6 ± 0.3, 5.7 ± 1.0, and 2.6 ± 0.5 cm for women, during rest, deep breathing and volunteer sniffing, respectively[61].
Excursion of the diaphragm dome evaluated via M-mode ultrasound is useful for predicting extubation outcomes[62].
Diaphragm thickening during inspiration indicates diaphragm shortening, and is analogous to the “ejection fraction” of the heart.
Acute respiratory distress is a very severe condition. The correction and immediate reorganization of acute respiratory failure are two fundamental steps in the correct management of patients in critical condition in the ICU, as well as acting to avoid initial mistakes and their deleterious consequences.
A study published in 2008 by Lichtenstein et al[63] assessed the utility of TUS in patients admitted to the ICU with respiratory distress. It was deduced that TUS can assist the physician in making an easier diagnosis in patients with acute respiratory distress; Lichtenstein called this protocol the “Bedside Lung Ultrasound in Emergency (BLUE) protocol”.
This observational study was conducted via ultrasonography on 260 consecutive dyspneic patients admitted to the ICU for acute respiratory distress; the conclusion of the ultrasound evaluation at first admittance of the dyspneic patient to the ICU was compared to the last diagnosis at patient discharge. Unclear diagnoses and uncommon patterns (frequency less than 2%) were excluded from the study. Three fundamental elements were evaluated: Artifacts (A-lines or B-lines), lung sliding, and alveolar consolidation and/or pleural effusion; all items were combined with venous analysis and clustered to the ultrasound outline value (Table 2).
| Profile | Characteristic items | Diagnosis |
| A’ profile | Lack of lung sliding, and presence of lung point | Pneumothorax |
| B profile | Anterior lung sliding, with presence of lung comet tails | Acute pulmonary edema |
| B’ profile | Lung comet tails, with abolished anterior lung sliding | Pneumonia |
| A/B profile | Anterior predominant B lines on one side, and predominant A lines on the other | Pneumonia |
| C profile | Anterior alveolar consolidations | Pneumonia |
| A profile | Anterior lung sliding with A lines, and the presence of DVT | Pulmonary embolism |
| A-V-PLAPS-profile | Anterior lung sliding with A lines, PLAPS, absence of DVT | Pneumonia |
| Nude profile | Anterior lung sliding with A lines, absence of DVT or PLAPS | Severe asthma or exacerbated COPD |
In the BLUE protocol, the first step is to check anterior lung sliding, as its presence rules out the diagnosis of PNT.
The A’ profile (A-profile with absence of lung sliding) and lung point revealed PNT (81% sensitivity and 100% specificity). However, if lung point was not present, additional diagnosis modalities were necessary.
The second step in the BLUE protocol is looking for anterior B-lines.
The B profile (presence of anterior lung sliding with lung comet tails) indicates the presence of acute pulmonary edema (97% sensitivity and 95% specificity).
The B’ profile (B profile with absence of lung sliding), A/B profile (anterior prevailing B-lines on one side and prevailing A-lines on the other), and C profile (identified anterior alveolar consolidations) suggest the presence of pneumonia (89% sensitivity and 94% specificity).
The A profile (characterized by anterior lung sliding with A-lines) indicates the possibility of deep vein thrombosis and the potential for pulmonary embolism (81% sensitivity and 99% specificity). If deep vein thrombosis is absent, posterior and/or lateral alveolar and/or pleural syndrome (PLAPS) is evaluated. The combination of A-profile, free veins, and PLAPS is referred to as the A-V-PLAPS-profile, and is potentially characteristic of pneumonia.
An A-profile with the absence of DVT or PLAPS (called the “nude profile”) and with preserved lung sliding is likely an exacerbation of chronic obstructive pulmonary disease (COPD) or severe asthma (89% sensitivity and 97% specificity).
The application of the aforementioned settings would provide the correct diagnosis in about 90.5% of conditions.
The BLUE protocol typically uses a convex or sector probe, although any available would be suitable. In order to give better results, the BLUE protocol must be initiated just after the physical examination, and must be integrated into the clinical approach. Cardiac analysis via echocardiography completes this approach.
A recent multicenter study, which consisted of 1005 enrolled patients, tested the hypothesis that an integrated method implementing TUS with clinical examination would have superior diagnostic precision than standard evaluation in discriminating acute decompensated heart failure from non-cardiogenic dyspnea in the ICU. The TUS-implemented method had prominently superior precision (sensitivity, 97%; specificity, 97.4%) in differentiating heart distress from a non-cardiac pattern of acute dyspnea than early clinical examination (sensitivity, 85.3%; specificity, 90%), CR alone (sensitivity, 69.5%; specificity, 82.1%), or natriuretic peptides (sensitivity, 85%; specificity, 61.7%)[64].
Although TUS has many advantages (Table 3), there are physician, patient, and disease limitations that are important to keep in mind.
| Thoracic ultrasound advantages |
| Rapid diagnosis |
| No limitation with setting, patient position, or clinical conditions |
| Differential diagnosis (e.g., chest pain, pulmonary edema, exacerbation of chronic obstructive pulmonary disease, subpulmonary effusion, subphrenic fluid accumulation, and tumors) |
| Diagnose presence and nature of pleural effusions |
| Guide invasive procedures (e.g., thoracentesis, chest tube placement, and biopsy) |
| Diagnose diaphragm paralysis |
| Diagnose localized pleural tumors or pleural thickening, assess invasion of the pleura and chest wall |
| Diagnose pneumothorax, drainage, or verify lung expansion |
| Few limitations in ventilated patients |
Concerning physician limitations, thoracic ultrasound is restricted by interobserver variability and time spent until acquisition. Moreover, TUS evaluation and the correct evaluation of the images produced require direct training in order to reach the required level of expertise and ability. Due to the serious consequences of TUS on patient management, access to an emergency clinician and intensivist should be of vital importance[19].
With regards to patient limitations in ICU, patients who are under mechanical ventilation as a consequence of the presence of an inflated lung between the heart and thoracic wall, sonographic imaging could be inadequate. Other elements that can limit imaging acquisition are related to surgical injury and chest dressings that can alter or preclude the transmission of ultrasound beams to the lung, with obesity and COPD also potentially worsening the quality of the images[7].
Absence of patient compliance and difficulty in moving patients into the ideal position makes several studies technically inadequate[65].
Finally, there are the disease limitations. Lung disease can only be revealed by TUS if the location of pulmonary disease is peripheral and has reached the pleura, if air is not present in the pleural space, if subcutaneous air is present, and if the lesion is not covered by bones. These physical limitations are especially significant when ruling out consolidations (especially for tumors) that can be placed in a medial position or bordered by normally aerated lung. In fact, is important to stress that centrally placed lesions typically avoid detection via ultrasound examination, which is the most important limitation of TUS [25].
TUS permits accurate, fast, and easy evaluation of many important acute respiratory diseases, even at the bedside (Tables 4 and 5)[19,26,66].
| Pleural effusion | Pleural effusion is an echo-free zone (dark zone) that causes lung consolidation and floating in the pleural effusion |
| TUS allows the nature of the fluid to be distinguished: | |
| Transudate: Anechoic and echo-free pattern | |
| Exudate: Echogenic, with small moving dots (e.g., leukocytes, erythrocytes, fibrin, and protein particles), fibrous strings, and mobile or immobile septations with encapsulated liquid | |
| TUS allows for the quantification of pleural effusion volume | |
| Ultrasound may guide thoracentesis and biopsy of the parietal pleura | |
| Pneumothorax | The interposition of gas between the visceral and parietal pleural layers, lack of lung sliding, and B-lines; only horizontal A-lines can be seen. Stratosphere sign is the characteristic pattern of the lack of lung sliding evaluated by M-mode. The lung point is the precise area of the chest wall where visceral and parietal pleura regain contact with each other, as well as where the regular reappearance of lung sliding replaces the pneumothorax pattern |
| Diaphragmatic function | A diaphragm study can be made by placing the probe below the costal margin and using M-mode to display the motion of the anatomical structures; normal inspiratory diaphragmatic movement is caudal, while normal expiratory trace is cranial. In M-mode, diaphragmatic excursion, speed of diaphragmatic contraction, inspiratory time, and duration of the cycle can be measured |
| A-lines | B-lines | Lung sliding | Pulse | Particular characteristics | |
| Normal | Present | Rare | Present | Present | |
| Pneumothorax | Present | Never | Absent | Absent | Lung point |
| Pleural effusion | Absent | Absent | Absent | Absent | Presence of B-lines in cases of concomitant interstitial syndrome or pneumonia |
| Interstitial syndrome | Absent | Multiple | Present | Present | B-lines crowded and confluent (white lung) |
TUS is fast, non-invasive, inexpensive, reliable, flexible, available for bedside use, repeatable, and does not employ radiation or contrast medium; for all these reason TUS is suitable for every patient, regardless of age, pregnancy, renal failure, or allergies. A portable ultrasound machine also permits ultrasound examination at any moment and at any site, owing to its transportability and the possibility of executing moving imaging[20].
TUS represents a new and useful tool for emergency physicians to use at all stages of diagnosis, as well as for the management of critically ill patients. Moreover, TUS is vitally important for the differential diagnosis of patients admitted to the ICU in order to differentiate between different aliments, such as dyspnea[38], pulmonary consolidation, pleural effusion, alveolar-interstitial syndrome, and pneumothorax, which can all have similar clinical presentations.
TUS performed by the clinician in charge of an ICU looks to be one of the most promising skills for respiratory and therapeutic monitoring, as well as assisting with the prevention of any kind of delay in the management of critical patients.
For all these reasons, TUS is rapidly expanding in our departments[7], and is fast becoming an essential part of emergency patient assessment and of the ICU’s armamentarium[19,67].
The authors are grateful to Dr. Ilario de Sio and Lucia Morelli for their cooperation in the figures section.
P- Reviewer: Chello M, den Uil CA, Ferrer-Hita JJ, Lazzeri C S- Editor: Gong XM L- Editor: Rutherford A E- Editor: Li D
| 1. | Elia F, Panero F, Molino P, Ferrari G, Aprà F. Ultrasound to reduce cognitive errors in the ED. Am J Emerg Med. 2012;30:2030-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53:550-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 410] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 3. | Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1885] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 4. | Bekemeyer WB, Crapo RO, Calhoon S, Cannon CY, Clayton PD. Efficacy of chest radiography in a respiratory intensive care unit. A prospective study. Chest. 1985;88:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Zanobetti M, Poggioni C, Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest. 2011;139:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intra-hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian Incident Monitoring Study in Intensive Care. Intensive Care Med. 2004;30:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 293] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: Bedside lung ultrasound in critical care practice. Crit Care. 2007;11:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Raimondi F, Cattarossi L, Copetti R. Pediatric chest ultrasound versus conventional radiology: experimental evidence first. Pediatr Radiol. 2014;44:900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Herth FJ, Becker HD. Transthoracic ultrasound. Respiration. 2003;70:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35:S250-S261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, Fava C, Frascisco M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 393] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 12. | Volpicelli G, Silva F, Radeos M. Real-time lung ultrasound for the diagnosis of alveolar consolidation and interstitial syndrome in the emergency department. Eur J Emerg Med. 2010;17:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Fauci AS. Harrison’s principles of internal medicine. New York: McGraw-Hill 2008; . |
| 14. | Turner JP, Dankoff J. Thoracic ultrasound. Emerg Med Clin North Am. 2012;30:451-473, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Zanforlin A, Giannuzzi R, Nardini S, Testa A, Soldati G, Copetti R, Marchetti G, Valente S, Inchingolo R, Smargiassi A. The role of chest ultrasonography in the management of respiratory diseases: document I. Multidiscip Respir Med. 2013;8:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Kirkpatrick AW, Breeck K, Wong J, Hamilton DR, McBeth PB, Sawadsky B, Betzner MJ. The potential of handheld trauma sonography in the air medical transport of the trauma victim. Air Med J. 2005;24:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Sargsyan AE, Hamilton DR, Jones JA, Melton S, Whitson PA, Kirkpatrick AW, Martin D, Dulchavsky SA. FAST at MACH 20: clinical ultrasound aboard the International Space Station. J Trauma. 2005;58:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Schabrun S, Chipchase L, Rickard H. Are therapeutic ultrasound units a potential vector for nosocomial infection? Physiother Res Int. 2006;11:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Reissig A, Copetti R, Kroegel C. Current role of emergency ultrasound of the chest. Crit Care Med. 2011;39:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Beckh S, Bölcskei PL, Lessnau KD. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest. 2002;122:1759-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Lichtenstein DA, Mezière G, Lascols N, Biderman P, Courret JP, Gepner A, Goldstein I, Tenoudji-Cohen M. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 23. | Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 704] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 24. | Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 445] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 25. | Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 26. | Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 27. | Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med. 2003;22:173-180. [PubMed] |
| 28. | Lichtenstein D. Lung ultrasound application. General ultrasound in the critically ill. Heidelberg: Springer-Verlag 2005; 129-133. |
| 29. | Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 30. | Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT Scan ARDS Study Group. Intensive Care Med. 2000;26:857-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 246] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 33. | Bouhemad B, Liu Z, Zhang M, Lu Q, Rouby JJ. Lung ultrasound detection of lung reaeration in patients treated for ventilator associated pneumonia. Intensive Care Med. 2006;32:S221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 34. | Smargiassi A, Inchingolo R, Soldati G, Copetti R, Marchetti G, Zanforlin A, Giannuzzi R, Testa A, Nardini S, Valente S. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med. 2013;8:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, Kort S, Porter TR, Spencer KT, Tayal VS, Wei K. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 36. | Via G, Hussain A, Wells M, Reardon R, Elbarbary M, Noble VE, Tsung JW, Neskovic AN, Price S, Oren-Grinberg A. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27:683.e1-683.e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 360] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 37. | Expert Round Table on Echocardiography in ICU. International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med. 2014;40:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 38. | Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 39. | Hansell DM. Thoracic imaging. Respiratory medicine. London, UK: WB Saunders 1995; 282-284. |
| 40. | Kinasewitz GT. Pleural fluid dynamics and effusions. Fishman’s pulmonary diseases and disorders. New York, NY: McGraw-Hill 1998; 1396-1397. |
| 41. | Reuss J. Sonographic imaging of the pleura: nearly 30 years’ experience. Eur J Ultrasound. 1996;3:25-39. |
| 42. | Mathis G. Thoraxsonography--Part I: Chest wall and pleura. Ultrasound Med Biol. 1997;23:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Balik M, Plasil P, Waldauf P, Pazout J, Fric M, Otahal M, Pachl J. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 44. | Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hörmann MF, Grabenwöger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Roch A, Bojan M, Michelet P, Romain F, Bregeon F, Papazian L, Auffray JP. Usefulness of ultrasonography in predicting pleural effusions & gt; 500 mL in patients receiving mechanical ventilation. Chest. 2005;127:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Vignon P, Chastagner C, Berkane V, Chardac E, François B, Normand S, Bonnivard M, Clavel M, Pichon N, Preux PM. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 47. | Broaddus VC, Light RW. Disorders of the pleura: general principles and diagnostic approach. Textbook of respiratory medicine. Philadelphia, PA: WB Saunders 1994; 2156-2160. |
| 48. | Woodcock A, Viskum K. Pleural and other investigations. Respiratory medicine. London, UK: WB Saunders 1995; 384-385. |
| 49. | Morris V, Wiggins J. Current management of pleural disease. Br J Hosp Med. 1992;47:753-758. [PubMed] |
| 50. | Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Mezière G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 52. | Dulchavsky SA, Schwarz KL, Kirkpatrick AW, Billica RD, Williams DR, Diebel LN, Campbell MR, Sargysan AE, Hamilton DR. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma. 2001;50:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Liu DM, Forkheim K, Rowan K, Mawson JB, Kirkpatrick A, Nicolaou S. Utilization of ultrasound for the detection of pneumothorax in the neonatal special-care nursery. Pediatr Radiol. 2003;33:880-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG, Hameed SM, Brown R, Simons R, Dulchavsky SA. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the Extended Focused Assessment with Sonography for Trauma (EFAST). J Trauma. 2004;57:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 55. | Chiles C, Ravin CE. Radiographic recognition of pneumothorax in the intensive care unit. Crit Care Med. 1986;14:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Lichtenstein D, Mezière G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 427] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 57. | Lerolle N, Guérot E, Dimassi S, Zegdi R, Faisy C, Fagon JY, Diehl JL. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest. 2009;135:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Tobin MJ, Laghi F, Brochard L. Role of the respiratory muscles in acute respiratory failure of COPD: lessons from weaning failure. J Appl Physiol (1985). 2009;107:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest. 2012;142:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 60. | Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med. 2001;20:597-604. [PubMed] |
| 61. | Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 466] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 62. | Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39:2627-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 63. | Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1193] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 64. | Pivetta E, Goffi A, Lupia E, Tizzani M, Porrino G, Ferreri E, Volpicelli G, Balzaretti P, Banderali A, Iacobucci A. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest. 2015;148:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 65. | Beaulieu Y, Marik PE. Bedside ultrasonography in the ICU: part 1. Chest. 2005;128:881-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | van der Werf TS, Zijlstra JG. Ultrasound of the lung: just imagine. Intensive Care Med. 2004;30:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Piccoli M, Trambaiolo P, Salustri A, Cerquetani E, Posteraro A, Pastena G, Amici E, Papetti F, Marincola E, La Carruba S. Bedside diagnosis and follow-up of patients with pleural effusion by a hand-carried ultrasound device early after cardiac surgery. Chest. 2005;128:3413-3420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |