Published online Aug 28, 2015. doi: 10.4329/wjr.v7.i8.202
Peer-review started: July 19, 2014
First decision: November 27, 2014
Revised: February 13, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: August 28, 2015
Processing time: 411 Days and 8.4 Hours
Early detection of skeletal metastasis is critical for accurate staging and optimal treatment. This paper briefly reviews our current understanding of the biological mechanisms through which tumours metastasise to bone and describes the available imaging methods to diagnose bone metastasis and monitor response to treatment. Among the various imaging modalities currently available for imaging skeletal metastasis, hybrid techniques which fuse morphological and functional data are the most sensitive and specific, and positron emission tomography (PET)/computed tomography and PET/magnetic resonance imaging will almost certainly continue to evolve and become increasingly important in this regard.
Core tip: Early detection of skeletal metastasis is critical for accurate staging and optimal treatment. This paper briefly reviews our current understanding of the biological mechanisms through which tumours metastasise to bone and describes the available imaging methods to diagnose bone metastasis and monitor response to treatment.
- Citation: O’Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: An update. World J Radiol 2015; 7(8): 202-211
- URL: https://www.wjgnet.com/1949-8470/full/v7/i8/202.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i8.202
Metastasis of malignant neoplasms to bone is common with metastases being far more prevalent than primary bone malignancies[1,2]. Indeed, bone is the third most common organ affected by metastasis, surpassed only by the lungs and liver[2-4], and is the most common site of distant metastasis from primary breast carcinoma[5].
Over the past twenty years, advances in our understanding of tumour biology have led to the development of improved treatment strategies for many cancers. As a result, many patients are living longer with metastatic disease and the incidence of skeletal metastasis is continuing to rise. Based on post-mortem findings, approximately 70% of patients with breast or prostate cancer have bone metastases[1,4]. Commensurate with the increased prevalence of bone metastasis, there is potential for significant comorbidities such as pain, limited mobility, hypercalcaemia, spinal cord or nerve root compression, myelosuppression and pathologic fracture[2,6]. Therefore, early detection of skeletal metastasis is critical for (1) accurate staging and optimal treatment; and (2) to allow the implementation of treatment strategies such as surgical fixation, radiotherapy, or bisphosphonate therapy to reduce the risk of complications and improve quality of life[7,8].
This paper briefly reviews our current understanding of the biological mechanisms through which tumours metastasise to bone and describes the available imaging methods to diagnose bone metastasis and monitor response to treatment.
Certain primary malignant neoplasms such as breast carcinoma and prostate adenocarcinoma have a propensity for metastasising to bone and are, therefore, termed osteotropic. Conversely, patients with cervical, endometrial, bladder and gastrointestinal tract tumours rarely develop skeletal metastases[9]. The selective deposition and proliferation of discrete circulating malignant cells within the skeleton relates to the “seed and soil” hypothesis of tumour biology originally conceptualised by Stephen Paget in the late 19th century. In accordance with this hypothesis, the bone environment represents a “fertile soil” in which some, but not all, cancer cell types (seeds) can flourish.
Metastasis to bone can occur via direct extension, arterial or venous spread with the latter representing the most common form. Once in the circulation, entry of the cancer cells into the venous circulation of the bone marrow is facilitated by the slow blood flow and the fact that hematopoietically active bone marrow is well vascularised[1]. Adhesion molecules produced by tumour cells bind to marrow stromal cells and bone matrix[8]. The normal remodelling process of bone provides chemotactic and growth factors which support these cancer cells once in place[1]. After skeletal colonisation, the malignant cells interrupt normal bone cell turnover by releasing local cytokines and growth factors. Certain tumours release factors which upregulate osteoclast activity such as parathyroid hormone-related protein, tumour necrosis factor α or β, and other cytokines such as interleukin-1 and interleukin-6 which results in net osteolysis. Other cancer cell types release factors such as epidermal growth factor, transforming growth factor α and β, and insulin-like growth factors which upregulate osteoblasts resulting in net osteosclerosis[8,10]. Thus, osseous metastases can be osteoblastic (bone forming) or osteolytic (bone destructive), however, a combination of both processes occurs in most cancers[4]. Osseus metastases from kidney, thyroid and lung maligancies are predominantly osteolytic, while osteoblastic lesions are usually seen in prostate cancer and breast cancer[7]. Furthermore, osteolytic metastases tend to be aggressive, whereas sclerotic metastases typically demonstrate slower progression. An important point to realise is that tumour cell proliferation within the bone marrow invariably predates bone destruction which is, consequently, a relatively delayed manifestation in bone metastasis which has important implications in terms of diagnosis[6].
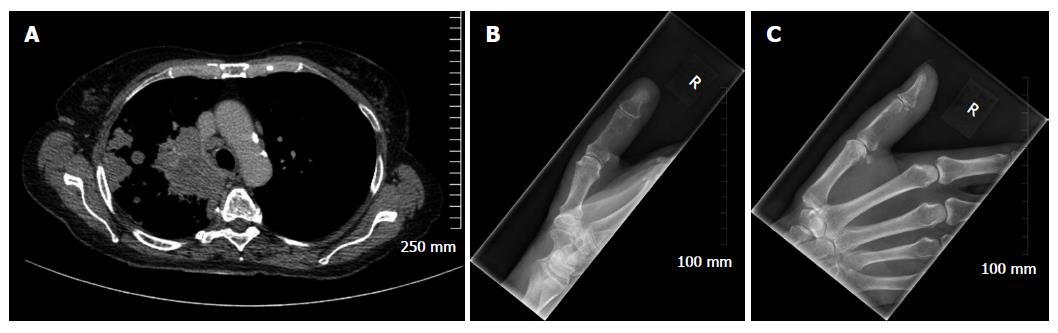
Considering benign osseous lesions and bone metastases oftentimes have similar imaging features, the location of a lesion in the skeleton can sometimes be used to help distinguish between the two in equivocal cases. The vertebrae, pelvis, ribs and the ends of long bones are preferred destinations of metastases because of their high red marrow content[1,9,11]. Within the spine, most metastases are located in the lumbar spine, less frequently in the thoracic spine, and rarely in the cervical spine (52%, 36% and 12% respectively)[12]. Less frequent metastatic sites include the mandible, patella, and dital extremities. In the majority of instances, metastases in the appendicular skeleton are secondary to lung cancer and are typically located in the scaphoid, lunate or phalanges[7] (Figure 1).
Plain radiographs are recommended to assess abnormal radionuclide uptake or the risk of pathological fracture and as initial imaging studies in patients with bone pain[5]. However, radiography is considered insensitive to screen for asymptomatic metastases[9]. Limited contrast in trabecular bone vis a vis cortical bone renders radiographic detection of lesions in the former more difficult and studies have shown that more than 50% to 70% of bone must be destroyed to be reliably detected by plain radiographs[2,7]. Osteolytic lesions typically demonstrate thinning of trabeculae and ill-defined margins with the latter representing abnormal trabeculae between the centre of the lesion and the radiologically normal bone. Conversely, sclerotic metastases classically appear as nodular, rounded and fairly well circumscribed lesions secondary to thickened coarse trabeculae[8].
Skeletal metastases may respond to treatment with reactive new bone formation, or sclerosis. Sclerosis tends to be initiated at the margins of the lesion and progress over time towards the centre. Sclerotic change in an osteolytic metastasis usually indicates a healing response to therapy, whereas worsening or developing osteolysis within sclerotic or mixed lesions, or progressive enlargement of an existing lesion, are indicators of disease progression[7]. Disadvantages of plain film for monitoring treatment response are that (1) typically 3-6 mo are required before any changes manifest radiographically; and (2) plain films only reveal structural bone alterations, and do not provide information on the malignant cells within the metastatic soft tissue deposit. Furthermore, differentiating new sclerotic metastases secondary to disease progression from sclerotic lesions caused by healing and re-ossification is often challenging[3,6].
Computed tomography (CT) provides excellent resolution of cortical and trabecular bone and is the imaging modality of choice for evaluating the ribs which have a high cortex to marrow ratio. The ability to apply dedicated bone algorithms to acquired images, adjust the window width and level, and view the skeleton in multiple planes using multiplanar reformatted images all serve to maximise the conspicuity of bone lesions and results in a higher sensitivity of CT compared to plain radiography in detecting both osteolytic and osteosclerotic metastases. The sensitivity and specificity of CT for detection of bone metastasis is 74% and 56%, respectively (Table 1). A major advantage of CT is that investigation for skeletal metastasis or evaluating treatment response can be performed at the time of staging or restaging other organs which reduces the burden of imaging for the patient. Despite the limited soft tissue resolution of CT vis a vis magnetic resonance imaging (MRI), in many instances, CT can demonstrate bone marrow metastases before bone destruction occurs which results in earlier diagnosis and can improve prognosis and prevent complications[6]. A further advantage of CT is that it can used to guide percutaneous biopsy when a tissue diagnosis is required[7].
Clinical trials have demonstrated a role for CT in evaluating for sclerotic change within a metastatic deposit which can occur in response to treatment of skeletal metastases with chemo/radiotherapy. Specifically, reactive sclerosis may be quantified by calculating the change in Hounsfield units within metastatic deposits following bisphosphonate therapy, thereby providing a valid objective measure of treatment response[3].
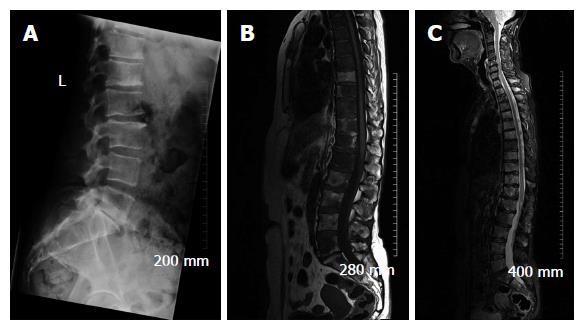
Due to its excellent soft tissue resolution, MRI is the imaging modality of choice for assessing metastatic spread in the marrow cavity, extension of tumour from the marrow cavity and involvement of surrounding structures[5]. Furthermore, MRI is highly sensitive for detecting skeletal metastasis as it has the capability to demonstrate an intramedullary metastatic deposit in advance of cortical destruction occurs and before a pathologic osteoblastic process manifests as focal accumulation of radiotracer on a bone scan (Figure 2)[6,8]. The sensitivity and specificity of MRI for detection of bone metastasis is 95% and 90%, respectively (Table 1). In addition, MRI is the technique of choice in suspected cases of cord compression from pathologic vertebral body fracture where a compromised oedematous spinal cord will demonstrate abnormal focal high T2 and turbo-short tau inversion recovery (STIR) signal. Given that MRI does not involve ionising radiation, it is especially suited for the investigation of suspected bony metastasis in pregnant women.
Normal bone marrow contains a high percentage of fat and demonstrates high signal intensity on T1- weighted sequences. Osseous metastases usually manifest as discrete foci of low T1 signal, corresponding to the replacement of normal fatty marrow by malignant cells. On a T2-weighted sequence, bone metastases usually demonstrate T2 hyperintensity due to their elevated water content and gadolinium enhancement due to increased vascularity[4,7].
The development of whole-body MRI in recent years, which uses fast pulse sequences over multiple anatomic stations to achieve a survey of the entire body, has resulted in the ability to use unenhanced T1-weighted spin echo and STIR sequences to screen the whole body for marrow abnormalities with a sensitivity and specificity superior to skeletal scintigraphy[5-7]. One limitation of MRI is that cortical bone, with its very short T2 relaxation time, is very poorly interrogated. Therefore, bones with a low marrow volume such as the ribs are better evaluated with CT as described above[6].
An advantage of MRI is that it can sometimes be used to distinguish osteoporotic from malignant vertebral compression fractures. Oedema from osteoporotic compression fractures should subside in within 3 mo. If marrow oedema persists on a follow-up MRI study performed at least 12 wk after the initial scan, a pathologic fracture is likely[5], however, this correlation can be inconsistent and determining if marrow signal changes are due to fracture or tumour remains a diagnostic challenge using MRI alone[4].
MRI can be used to assess treatment response by evaluating the size and number of osseous metastases over time. It is important to note, however, that alteration in signal intensity alone on a T1-weighted sequence does not constitute a response to therapy. Recent studies suggest that quantitative diffusion weighted imaging (DWI) can be used to evaluate treatment response before a change in the tumour burden can be seen using non quantitative assessment. More specifically, early reduction in tumour cell volume following cell death with a corresponding increase in the extracellular space is manifested on DWI as an increase in the apparent diffusion coefficient (ADC) value of the metastatic deposit[6]. However, further studies are needed to define the precise imaging criteria, for example T1 and DWI signal characteristics and/or percentage signal change pre and post contrast, which should be used to evaluate the treatment response[3].
Morphological imaging techniques such as plain film, CT and MRI described above interrogate the structure of a lesion within bone. Conversely, nuclear medicine techniques quantitatively assess the function of bone or tumour cells[6]. Prior to describing the role of the nuclear medicine imaging modalities most commonly used for imaging skeletal metastases, it is pertinent to briefly review the various radioisotopes that are employed in these studies. For more comprehensive coverage of this topic the reader is referred to the recent review by Cuccurullo et al[2].
Osteotropic radioisotopes are bone seeking agents that accumulate at the site of active bone production regardless of whether the aetiology is benign or malignant. The predominant osteotropic agents used in skeletal scintigraphy are metastable technetium 99 labelled diphosphonates, among which methylene diphosphonate (99mTc-MDP) is used most commonly based on its effectiveness, low cost, widespread availability and favourable dosimetry. 18Flabelled sodium fluoride (NaF) is an osteotropic compound used in positron emission tomography (PET) which has a higher first pass extraction rate than 99mTc-MDP. Indeed, studies indicate that the regional extraction of 18F NaF from plasma to bone is on average approximately three times higher in metastatic lesions than in adjacent normal bone tissue. Consequently, 18F NaF has very high selectivity for bone metastases, however its relatively low specificity when not used in conjunction with morphological imaging techniques (see hybrid imaging below) and the requirement of a cyclotron for production are limiting factors in its use[2].
In contrast to osteotropic agents, which have a high affinity for calcium, oncotropic radioisotopes demonstrate uptake into malignant cells and are classified as either specific or non-specific. Specific oncotropic agents are available to investigate for bone metastases from neuroendocrine tumours. For example, metaiodobenzylguanidine is a noradrenaline analogue, taken up specifically by the sympathetic nervous system and related tumours. When labelled with Iodine 123 or Iodine 131 it may detect bone metastases from pheochromocytomas and paragangliomas. In addition, somatostatin receptor scintigraphy with Indium 111 pentetreotide (octreoscan) and PET-CT using Gallium 68 labelled somatostatin analogues can be used to diagnose both organ confined and metastatic neuroendocrine malignancies. Further information regarding available specific oncotropic tracers can be found on the Molecular Imaging and Contrast Agent Database http://www.ncbi.nlm.nih.gov details. The most commonly used non-specific oncotropic radioisotope is the glucose analogue 18F labelledfluorodeoxyglucose (18F FDG). Uptake of 18F FDG occurs in cells with increased glucose metabolism such as neurons and mitotic neoplastic cells. Therefore, similar to osteotropic compounds, and as their name suggests, non-specific oncotropic radioisotopes are sensitive but not specific for skeletal metastasis.
Bone scintigraphy continues to be the most widely used radionuclide technique for investigation of skeletal metastasis primarily due to its widespread availability[2]. Radiotracer uptake depends on local blood flow, osteoblastic activity and extraction efficiency. Once accumulated in bone diphosphonates are absorbed by hydroxyapatite crystals on mineralizing bone surfaces[13].
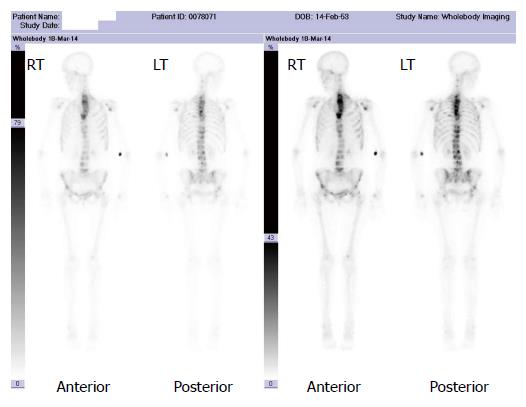
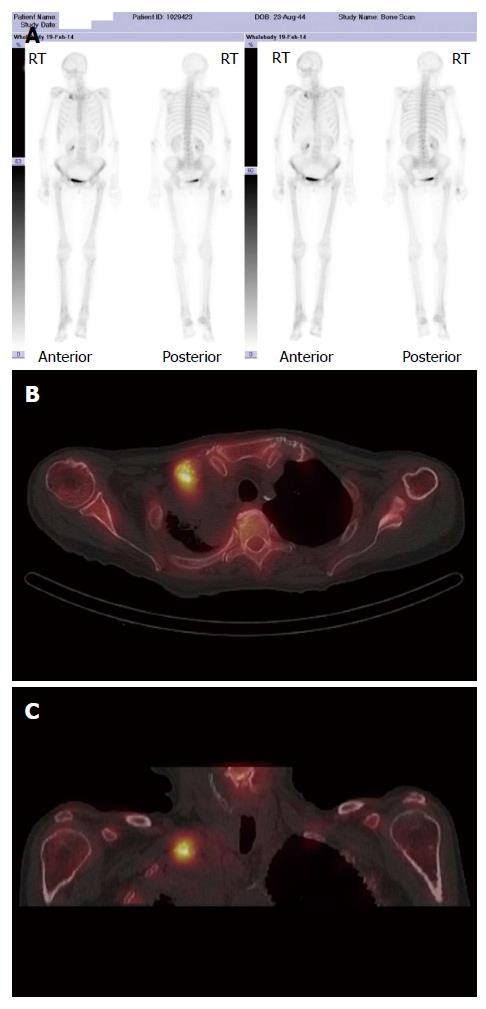
A major advantage of radionuclide bone scanning is that imaging of the whole skeleton can be performed (Figure 3). This is important given that metastatic lesions can occur in regions of the appendicular skeleton that are not routinely included in a skeletal survey[9]. A further advantage relates the high sensitivity of scintigraphy which enables earlier detection of osseous metastases. The sensitivity and specificity of bone scintigraphy for detection of bone metastasis is 78% and 48%, respectively (Table 1). In particular, studies indicate that only a 5%-10% alteration in the ratio of lesion to normal bone is necessary to manifest abnormal tracer accumulation on a bone scan. As a result, osteosclerotic bone metastases can be detected on bone scintigraphy up to 18 mo earlier than on plain radiographs[7].
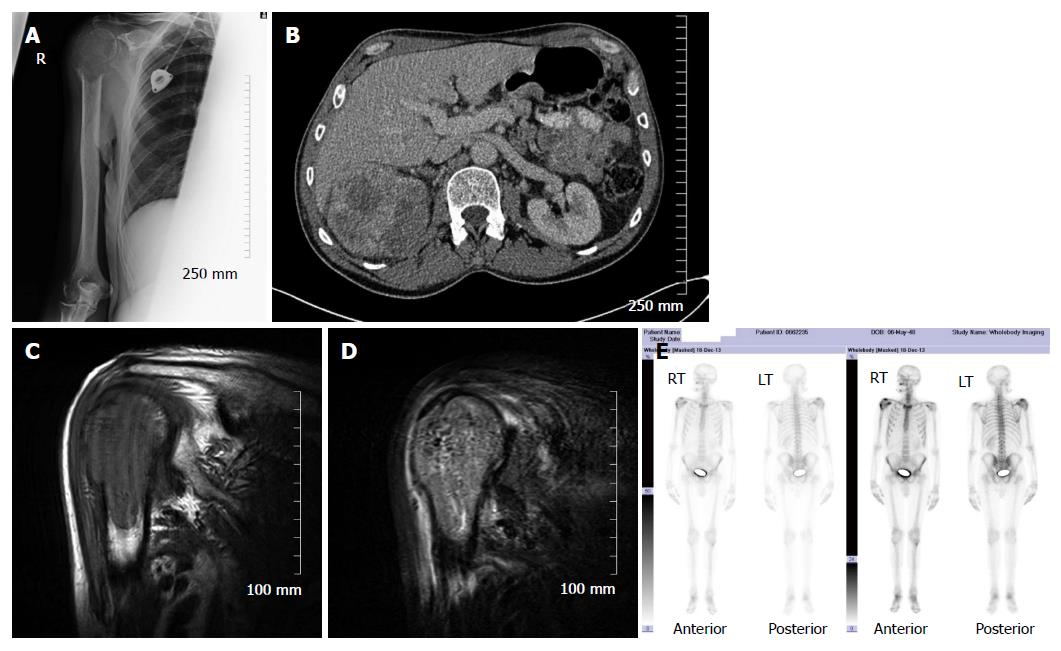
Skeletal scintigraphy has some notable limitations. For example, bone scintigraphy is non-specific and multiple benign osseous lesions, such as eosinophilic granuloma fibrous dysplasia and enchondroma, can lead to a false positive diagnosis of bone metastasis[14]. Interpreting focal accumulation of radiotracer in the spine can be particularly problematic as degenerative disease may be indistinguishable from bone metastases. Consequently, other imaging modalities such as plain radiography, CT or MRI are often required for correlation to exclude benign causes[8]. Secondly, the spatial resolution of scintigraphy is poor measuring approximately 1 cm and can result in difficulty determining the precise location of a lesion within a bone which can be of diagnostic significance[2]. Thirdly, bone scintigraphy assesses osteoblastic processes rather than tumour proliferation and, consequently, false negative results can occur[8]. Furthermore, primarily osteolytic lesions with limited reactive osteoblastic reaction, such as renal cell carcinoma metastases, typically demonstrate low or absent tracer accumulation leading to a false negative result (Figure 4)[6]. Finally, when bone metastases are extensive and diffuse, a bone scan on first inspection may appear normal due to the confluent nature of the lesions (referred to as a super scan because of the apparent good quality of the scan) and can be misinterpreted as a negative study[9,13]. It is therefore import to carefully assess for uptake in the kidneys on skeletal scintigraphy indicative of renal excretion of radiotracer which is characteristically absent on a super scan.
Certain clues and techniques can help to determine if focal uptake of radiotracer is secondary to a benign osseous lesion or metastasis. For example, vertebral body fractures have a characteristic appearance on bone scintigraphy, showing a horizontal linear pattern of increased tracer accumulation. Multiple linear abnormalities of varying intensity favour a benign aetiology with presumed osteoporotic fracture occurring at different time points. In addition, a short interval follow-up scan that shows reducing activity at a vertebral fracture site suggests a benign aetiology and a healing fracture. Secondly, lesions that extend from the vertebral body into the posterior vertebral elements or involve the pedicle are more likely to represent metastases[13]. Finally, linear uptake of radiotracer in contiguous ribs is highly suggestive of trauma and not metastasis.
Bone metastases responding to treatment will demonstrate reduced or absent radiotracer uptake when compared with the pretreatment scan[6]. It is important to recognise, however, that early in the course of treatment a flare response can occur, which is characterized by a transient elevation in radiotracer accumulation secondary to the stimulation of osteoblasts during the repair process which can be misinterpreted as treatment failure, as it can have an imaging appearance indistinguishable from disease progression[7]. The flare response is most commonly associated with hormone based therapies and may last for up to 6 mo after therapy[13]. Progression of disease is suggested when new deposits develop or there is an interval increase in the is activityor size of existing deposits[3].
Single photon emission CT (SPECT) imaging of the skeleton uses 99mTc-MDP, the same radionuclide used in conventional skeletal scintigraphy, however images are acquired in a cross-sectional rather than a planar fashion. Whereas planar imaging is limited by superimposition of structures, SPECT can show axial slices through the body, providing better localisation of abnormal radionuclide uptake[5,7]. The sensitivity and specificity of SPECT for detection of bone metastasis is 87% and 91%, respectively (Table 1). A limitation of SPECT when compared with other available nuclear medicine technique is an inability to generate absolute quantification values[6].
PET is a nuclear medicine technique that produces high-resolution tomographic images through the detection of high-energy photon pairs emitted during positron decay of a radioisotope. PET is superior to conventional bone scanning in terms of spatial resolution. For skeletal metastases, 18F NaF or 18F FDG are the radiopharmaceuticals most frequently employed[7].
The uptake mechanism of 18F NaF is similar to that of 99mTc-MDP. Specifically, following diffusion through the capillary wall into the extracellular fluid, fluoride ions undergo gradual exchange with the hydroxyl groups of hydroxy-apatite crystal within bone to form fluoro-apatite and subsequently deposited primarily on the surface of bone where re-modelling is maximal. Therefore, 18F NaF-PET demonstrates radiotracer accumulation at foci of osteoblastic activity[6,7]. The available literature indicates that 18F NaF-PET is substantially more sensitive and specific than skeletal scintigraphy and SPECT for detection of metastases, particularly for osteolytic lesions[4,15]. In addition, comparative studies have demonstrated that 18F NaF-PET demonstrates higher sensitivity for detection of bone lesions when compared with 18F FDG-PET[8].
18F FDG-PET is a functional rather than anatomic imaging method that detects cellular metabolism of a glucose analogue. Many radiopharmaceuticals are available that can be imaged with PET, but 18F FDG is commonly used in oncology because of the high glucose uptake by many tumours[5]. Accumulation of 18F FDG is predominantly related to the amount of viable tumour cells. However, the sensitivity of 18F FDG-PET may vary among different histologies[4]. For example, it has been established that certain well-differentiated and indolent tumours, such as neuroendocrine and bronchial tumours, go undetected by 18F FDG because of the poor 18F FDG accumulation. Furthermore, in patients with primarily osteosclerotic metastases from prostate cancer, 18F FDG-PET has reduced sensitivity for the detection of skeletal metastases compared with 99mTc-MDP scintigraphy[6]. This is due to the reduced metabolic activity in sclerotic bone metastases. The sensitivity and specificity of 18F FDG-PET for detection of bone metastasis is 98% and 56%, respectively (Table 1).
A major advantage of 18F FDG-PET is the ability to compare the maximum standardised uptake value of a metastatic skeletal deposit between studies which provides an objective measure of the response to treatment. However, similar to skeletal scintigraphy, a potential limitation of 18F FDG-PET in assessing the treatmentresponse of metastatic bone disease is the flare phenomenon (described above) which may be seen after hormone therapy, which can be challenging to distinguish from bone marrow replacement by malignant cells, and result in false positive findings[3,6].
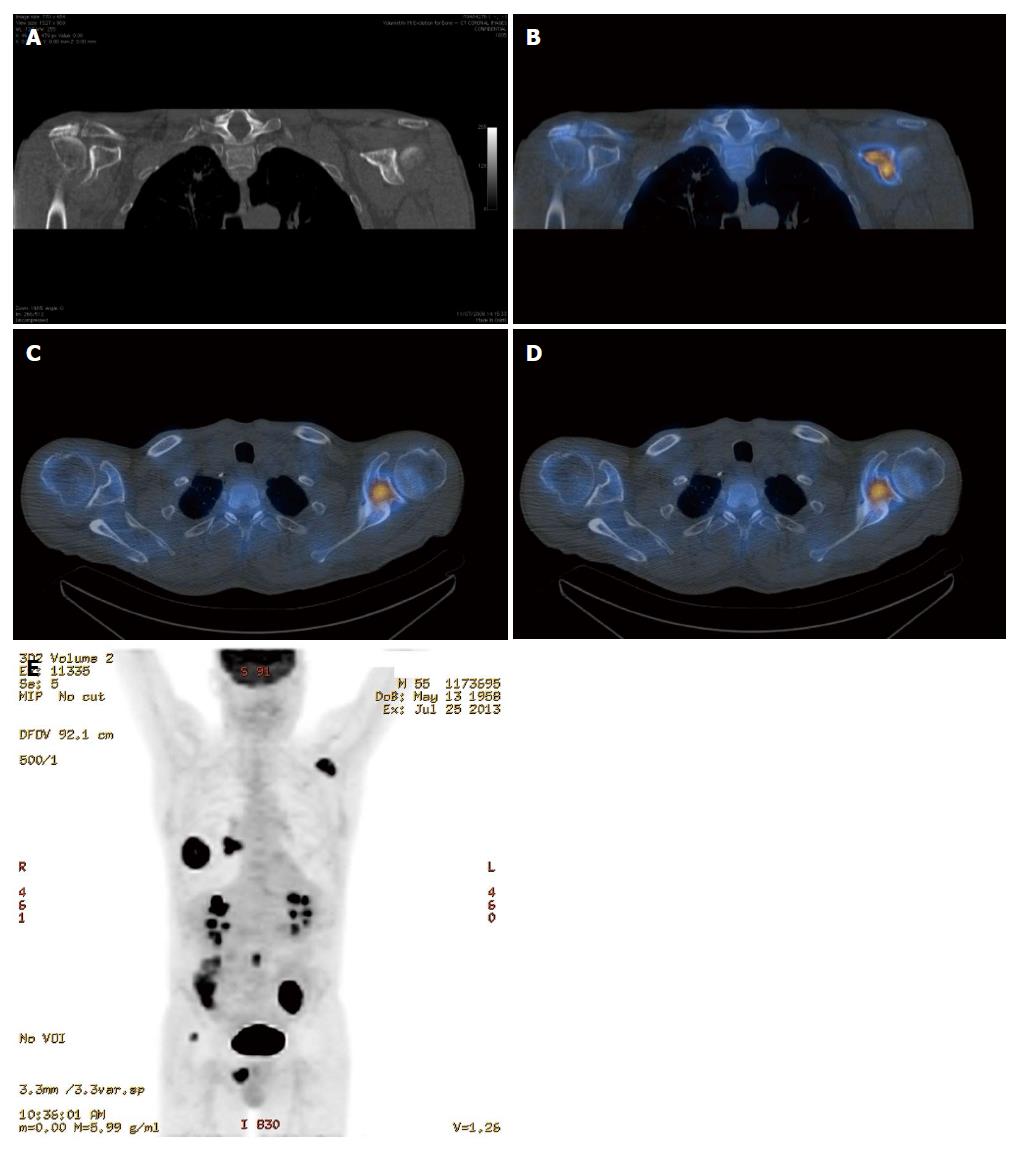
It is clear from the preceding sections that the various imaging modalities traditionally used to investigate skeletal metastasis have idiosyncratic strengths and weaknesses. For example, an alteration in the structure of bone in response to treatment may be well demonstrated on CT, whereas tumour cell response is usually best evaluated using PET[6]. It is intuitive, therefore, that combining imaging modalities can increase sensitivity and specificity to improve diagnostic accuracy. The sensitivity and specificity of 18F NaF-PET/CT for detection of bone metastasis is 100% and 97%, respectively (Table 1). Indeed, technological advances have enabled the development of hybrid imaging techniques including SPECT/CT, PET/CT (Figures 5 and 6) and, more recently, PET/MRI. These techniques are (semi-) quantitative providing a standardized uptake value and allow the fusion of anatomic data from cross sectional imaging with functional information from nuclear medicine studies. As a result, the radiologist can determine if focal radiotracer uptake on a nuclear medicine study corresponds to a discrete skeletal lesion. Similarly, diagnostic confidence increases when an osseous lesion suspicious for metastasis on cross sectional imaging avidly accumulates radiotracer. A recent meta-analysis by Liu et al[16] found that 18F FDG-PET was the best modality to detect bone metastasis in patients with lung cancer, both on a per-patient and per-lesion basis while MRI had the highest specificity on a per-lesion basis. Furthermore, PET/CT was shown to be better than PET alone.
The highest potential for early diagnosis of skeletal metastasis should, therefore, involve a combination of MRI and PET. To our knowledge, there is currently no published article comparing the accuracy of PET/CT and PET/MRI in diagnosing skeletal metastases and work in this area is warranted. One disadvantage of the hybrid imaging techniques involving CT is the radiation dose incurred by the patient, with a typical effective dose of approximately 22 mSv[5]. A low dose CT protocol can be used without significantly affecting the improved spatial localisation afforded by PET/CT vs PET alone, however, much of the precise anatomic detail is lost. Recent improvements in iterative reconstruction techniques are enabling low dose image acquisition while maintaining excellent contrast resolution and continued progress in this regard is likely.
In this overview of imaging skeletal metastasis, it seems appropriate to briefly highlight experimental imaging strategies currently being explored that may influence the future of oncologic imaging.
Optical imaging techniques which involve transgenic expression of bioluminescent or fluorescent proteins in cancer cell lines are yielding novel information on how tumour cells invade, spread, proliferate and respond to treatment in small animal models of bone metastasis[17,18]. While such advances are critical to advancing our understanding of tumour biology, it will likely take many years before the results of this research manifest clinically.
Imaging research focused on tumour stimulated angiogenesis may well lead to improvements in imaging skeletal metastasis in the near future. Vascularity of osseous metastases can be visualised by cross sectional imaging and quantitative data obtained. Specifically, dynamic contrast-enhanced (DCE) MRI or CT can be employed to quantify variables in tissue vascularity, such as blood volume and perfusion. DCE imaging can be achieved by sequentially imaging the distribution of a systemically administered contrast agent producing imaging biomarkers that which can then be used to evaluate the response of a tumour to therapies designed to inhibit angiogenesis. Using this approach, potential treatment responses can be detected at an early stage using MRI and CT, before a change in the tumour volume can be reliably detected[6]. Therefore, DCE will likely continue to develop as a sensitive method to evaluate early tumour response.
The availability of improved chemotherapy regimens for many cancers together with a more aggressive approach by surgical oncologists means that many patients are now living longer with metastatic disease. Prolonged survival of patients with cancer results in a greater likelihood of developing distant metastasis which has, in turn, led to a higher prevalence of skeletal metastasis[19]. In line with these changes, considerable advances in imaging technology have enabled more reliable evaluation of bone metastases and treatment response. Among the various imaging modalities currently available for imaging skeletal metastasis, hybrid techniques which fuse morphological and functional data are the most sensitive and specific, and PET/CT and PET/MRI will almost certainly continue to evolve and become increasingly important in this regard. At present, however, no single imaging strategy is consistently superior for the assessment of metastatic bone disease across all tumour types and clinical scenarios[9]. The future of imaging bone metastasis will likely involve the development of an array of new radiotracers which will be tumour specific and greatly increase diagnostic accuracy.
P- Reviewer: Boy C, Kara PO, Sakamoto A S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Cuccurullo V, Cascini GL, Tamburrini O, Rotondo A, Mansi L. Bone metastases radiopharmaceuticals: an overview. Curr Radiopharm. 2013;6:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 3. | Vassiliou V, Andreopoulos D, Frangos S, Tselis N, Giannopoulou E, Lutz S. Bone metastases: assessment of therapeutic response through radiological and nuclear medicine imaging modalities. Clin Oncol (R Coll Radiol). 2011;23:632-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Yu HH, Tsai YY, Hoffe SE. Overview of diagnosis and management of metastatic disease to bone. Cancer Control. 2012;19:84-91. [PubMed] |
| 5. | Costelloe CM, Rohren EM, Madewell JE, Hamaoka T, Theriault RL, Yu TK, Lewis VO, Ma J, Stafford RJ, Tari AM. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Bäuerle T, Semmler W. Imaging response to systemic therapy for bone metastases. Eur Radiol. 2009;19:2495-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Choi J, Raghavan M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer Control. 2012;19:102-112. [PubMed] |
| 8. | Rajarubendra N, Bolton D, Lawrentschuk N. Diagnosis of bone metastases in urological malignancies--an update. Urology. 2010;76:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Roberts CC, Daffner RH, Weissman BN, Bancroft L, Bennett DL, Blebea JS, Bruno MA, Fries IB, Germano IM, Holly L. ACR appropriateness criteria on metastatic bone disease. J Am Coll Radiol. 2010;7:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Longo V, Brunetti O, D’Oronzo S, Ostuni C, Gatti P, Silvestris F. Bone metastases in hepatocellular carcinoma: an emerging issue. Cancer Metastasis Rev. 2014;33:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Saha S, Burke C, Desai A, Vijayanathan S, Gnanasegaran G. SPECT-CT: applications in musculoskeletal radiology. Br J Radiol. 2013;86:20120519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Talbot JN, Paycha F, Balogova S. Diagnosis of bone metastasis: recent comparative studies of imaging modalities. Q J Nucl Med Mol Imaging. 2011;55:374-410. [PubMed] |
| 13. | Gnanasegaran G, Cook G, Adamson K, Fogelman I. Patterns, variants, artifacts, and pitfalls in conventional radionuclide bone imaging and SPECT/CT. Semin Nucl Med. 2009;39:380-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Zhao C, Liu H, Hou H, Zhang H. Multiple metastasis-like bone lesions in scintigraphic imaging. J Biomed Biotechnol. 2012;2012:957364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Liu T, Xu JY, Xu W, Bai YR, Yan WL, Yang HL. Fluorine-18 deoxyglucose positron emission tomography, magnetic resonance imaging and bone scintigraphy for the diagnosis of bone metastases in patients with lung cancer: which one is the best?--a meta-analysis. Clin Oncol (R Coll Radiol). 2011;23:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Kaijzel EL, Snoeks TJ, Buijs JT, van der Pluijm G, Löwik CW. Multimodal imaging and treatment of bone metastasis. Clin Exp Metastasis. 2009;26:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Snoeks TJ, Khmelinskii A, Lelieveldt BP, Kaijzel EL, Löwik CW. Optical advances in skeletal imaging applied to bone metastases. Bone. 2011;48:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, Litsas G, McKay R, Podoloff DA, Srinivas S. NCCN Task Force Report: Bone Health In Cancer Care. J Natl Compr Canc Netw. 2013;11 Suppl 3:S1-S50; quiz S51. [PubMed] |