Published online Mar 28, 2016. doi: 10.4329/wjr.v8.i3.268
Peer-review started: September 17, 2015
First decision: October 30, 2015
Revised: November 16, 2015
Accepted: January 8, 2016
Article in press: January 11, 2016
Published online: March 28, 2016
Processing time: 187 Days and 21.4 Hours
Differentiation between neoplastic and nonneoplastic conditions magnetic resonance imaging (MRI) has established itself as one of the key clinical tools in evaluation of musculoskeletal pathology. However, MRI still has several key limitations which require supplemental information from additional modalities to complete evaluation of various disorders. This has led to the development hybrid positron emission tomography (PET)-MRI which is rapidly evolving to address key clinical questions by using the morphological strengths of MRI and functional information of PET imaging. In this article, we aim to review physical principles and techniques of PET-MRI and discuss clinical utility of functional information obtained from PET imaging and structural information obtained from MRI imaging for the evaluation of musculoskeletal pathology. More specifically, this review highlights the role of PET-MRI in musculoskeletal oncology including initial diagnosis and staging, treatment planning and post-treatment follow-up. Also we will review utility of PET-MRI in evaluating musculoskeletal infections (especially in the immunocompromised and diabetics) and inflammatory condition. Additionally, common pitfalls of PET-MRI will be addressed.
Core tip: Positron emission tomography-magnetic resonance imaging is a rapidly emerging technique which provides detailed anatomic and functional imaging simultaneously and allows for differentiation of neoplastic from non-neoplastic conditions. This modality can prove to be both time and cost effective means of evaluating complex cases in patients with coexisting neoplastic, infectious and/or inflammatory conditions. Additional benefits include reducing radiation exposure in patient cohort who is likely to undergo multiple radiologic evaluation over their life time for follow-up.
- Citation: Chaudhry AA, Gul M, Gould E, Teng M, Baker K, Matthews R. Utility of positron emission tomography-magnetic resonance imaging in musculoskeletal imaging. World J Radiol 2016; 8(3): 268-274
- URL: https://www.wjgnet.com/1949-8470/full/v8/i3/268.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i3.268
For the last few decades, magnetic resonance imaging (MRI) has been the “gold standard” for certain aspects of musculoskeletal imaging due to its capability of noninvasively providing high-resolution, high-tissue contrast images of osseous, articular and soft tissue structures[1]. MRI can reliably diagnose structural abnormalities (e.g., meniscal/ligament/tendon tears, occult fractures, myopathy/muscle atrophy, etc.)[2] and articular derangements (especially in osteoarthritis, rheumatoid arthritis)[1,2]. More recently, whole-body MRI has shown ability to potentially screen, diagnose and stage musculoskeletal neoplasms (such as multiple myeloma, skeletal and soft tissue sarcomas) and diffuse musculoskeletal processes (e.g., muscular dystrophy, inflammatory myopathies, etc.)[1,3].
While MRI is widely considered the best imaging modality to evaluate bone marrow and soft tissue pathology (especially in delineating tissue extent of the lesion), it frequently fails to characterize cortical involvement and evaluate cortex-based lesions (e.g., osteoid osteoma). In such cases, radiographs, computed tomography (CT) and positron emission tomography (PET) often remain preferred modalities[4-6].
Unlike MRI, PET provides biochemical and physiologic information which gives key diagnostic information uncovering pathology using various radiochemical agents. The most common PET imaging agent is 18F-fluorodeoxyglucose (FDG), which is commonly used to assess for glucose metabolism and forms the basis of tumor imaging. FDG is a positron emitter with a half-life of approximately 110 min. FDG is a glucose analog, which is taken up by cells. Once inside the cell, the FDG is phosphorylated and effectively trapped within the cell. Highly metabolic tissues will uptake more glucose in order to sustain their metabolic activity. Most malignant tumors as well as inflammatory processes have a relatively high degree of metabolic activity amongst their cellular background. This forms the basis of PET-FDG imaging. Of note, particular organs a relatively high baseline glucose metabolism will inherently uptake more FDG relative to the remainder of the body. For example, the brain has a normal intense uptake of glucose and will concentrate a significant portion of the injected FDG. Similarly, FDG is concentrated in the urine, and as such, the kidneys, ureters, and bladder will appear hyperintense[1,2,4-9].
Over the last forty years, PET has played a critical role in evaluation of oncologic processes (tumor diagnosis and follow-up), cardiac perfusion imaging and brain perfusion imaging. PET can aid in initial staging and follow up of patients who receive surgery, radiation, or chemotherapy. However, PET has several shortcomings and limitations, with one of the most important being its low spatial resolution[6]. Fusion of PET-acquired images with CT images has allowed radiologists to alleviate some of this spatial resolution limitation. As important as PET-CT has been in evaluation of oncologic diseases, its utility for complete characterization of musculoskeletal disorders (including neoplasms) has not been as extensive, largely due to the comparatively lower soft tissue resolution of CT compared to MRI[1,2]. The advent of PET-MRI therefore provides a potential highly useful imaging modality for musculoskeletal imaging, as it couples the molecular and physiologic information acquired from PET with the unparalleled soft tissue resolution of MRI to provide a superior level of anatomic and functional patient information. Also, two major benefits of utilizing a combined PET-MRI unit include reduction of image acquisition time and misregistration artifacts. Additionally, using PET-MRI over PET-CT has the obvious benefit of substantial reduction of patient ionizing radiation exposure, especially in those who require multiple follow-up examinations[1]. PET-MRI exam radiation dose is approximately 4.6 mSv which is about 20% of an average PET-CT[7,8], a statistic that is particularly important for pediatric patients due to their increased radiation sensitivity[2,7,8].
In this review article, we aim to review clinical utility of PET-MRI in musculoskeletal imaging with emphasis on clinical value in oncologic, inflammatory and infectious processes.
Current MRI technique is limited in evaluation of small pulmonary nodules[4]. Additionally, there are inherent limitation of MRI-based modalities that can preclude imaging of patients with implanted medical devices such as some defibrillators and pacemakers.
Staging of neoplasms is one of the key determinants in identifying patient treatment options and determining overall prognosis. TNM staging is the most commonly used system to evaluate extent of neoplastic burden in a patient and is based on tumor size (T), degree/number of lymph node involvement (N) and presence or absence of metastasis (M). Imaging evaluation allows to assess for all three of these factors (T, N, M) and therefore diagnostic precision and accuracy are key determinants in developing appropriate treatment plan for the patient. PET-MRI can provide high tissue contrast detail allowing for tumor detection with increased sensitivity (PET) and specificity (MRI)[10].
There are no randomized controlled studies performed using PET-MRI to define standardized uptake value (SUV) cut-off value for discern benign and malignant lesions. Data from prior PET and PET-CT has been used to use very low SUV value of less than 2 to 2.5 as the cut-off threshold value[11]. It should be noted, that though this cut-off value is helpful, it is by no means definitive and therefore, in cases with high index of suspicion where SUV values appear discordant with clinical findings, a tissue biopsy and/or interval follow-up may be warranted for a more definitive diagnosis.
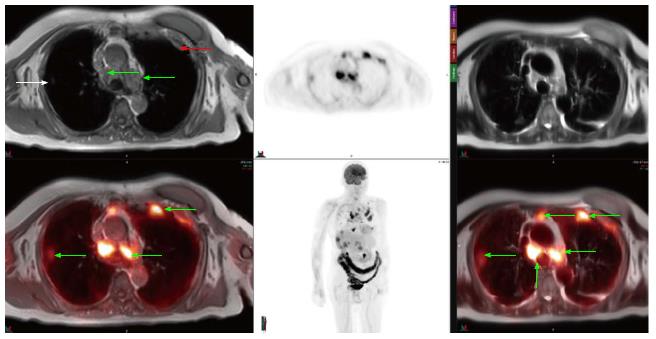
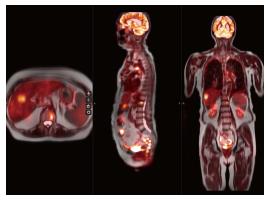
Approximately 2% of all cancer related deaths are due to sarcomas (soft tissue and osseous) which account for less than 1% of all cancers and carry overall five-year survival of 60%[6]. MRI is the initial modality of choice for evaluation of sarcomas and contrast-enhanced MRI can provide fairly accurate assessment of sarcomas. However, occasionally it is difficult to discriminate between infiltrative tumor vs edema on both initial and follow-up evaluations, and it is also sometimes challenging to discern post-surgical change from tumor recurrence. Our initial clinical experience with PET-MRI using the SUV obtained from PET in conjunction with soft tissue detail of MRI to help discern neoplastic from non-neoplastic tissue has yielded a fair amount of cases which would previously have been equivocal and prompted tissue biopsy to exclude tumor recurrence (Figures 1 and 2).
Whole-body PET-MRI allows for simultaneous loco-regional examination and whole-body evaluation for distant disease[1,2,4-6]. PET-MRI is especially helpful in overcoming PET-CT limitations of evaluation of disease in organs with high background PET activity such as the brain, liver, kidney and spinal canal[1,5,6].
The most common PET imaging agent is FDG, which is commonly used to assess for glucose metabolism and forms the basis of tumor imaging. FDG is a positron emitter with a half-life of approximately 110 min. FDG is a glucose analog, which is taken up by cells. Once inside the cell, the FDG is phosphorylated and effectively trapped within the cell. Highly metabolic tissues will uptake more glucose in order to sustain their metabolic activity. Most malignant tumors as well as inflammatory processes have a relatively high degree of metabolic activity amongst their cellular background. This forms the basis of PET-FDG imaging. Of note, particular organs a relatively high baseline glucose metabolism will inherently uptake more FDG relative to the remainder of the body. For example, the brain has a normal intense uptake of glucose and will concentrate a significant portion of the injected FDG. Similarly, FDG is concentrated in the urine, and as such, the kidneys, ureters, and bladder will appear hyperintense[1,2,5-8,12].
PET-FDG has proven itself to be the standard radiotracer for PET imaging, given its significant versatility for both pretreatment and follow-up clinical situations. Most other radiotracers in practice have been developed for non-MSK purposes, including myocardial perfusion imaging (for example, Rubidium-82, Nitrogen-13 ammonia)[4] or for brain perfusion imaging (for example, Oxygen-15 water)[9]. Several other radiotracers are still under investigation, including 11C-choline, which has yielded encouraging results in providing more accurate lymph node involvement (N-staging) when used in conjunction with diffusion weighted imaging (DWI) or short-tau inversion recover (STIR) sequences. There are other investigational radiotracers are currently under study for tumor characterization, such as 18F-fluoromisonidazole (FMISO), which is a marker for tumor hypoxia. Many of these emerging radiotracers are still under investigation[13].
The ability to calculate the change of PET agent uptake between initial and follow-up exams can provide information on treatment response, which is especially important in patients who receive adjuvant therapy where tumor size does not change and MRI alone would have otherwise failed to discern positive or negative treatment response[5]. It should be noted, however, that randomized controlled studies are required to assess for overall impact of PET-MRI based staging on overall survival.
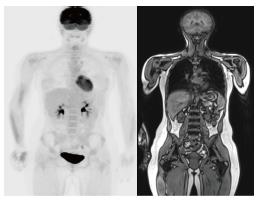
As with sarcoma, precise staging on initial and follow-up treatment is critical to patient care. PET-CT can provide valuable information on focal lesional activity which correlates with disease activity[10,11,14]. However, unlike PET-CT, whole-body MRI is superior to more accurately characterize extent of bone marrow involvement in myeloma[15] especially in small lesions that are not well characterized on PET-CT[10,15-17]. Shortt et al[15] showed that combination of data acquired from PET and whole-body MRI can improved specificity and positive predictive value. Our initial anecdotal experience with PET-MRI suggests myeloma lesions to be T1-hypointense with variable degree of contrast enhancement and SUV uptake (Figure 3).
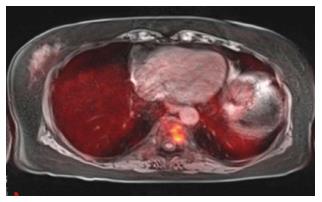
PET, PET-CT and MRI are all used in various clinical settings for evaluation of osseous metastasis, with each modality offering high sensitivity with variable degrees of specificity. The major shortcoming of PET and PET-CT are their higher number of false positives and lower positive predictive value due to suboptimal soft tissue resolution. Additionally, PET and PET-CT yield higher rates of false positives for lesions that are 5 mm or smaller[12]. MRI, especially with diffusion weighted imaging, provides precise pre-treatment evaluation, but this information following adjuvant treatment is less sensitive and specific. In such instances, PET-MRI can help provide the superior MRI tissue contrast detailing anatomic extent of disease while PET helps discern presence or absence of treatment response based on changes in neoplasm metabolic activity (Figures 4 and 5)[12].
PET-CT has solidified itself as the imaging modality of choice for initial staging, monitoring of treatment response and follow-up of patients with lymphoma[12]. PET-CT can detect lymphoma with 90% sensitivity and 91% specificity, although overall positive predictive value is low especially for disease progression[12]. Increasing number of false positive PET-CT results has been noted in patients on rituximab[12]. MRI, to a certain extent, can provide information about changes in cellular content of lesions (e.g., on DWI and MRI spectroscopy), and the combination of MRI information with PET has shown promising results in initial studies[12,18]. Additionally, PET-MRI can be used in evaluating patients for post-treatment changes (e.g., thymic hyperplasia, nodal necrosis, etc.) in addition to the standard changes in SUV values seen on PET and PET-CT[12,19].
Clinical response to therapy (including chemotherapy, radiotherapy, surgery) as evaluated on imaging can provide prognostic information of disease-free survival especially in patients being evaluated for sarcomas[12]. At present, for most oncologic diseases, functional information derived from PET-based imaging units (PET and PET-CT) provides this information with the notion that change in radiotracer uptake is theorized to directly correlate with treatment response, so that decreased radiotracer uptake on PET is indicative of positive treatment response and conversely increased PET uptake is indicative of an insufficient or lack of a treatment response[12]. In the post-surgical setting, however, this is less true, as a reparative response can generate a variable degree of radiotracer uptake in the surgical bed with SUV of greater than 2.5. This is further complicated by distortion of normal anatomy in post-surgical cases where the lack of distinction between neoplasm and edema makes definitive diagnosis enigmatic. In such scenarios, multisequence MRI (including DWI, spectroscopy, etc.) along with PET information can improve specificity of findings[12].
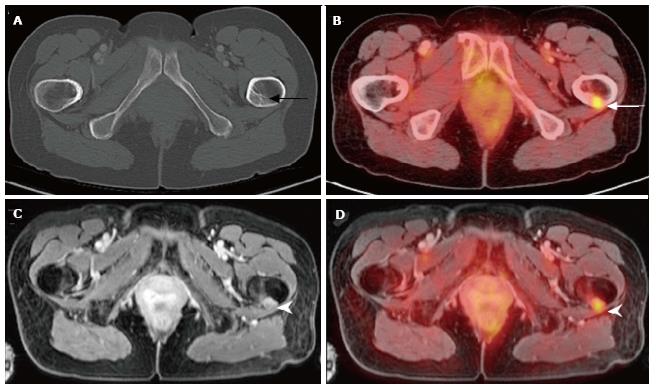
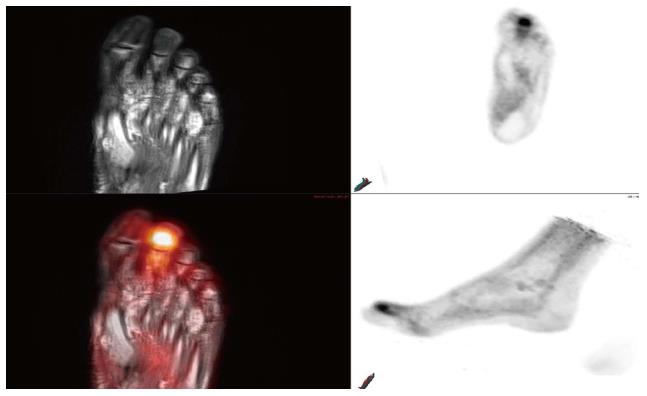
PET and PET-CT studies have shown that radiotracer uptake can be seen in non-neoplastic conditions including infectious and inflammatory conditions. Generally speaking, SUV of less than 2.5 has been used as a rule-of-thumb to suggest non-neoplastic conditions. However, many infectious processes and inflammatory conditions can cause SUV values of greater than 2.5. Therefore, as always, correlation with clinical history is imperative for optimal interpretation of PET, PET-CT and PET-MRI. PET-CT has a limited role with conditions such as Charcot arthropathy or organ system disorders (i.e., CNS, liver, spleen) where CT cannot provide the soft tissue contrast needed to fully characterize the clinical process[1,12], and our initial experience with PET-MRI has shown its advantage to PET-CT for the evaluation osteomyelitis in patients with Charcot arthropathy with better diagnostic yield (Figure 6).
Caution must be used when administering gadolinium-based contrast for MRI in patients. Nephrogenic system fibrosis (NSF) is a rare but serious disease of fibrosis of the skin and organs that may develop in patients with poor renal function and a low glomerular filtration rate (GFR). Additionally, care should also be taken for patients who require multiple follow-up contrast-enhanced MRI studies. A recent FDA report in July 2015 demonstrated potential gadolinium deposits in the brain. This is of uncertain clinical significance, however, referring clinicians should be aware of this fact. However, reducing the contrast need may be one the potential unseen benefits of PET-MRI; the inherent improved soft tissue resolution with the combined PET-tumor characterization may obviate the need for contrast in certain clinical situations[20].
Although data on PET-MRI utility is limited at present, emerging studies demonstrate positive application of this hybrid modality, especially in oncologic diagnosis and follow-up. PET-MRI provides detailed anatomic (MRI) and functional (PET) information which shows promise in improving sensitivity and specificity, which will facilitate a more accurate and reliable TNM-staging of various primary and secondary musculoskeletal neoplasms as these conditions require diagnostic modalities with high tissue contrast and resolution for accurate diagnosis. There also is the added benefit of substantially reduced patient radiation exposure. Larger studies are required to evaluate the overall impact on patient survival, which serves as the clinical benchmark for all diagnostic and therapeutic interventions. In addition to accuracy, cost-benefit analyses comparing PET-CT and PET-MRI are required, which should factor in both monetary and health-risk (i.e., radiation exposure) cost.
P- Reviewer: Morbelli SD, Schoenhagen P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Disselhorst JA, Bezrukov I, Kolb A, Parl C, Pichler BJ. Principles of PET/MR Imaging. J Nucl Med. 2014;55:2S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Werner MK, Schmidt H, Schwenzer NF. MR/PET: a new challenge in hybrid imaging. AJR Am J Roentgenol. 2012;199:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Chatal JF, Rouzet F, Haddad F, Bourdeau C, Mathieu C, Le Guludec D. Story of Rubidium-82 and Advantages for Myocardial Perfusion PET Imaging. Front Med (Lausanne). 2015;2:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | National Cancer Institute. Cancer staging: TNM staging. Available from: http://www.cancer.gov/about-cancer/diagnosis-staging/staging/staging-fact-sheet#q3. |
| 5. | Partovi S, Kohan AA, Zipp L, Faulhaber P, Kosmas C, Ros PR, Robbin MR. Hybrid PET/MR imaging in two sarcoma patients - clinical benefits and implications for future trials. Int J Clin Exp Med. 2014;7:640-648. [PubMed] |
| 6. | Fahey FH, Treves ST, Adelstein SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med Technol. 2012;40:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Hirsch FW, Sattler B, Sorge I, Kurch L, Viehweger A, Ritter L, Werner P, Jochimsen T, Barthel H, Bierbach U. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013;43:860-875. [PubMed] |
| 8. | Chawla SC, Federman N, Zhang D, Nagata K, Nuthakki S, McNitt-Gray M, Boechat MI. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol. 2010;40:681-686. [PubMed] |
| 9. | Watabe T, Shimosegawa E, Kato H, Isohashi K, Ishibashi M, Tatsumi M, Kitagawa K, Fujinaka T, Yoshimine T, Hatazawa J. Paradoxical reduction of cerebral blood flow after acetazolamide loading: a hemodynamic and metabolic study with (15)O PET. Neurosci Bull. 2014;30:845-856. [PubMed] |
| 10. | Breyer RJ, Mulligan ME, Smith SE, Line BR, Badros AZ. Comparison of imaging with FDG PET/CT with other imaging modalities in myeloma. Skeletal Radiol. 2006;35:632-640. [PubMed] |
| 11. | Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457-1463. [PubMed] |
| 12. | Lee IS, Jin YH, Hong SH, Yang SO. Musculoskeletal applications of PET/MR. Semin Musculoskelet Radiol. 2014;18:203-216. [PubMed] |
| 13. | Jennings M, Marcu LG, Bezak E. PET-specific parameters and radiotracers in theoretical tumour modelling. Comput Math Methods Med. 2015;2015:415923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Schirrmeister H, Bommer M, Buck AK, Müller S, Messer P, Bunjes D, Döhner H, Bergmann L, Reske SN. Initial results in the assessment of multiple myeloma using 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:361-366. [PubMed] |
| 15. | Shortt CP, Gleeson TG, Breen KA, McHugh J, O’Connell MJ, O’Gorman PJ, Eustace SJ. Whole-Body MRI versus PET in assessment of multiple myeloma disease activity. AJR Am J Roentgenol. 2009;192:980-986. [PubMed] |
| 16. | Bredella MA, Steinbach L, Caputo G, Segall G, Hawkins R. Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol. 2005;184:1199-1204. [PubMed] |
| 17. | Lecouvet FE, Dechambre S, Malghem J, Ferrant A, Vande Berg BC, Maldague B. Bone marrow transplantation in patients with multiple myeloma: prognostic significance of MR imaging. AJR Am J Roentgenol. 2001;176:91-96. [PubMed] |
| 18. | Lin C, Itti E, Luciani A, Zegai B, Lin SJ, Kuhnowski F, Pigneur F, Gaillard I, Paone G, Meignan M. Whole-body diffusion-weighted imaging with apparent diffusion coefficient mapping for treatment response assessment in patients with diffuse large B-cell lymphoma: pilot study. Invest Radiol. 2011;46:341-349. [PubMed] |
| 19. | Inaoka T, Takahashi K, Mineta M, Yamada T, Shuke N, Okizaki A, Nagasawa K, Sugimori H, Aburano T. Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology. 2007;243:869-876. [PubMed] |
| 20. | FDA Drug Safety Communication. FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). Available from: http://www.fda.gov/Drugs/DrugSafety/ucm455386.htm. |