Published online Dec 28, 2015. doi: 10.4329/wjr.v7.i12.424
Peer-review started: May 20, 2015
First decision: September 8, 2015
Revised: September 15, 2015
Accepted: November 13, 2015
Article in press: November 17, 2015
Published online: December 28, 2015
Processing time: 226 Days and 11 Hours
Pancreatic cancer is one of the most common malignant tumors and remains a treatment-refractory cancer with a poor prognosis. Currently, the diagnosis of pancreatic neoplasm depends mainly on imaging and which methods are conducive to detecting small lesions. Compared to the other techniques, magnetic resonance imaging (MRI) has irreplaceable advantages and can provide valuable information unattainable with other noninvasive or minimally invasive imaging techniques. Advances in MR hardware and pulse sequence design have particularly improved the quality and robustness of MRI of the pancreas. Diffusion MR imaging serves as one of the common functional MRI techniques and is the only technique that can be used to reflect the diffusion movement of water molecules in vivo. It is generally known that diffusion properties depend on the characterization of intrinsic features of tissue microdynamics and microstructure. With the improvement of the diffusion models, diffusion MR imaging techniques are increasingly varied, from the simplest and most commonly used technique to the more complex. In this review, the various diffusion MRI techniques for pancreatic cancer are discussed, including conventional diffusion weighted imaging (DWI), multi-b DWI based on intra-voxel incoherent motion theory, diffusion tensor imaging and diffusion kurtosis imaging. The principles, main parameters, advantages and limitations of these techniques, as well as future directions for pancreatic diffusion imaging are also discussed.
Core tip: Magnetic resonance imaging (MRI) has irreplaceable advantages and can provide valuable information unattainable with other noninvasive or minimally invasive imaging techniques. Diffusion MR imaging serves as one of the common functional MRI techniques and is the only technique that can be used to reflect the diffusion movement of water molecules in vivo. In this review, the various diffusion MR imaging techniques for pancreatic cancer will be discussed, including conventional diffusion weighted imaging (DWI), multi-b DWI based on intra-voxel incoherent motion theory, diffusion tensor imaging and diffusion kurtosis imaging.
- Citation: Tang MY, Zhang XM, Chen TW, Huang XH. Various diffusion magnetic resonance imaging techniques for pancreatic cancer. World J Radiol 2015; 7(12): 424-437
- URL: https://www.wjgnet.com/1949-8470/full/v7/i12/424.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i12.424
Pancreatic cancer is one of the most common malignant tumors with a poor prognosis, of which the 5-year survival rate range is no more than 5%[1] and as low as 0.4% to 2%[2,3]. It is reported that there has been little improvement in survival rate over the past 30 years[4]. Because the pancreas is deep-seated, there is a lack of apparent symptoms in early pancreatic cancer. In most cases, the tumor is diagnosed at an advanced stage, at which point, it does not benefit from radical surgery[2]. The management of pancreatic cancer is still encountered as a significant and unresolved therapeutic challenge.
Currently, the diagnosis of pancreatic neoplasm depends mainly on the imaging, and which methods can be conducive to detecting small lesions. Despite the continuing advances in diagnostic techniques, the early precise diagnosis of pancreatic cancer remains unsatisfactory. Early detection followed by surgical resection offers hope for a cure and is the key to improving pancreatic cancer survival[5]. Unfortunately, only 20% of patients are resectable at the time of diagnosis[6]. Computed tomography (CT), Magnetic resonance imaging (MRI), transabdominal and endoscopic ultrasonography (US and EUS) and endoscopic retrograde cholangiopancreatography (ERCP) also play an important role in the diagnosis of pancreatic cancer[7-10]. Among them, MRI has irreplaceable advantages, especially, the advances in MR hardware and pulse sequence design that have improved the quality and robustness of MRI of the pancreas. Today, MRI is an indispensable tool for pancreatic disorders and can provide valuable information unattainable with other noninvasive or minimally invasive imaging techniques[11-13].
With the rapid development of the MRI, diffusion MR imaging, which is based on the microscopic mobility of water molecules in the tissues without contrast administration, is a promising technique that is widely applied in clinical practice. Diffusion MR imaging is also the only available method that can measure the diffusion properties of tissues noninvasively and quantitatively[14,15], such as the diffusion weighted imaging (DWI), and has been helpful for the detection and characterization of pancreatic conditions[16,17]. The DWI technique serves as an excellent adjunct to routine abdominal MR imaging[13], is noninvasive in contrast to EUS and ERCP, and does not employ ionizing radiation like CT[18].
The changes in the composition and/or cellularity of tissues influences the random thermal diffusion of water molecules[19]. Compared to normal pancreatic tissue, pancreatic cancer has a higher cell density, relatively smaller extracellular space and a different blood supply. Thus, the diffusion of molecules in the cancer would be different from that in the normal pancreatic tissue. DWI, one of the functional MRI techniques based on water molecule movement, can depict this change in diffusion and can quantitatively measure the parameters that can represent these diffusion properties. Thus, DWI can reflect biologic abnormalities at an early stage[20].
In this review, the various diffusion MR imaging techniques for pancreatic cancer will be discussed, including DWI, multi-b DWI based on intra-voxel incoherent motion (IVIM) theory, diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI). The principles, main parameters, advantages and limitations of each technique and the future directions for pancreatic diffusion imaging will also be discussed.
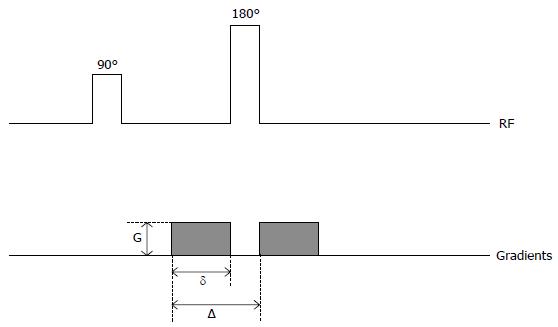
Single-shot spin-echo echo-planar (SE-EPI) sequence is the most widely applied in the DW MR imaging (Figure 1). Conventional DWI uses the 2 motion-probing bipolar gradients in 3 directions (x, y, z) and acquires the signal from the 3 directions. The final DW image is derived from the fusion of the 3 images. DWI exploits the random motion of water molecules in biologic tissues. The water molecules diffuse in 3 different compartments: The intracellular, the intravascular, and the interstitial compartment. The diffusion of water molecules depends on the interactions with cell membranes, tissue compartments, and intracellular content[21]. Consequently, the diffusion of water in tissues reflects, to various degrees, a combination of tissue cellularity, tortuosity of extracellular spaces, integrity of cell membranes, and viscosity of fluids[22].
DWI was originally described for the central nervous system[23,24], which is particularly good for the diagnosis of ischemic stroke. In recent years, DWI has presented promising results in the diagnosis of some illnesses of the lower abdomen, such as those of the prostate[25,26]. DWI of the upper abdomen has been a technical challenge due to respiration, bowel peristalsis, blood flow and long acquisition times[18]. The implementation of ultrafast imaging techniques, such as parallel imaging, has made DWI (a combination of pulses and strong gradients) of the upper abdomen a feasible option. It has also been found to be useful in the differentiation of malignancy from benign liver lesions[27,28].
The three main parameters in the DWI are D value, b value and apparent diffusion coefficient (ADC) value. The D value is the diffusion parameter representing the free molecular diffusion and is defined as the average displacement by molecules in a certain direction, per unit of time. The D value can be affected by a variety of physiological factors, including respiration, perfusion, pulse and movement. The b value is referred to as the gradient factor, which can reflect the effect of the diffusion gradient. In the conventional DWI, the various b values can be selected. The low b value is applied more in water molecules with rapid movement or long diffusion distance, but the high b value is applied more in water molecules with slow movement or a short diffusion distance. Thus, the high b value is good for reducing the effect of the movement of water molecules due to perfusion[29].
In vivo, there are many factors that can affect the diffusion movement of water molecules, including the b value, D value and T2 shine-through effect. The T2 shine- through effect occurs when tissue with a long T2 relaxation time is characterized by hyperintensity on DWI. The ADC results in standardizing by considering the above factors and would be used to reflect the state of diffusion of water molecules in vivo.
DWI, which can be used for the qualitative and quantitative assessment of tissue diffusivity, can be routinely applied in clinical practice[16]. Recent studies indicate that DWI is also promising in pancreatic imaging[30-34]. These popular research studies also reflect the value of DWI in the diagnosis of pancreatic cancer. Moreover, compared to the other techniques, the sensitivity and specificity for the diagnosis of pancreatic cancer is valuable. Kartalis et al[18] conducted research on the value of DWI for pancreatic cancer, and their results showed that the qualitative DWI of pancreatic cancer has an accuracy of 96%; further, DWI has been shown to have high sensitivity (92%) and specificity (97%), consistent with the findings of Ichikawa et al[30] (96.2% and 98.6%, respectively). Furthermore, a recent study shows that the addition of DWI to conventional MR imaging improves the sensitivity of cancer detection[35]. The sensitivity of DWI was close to that of dynamic gadolinium-enhanced MRI (97.7%), with a higher specificity (85.1%)[36]. Compared to the multidetector CT, positron emission tomography with CT and transabdominal ultrasound, the sensitivity and specificity of DWI are higher[18,37,38]. Although the sensitivity of EUS can reach 100%, it is invasive and has only 50% specificity[39]. Thus, it does not have wide application in clinical practice.
DWI can provide information regarding the cellular density and properties of the extracellular matrix[40,41]. The ADC values seem to reflect not only the underlying tissue microstructure but also the undirected movement of particles in thecapillaries[42]. The ADC value has been shown to be able to serve as a marker of cellularity[41,43]. The ADC value in the normal pancreas has been reported to range from 1.0 to 2.0 × 10-3 mm2/s[44]. Many studies have shown that the ADC value of pancreatic cancer is lower than that of the normal pancreas[13,31,45-52]. There are three reasons that may explain these results[53]. First, tumor cell growth is rapid with high cellularity. Second, tumor cell atypia and the richness of organelles are positively correlated in the pancreatic tumor cells, and the nucleus and organelles are bulkier than that of normal pancreas cells. Thus, to some extent, tumor cell growth may limit the diffusion of water molecules. Third, the decrease of extracellular space from dense cellularity and extracellular fibrosis may also account for the restricted water diffusion[13,20,54-56]. However, Wang et al[54] reported that there is no significant difference in the ADC value between the normal pancreas and pancreatic cancer. These different results were most likely due to the application of different DWI experimental protocols and processing means[57]. The most important factor is the choice of b value. Kim et al[58] conducted research to determine the effect of the magnitude of b values on the ADC. Their results showed that the calculated ADC value could be affected by the magnitude of the maximum b value and that the higher the maximum b value, the lower the ADC value.
Additionally, the diverse differentiation of pancreatic cancer can be differentiated using DWI. For example, poorly differentiated adenocarcinoma had significantly lower ADCs than those of well/moderately differentiated adenocarcinomas[59].
Recently, DWI techniques have been shown not to be uniform, this controversial conclusion needs further study and differences in the sequence parameters and b values chosen may affect the ADC results. Future prospective studies are required to better determine the most appropriate use of the b value of pancreatic disease. Comparing different b values in a larger series of patients with malignant lesions would probably be of value; the quantification of the ADC of various lesions will be more accurate is feasible b values are used. The ADC values may have considerable overlap between the benign and malignant lesions, indicating that qualitative DWI seems to be more accurate than the quantitative analysis and can be used as an accurate method for the detection of pancreatic cancer[18].
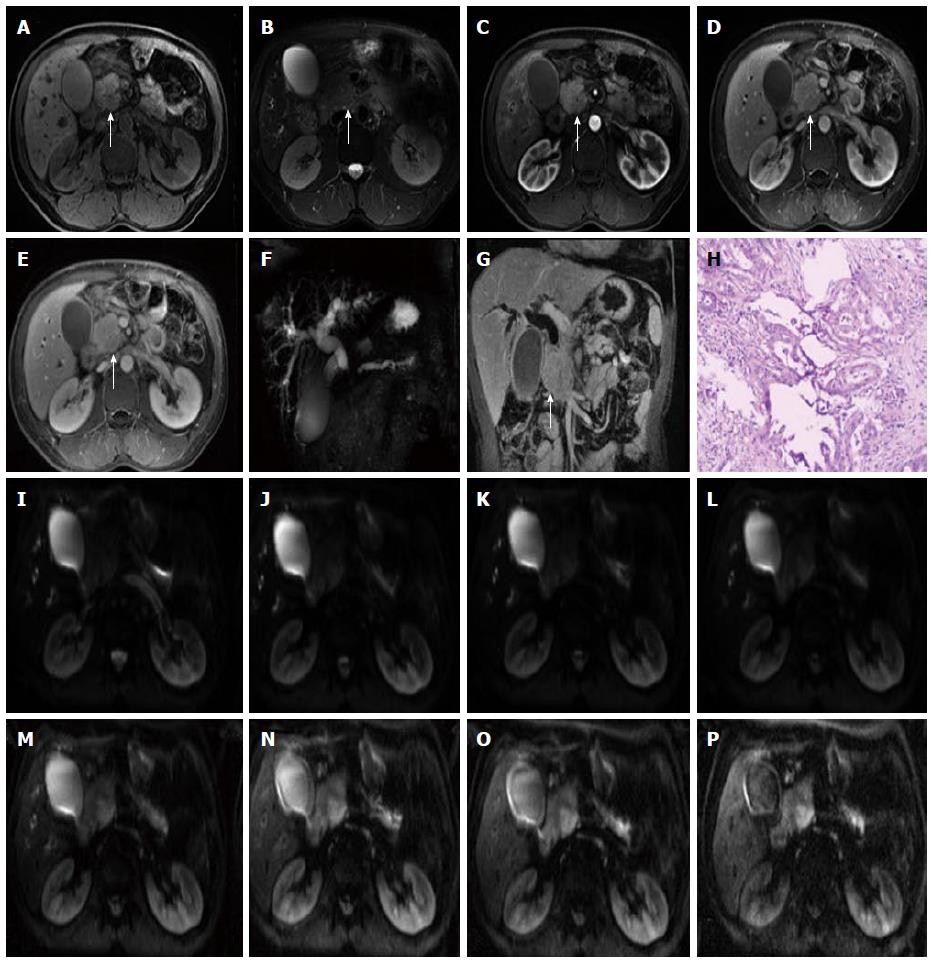
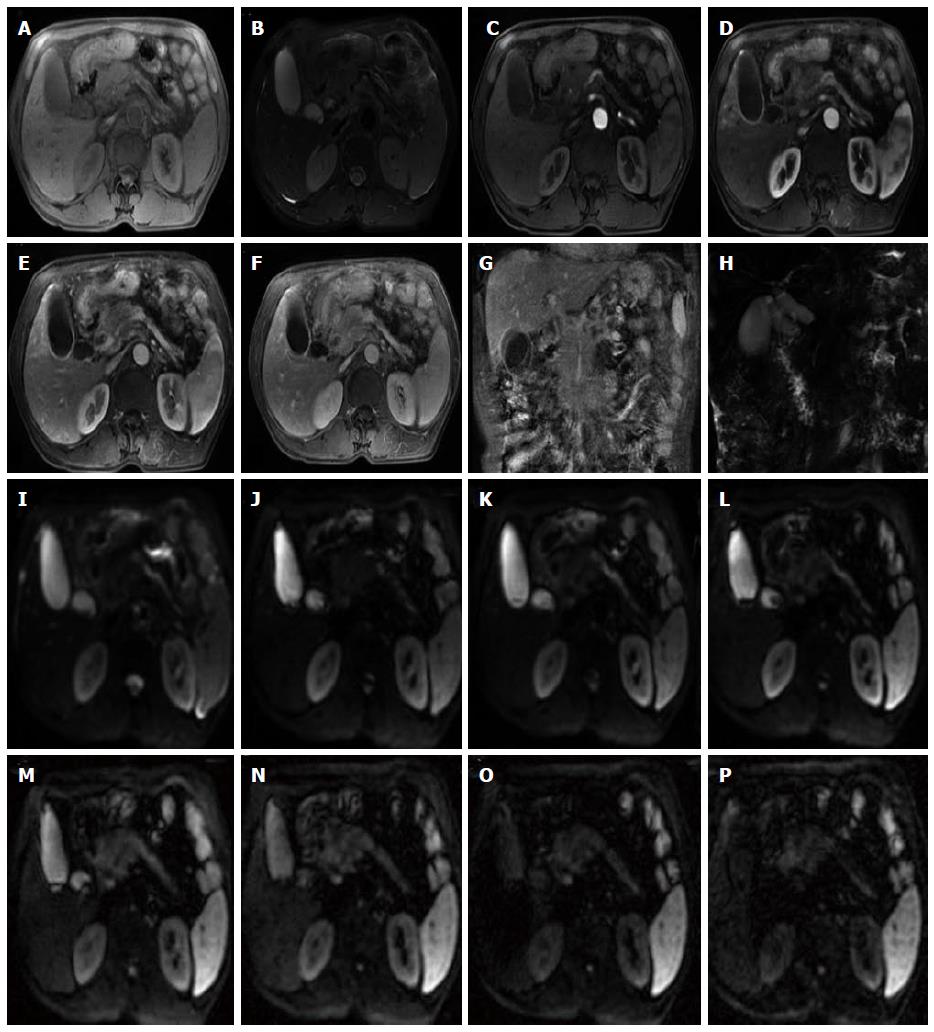
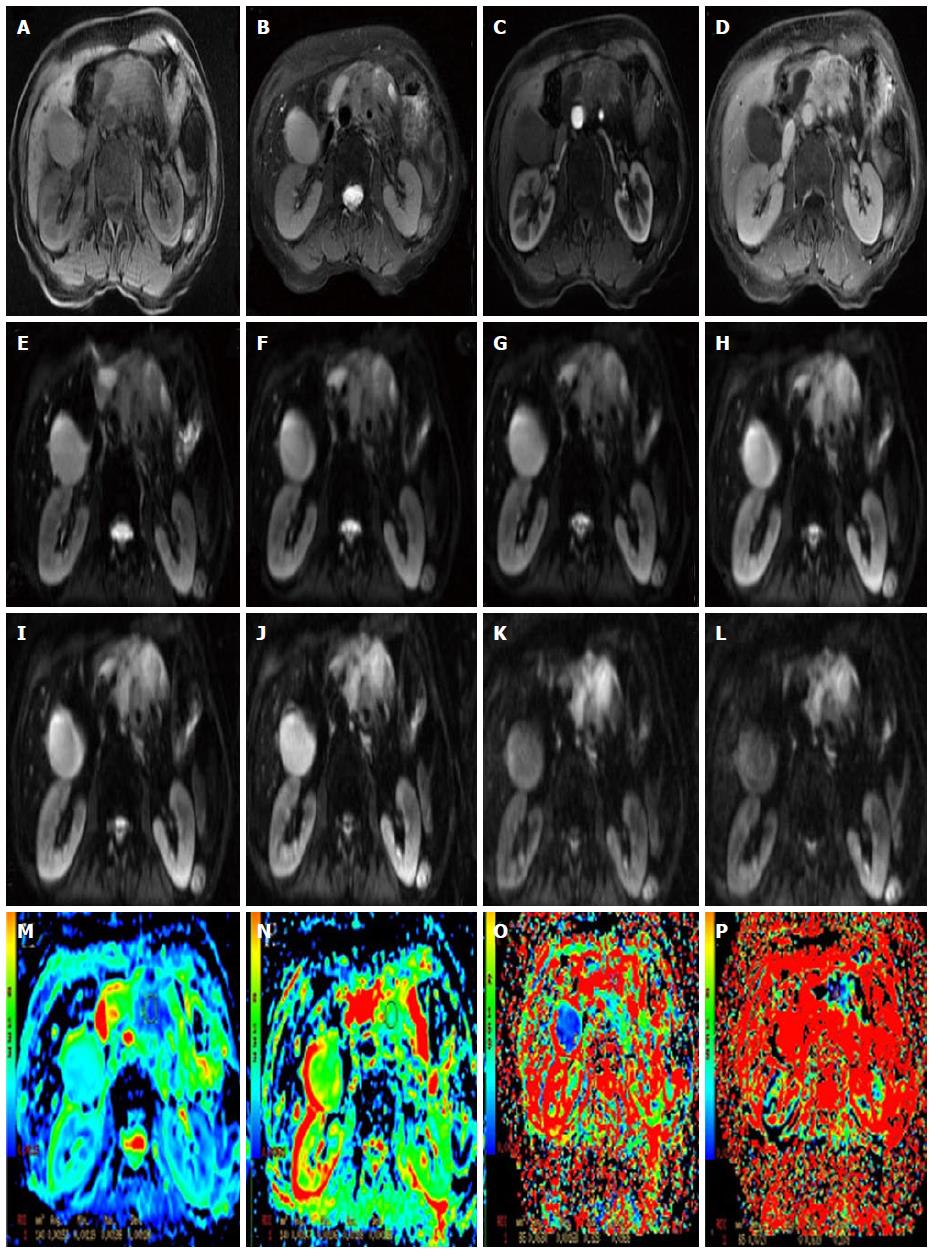
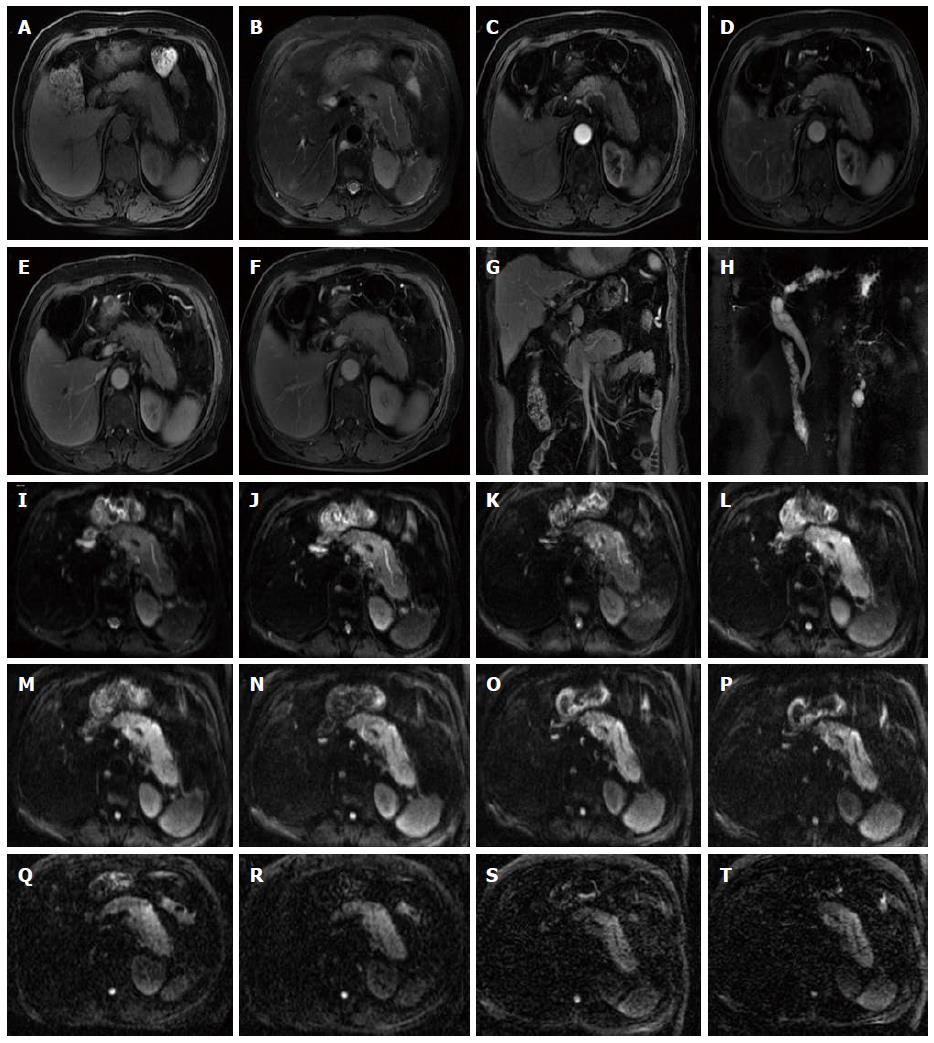
The rapid development of DWI monoexponential and biexponential models, of which the theoretical basis of the techniques is that the diffusion of water molecules is characterized by a normal distribution, has been applied to abdominal imaging using DWI. The monoexponential model is the most commonly used in daily practice. However, the biexponential model, which is based on the IVIM theory that was introduced as a technique to reflect both perfusion and diffusion by Le Bihan et al[20] can account for separating tissue diffusivity and tissue microcapillary perfusion. The unique feature of the multi-b DWI based on IVIM is the application of the multiple b value (Figures 2-4), which can be used in biexponential models to calculate the IVIM-derived parameters.
Monoexponential models are based on an assumption that the diffusion occurs in a free and unrestricted environment in biologic tissue, that is to say, the distribution of displacements obeys Gaussian law. Biexponential models reflect a combination of tissue perfusion and tissue diffusivity effects. It is now generally accepted that when the b value is relatively low (0-200 s/mm2), the signal of ADC contains two parts; one is the diffusion of water molecules, and the other is the perfusion of water molecules in the capillary in local microcirculation. Further, the effect of perfusion is more sensitive. When the b value is relatively high (200-1000 s/mm2), the attenuation of signal due to the effect of perfusion is slight, at this point, the signal of DWI only approximately reflects the diffusion of water molecules[60]. This is a basic principle of conventional DWI and is the reason that the high b value was selected.
IVIM can accurately describe the relationship between signal attenuation and b values in the DWI and relatively obtain the parameters that present the effect of diffusion and perfusion in tissue.
Standard ADC value (or conventional ADC value) can be obtained from IVIM. Additionally, there are three main parameters in the IVIM, including D value, D* value and f value (perfusion fraction). D value is the true diffusion coefficient, also called the structural diffusion constant D value or slow ADC value, which reflects the tissue microstructure[48] and is the actual diffusion effect of water molecules. The D* value, also referred as the pseudo diffusion coefficient, perfusion-related coefficient or fast ADC value, is the diffusion parameter due to the perfusion effect of the incoherent microcirculation within the voxel[61]. The diffusion and perfusion can affect the signal intensity attenuation on DWI, and the proportion of the perfusion effect is defined as the perfusion fraction (Figure 4).
Diffusion-based IVIM has recently gained interest as a method to detect and characterize pancreatic lesions, and multi-b DWI based on IVIM theory shows very promising results and should be further investigated[48,62]. The f values were reported to make a contribution to distinguishing between normal pancreatic parenchyma and pancreatic neoplasm[45,49,50,63,64], and the f value proved to be the superior DWI-derived parameter for the differentiation of mass-forming pancreatitis and pancreatic carcinoma[50].
Many studies indicated that the IVIM-derived parameter’s f value was a superior parameter for differentiating pancreatic tumors from the normal pancreas compared to the conventional ADC values and that the f value is lower in pancreatic cancer[48,51]. Lemke et al[63] conducted research to study the vascular contribution to the measured ADC value and to validate the IVIM theory; their results showed that the perfusion fraction f in the blood-suppressed pancreatic tissue decreased, possibly because the normal pancreas has a rich blood supply and will lead to a high f value. However, pancreatic cancer can destroy the normal pancreatic tissue and the vessel, and the decrease of vessel density may lead to the decreased f value, even if research shows that the f value in the IVIM-approach proved to be the best parameter for the differentiation between the normal pancreas and pancreatic cancer[48,51].
Compared to the f value, there is relatively less research on D value and D* value in pancreatic tumors. The structural diffusion constant D value reflects the tissue microstructure. The value of the D value for pancreatic cancer is controversial. Lemke et al[48] found that the D value showed no significant difference in pancreatic carcinoma and the healthy pancreas. Concia et al[49] found that the D’ value (D value was estimated by D’ value) hardly differed in neuroendocrine pancreatic tumors and chronic pancreatitis. Klauss et al[50] reported that the D value cannot distinguish pancreatic carcinoma from mass-forming chronic pancreatitis. Klauss et al[42] reported that D value correlates with the histopathological grade of fibrosis in pancreatic lesions, which is the most characteristic histopathological feature of pancreatic carcinoma, compared with healthy pancreatic tissue, and concluded that D value can be used to monitor novel therapy approaches that inhibit the formation of fibrosis. In 2014, Hwang et al[65] reported that the D value may be a better marker of cellularity than ADC. Until now, this was the only research on the D* value in pancreatic cancer. In 2014, Kang et al[51] used IVIM-derived parameters for the differentiation of common pancreatic tumors and concluded that the D* value and f values were more useful parameters in the differentiation of pancreatic adenocarcinomas from neuroendocrine tumors than were the ADC and D values.
The sequence of DTI is similar to that of the DWI, and both are a SE-EPI sequence. The DTI also applies the two motion-probing bipolar gradients on either side of the refocusing 180° pulse. The difference or the unique feature is that the DTI acquires images from multiple directions. Thus, in clinical practice, a minimum of 6 non-collinear images is needed, but 12 or more images are often collected to increase the accuracy of the measure (Figure 5, Figure 6Q and R).
The DTI based on the diffusion of water molecules is anisotropic, which can be illustrated by the fact that the diffusion can be greater in one direction than in other directions and is termed “anisotropic” due to some factors, such as cell membranes, fibers, and myelin[66]. Conversely, without barriers, the random Brownian movement of water molecules is uniform in all directions or “isotropic”. In general, DWI experiments yield an average ADC over three orthogonal directions, ignoring the anisotropy of tissue in the diffusion process[11,67].Though the DTI model also assumes the diffusion distribution to be Gaussian, the same as the DWI, the DTI can measure the magnitude and directionality of water diffusion in tissue quantitatively[66]. The “tensor” in DTI refers to a mathematical construct for representing the magnitude of directional water diffusion in a three-dimensional volume[17].
DTI can not only reveal the degree of the restriction of water molecules in the diffusion movement but can also evaluate the different direction of diffusion. In a recently popular model, the DTI can provide some details on the microstructure of tissues that are not available in conventional imaging[68-70]. The major advantages of DTI are that it can assess the directionality of the diffusion of water molecules in biological tissue[71]. Thus, the DTI can evaluate more comprehensively and accurately the diffusion movement of water molecules in tissue. DTI also provides another non-invasive characterization of tissue microstructural properties in vivo[68].
DTI can demonstrate the subtle abnormalities of some diseases, and degrees of anisotropy have been reported to correlate with the microstructural changes in neural tissues[72,73] and even the peripheral nervous system[70]. DTI is also applied in myocardial infarction[74], prostate cancer[75,76], kidneys[77], liver[78], breast cancer[79], to name just a few. Indeed, it was reported that DTI would provide significant characterization of tissue microstructure and pathophysiology[80-82]. Each voxel in a DTI data set contains vector information that reflects the directionality and magnitude of diffusion in the underlying tissue.
There are five main parameters in the DTI, including mean diffusivity (MD), three eigenvalues λ1, λ2, λ3, and fractional anisotropy (FA). MD is the average of the ADC in all directions and can represent the degree of diffusion. In theory, MD more truly reflects the water molecules’ diffusion ability than ADC, but in clinical practice, the mean diffusivity is expressed as ADC. The FA represents the fraction of the magnitude of tensor that is due to anisotropic water diffusion[83]. That is to say, FA represents the diversity of diffusion direction, which is calculated by the three above eigenvalues[84].
In 2014, Nissan et al[57] used the DTI for patients with pancreatic-ductal-adenocacinoma, and their results indicated that the parameters of DTI (λ1, λ2, λ3 and the ADC value) were lower than the values of the corresponding diffusion coefficients in the distal normal pancreatic tissue of the patients[57]; this outcome suggested that the fast diffusion component is dominated by the microcapillary perfusion process[63]. The results were consistent with those of previous DWI studies reporting lower ADC values in pancreatic cancer attributed to their higher cellularity[45-47].
The high b value is the most important feature of the DKI (Figure 6S and T). The DWI and IVIM are based on an assumption that the diffusion of water molecules obeys the normal distribution in vivo. However, Wu et al[85] reported that in biological tissue, complex cellular microstructures make water diffusion a highly hindered or restricted process, especially at high b values, where the distribution of displacements does not obey a Gaussian distribution. DKI was recently reported to be an extension to the Gaussian DT model[14], and it has become more popular in recent years.
DKI uses the same pulse sequences as that of conventional DWI, but with b values that are somewhat larger than those usually selected[86]. DKI is a straightforward extension of DTI, which requires only minor changes in data acquisition and processing[87,88]. The theory of DKI is based on the above principles, which describes the non-Gaussian diffusion behavior in tissues[14]. The literature has even reported that the DKI parameters, such as the radial or axial kurtosis, are more sensitive to brain physiology changes than the well-known DTI parameters in some white and gray matter structures[14]. In the white and gray matter structures, the DKI shows a better detection and characterization of various changes[89]. Hence, the DKI, which can measure the kurtosis excess of that distribution, allows for a more accurate description of the diffusion properties of neural tissues than the DTI model[87].
In the model, DKI can obtain the parameters that can also be derived from DWI and DTI, such as ADC and FA. Its main parameter is the mean kurtosis (MK). MK is a complex micro parameter that is associated with the complexity of the tissue structure. The high MK represents the more complex tissue structure[14].
There is less research on DKI for pancreatic cancer. However, in 2012, Rosenkrantz et al[90] used DKI in prostate cancer, and their preliminary findings suggest an increased value for DKI compared with that of standard DWI in prostate cancer assessment. In theory, the pancreatic cancer occurs with tumor cell invasion and the proliferation of interstitial cells and connective tissue. The change in the tissue structure leads to the change of MK value.
In pancreatic cancer, tumor cell growth will lead to changes in cellularity, tumor cell atypia, organelles, and extracellular space. All of these factors can change the water molecules’ movement and restrict water diffusion. Extending the diffusion MR imaging, the diffusion of water molecules can be described more accurately and comprehensively. The conventional DWI can reflect the diffusion in one direction. The multi-b value DWI is based on the IVIM theory, which is generated by the blood flow in the tortuous microcirculation of the normal pancreatic tissue[51] and thus can reflect both perfusion and diffusion. The DTI can measure the magnitude and directionality of water diffusion in tissue quantitatively. DKI describes the non-Gaussian diffusion behavior in tissues.
However, we should be aware of the limitations of this technique: (1) Generally, in daily work, abdominal MRI suffers from interference and motional artifacts due to breathing[91,92]; (2) The gradient eddy currents in the EPI protocols can lead to the B0 field inhomogeneity and susceptibility differences[93,94]. Using a dielectric pad and a bellows belt for respiratory triggering can reduce geometrical distortions. DWI has been mostly acquired using single-shot echo planar imaging (ss EPI) to minimize motion-induced artifacts[95]; and (3) the choice of b value can also limit the technique. Currently, the ADC of the pancreas still does not reach unanimity; some scholars think the ADC, which was derived from the low b value, presents only a small part of the diffusion movement, which leads to contamination of other forms of IVIM, such as perfusion in the capillary bed. The perfusion will affect the diffusion when the b value is low, even though it can characterize the anatomy and the details of the lesion[55]. Finally, low b values result in increased ADC values[19,20]. Conversely, Kim et al[58] indicated that the high b value can be useful in clinical practice. The high b value means that it needs a longer echo time (TE), implying that it will lead to decreasing the SNR and increasing artifacts. Poor image quality will affect observation[18]. Using a high b value, the ADC value may be closer to the real state. In clinical practice, the choice of b value is controversial. As a compromise, a b value of 500 s/mm2 was chosen[18]; however, the higher b value of 1000 s/mm2 has been reported as good for malignant abdominal tumors[96] and the detection of pancreatic adenocarcinoma[32,96]. The choice of b value to minimize motion artifacts and to improve the SNR in pancreas is very important. Higher b values may be more sensitive to reflect true diffusion[30,97]. In clinical practice, taking the two factors into the consideration, a feasible b value can be selected depending on your purpose of study.
The proposed method may hold great promise for the non-invasive, non-contrast-enhanced imaging of pancreas lesions and may eventually become a screening tool for pancreatic cancer. MR is well suited to the quantitative and non-invasive measurement of diffusion. Diffusion MR imaging techniques are increasingly varied, from the simplest and most commonly used techniques to the more complex, such as from DWI to DKI. The diffusion MR imaging for pancreatic cancer revealed valuable advantages, such as high sensitivity and specificity. Moreover, diffusion MR imaging can aid in differentiating the different type of differentiation. These techniques go beyond traditional macrostructural volumetric methods and provide valuable information about underlying tissue integrity and organization at the microstructural and biochemical levels.
At present, a major issue with diffusion MR imaging is the lack of standardization of the protocol[98]. The IVIM-derived parameters in pancreatic cancer are controversial. For example, it is unknown how fibrosis affects diffusion parameters[42,52]. Further studies evaluating the behavior of IVIM-derived parameters in the diagnosis and treatment of pancreatic cancer are needed for standardization. One important point to bear in mind in future studies is that larger sample sizes, including imaging and histopathological workup are needed. The clinical report of utilization of the DTI and DKI in pancreatic cancer is still rare, and the potential of DTI to reveal the complex microstructure and physiology of the pancreas and detect pathological changes has not been investigated. Much work remains in solving the challenges inherent to tractography, which may certainly be a very promising technique that may be likely to contribute greatly to our understanding of nerve invasion.
In addition to the use of advanced DTI or DKI for pancreatic cancer, future advancements will come from continued study. Further standard diffusion MR imaging can benefit the accurate detection and staging of pancreatic cancer and provide the imaging evidence for clinical treatment.
P- Reviewer: Hayano K, Sugimura H S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (3)] |
| 2. | Krechler T, Horejs J, Ulrych J, Zeman M, Macásek J, Dusková J, Zák A. Current status of pancreatic cancer diagnosis. Cas Lek Cesk. 2011;150:587-593. [PubMed] |
| 3. | Singh M, Maitra A. Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology. 2007;7:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 5. | Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto M, Cleeter DF. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother. 2014;10:3354-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1543] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 8. | Klimstra DS, Pitman MB, Hruban RH. An algorithmic approach to the diagnosis of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Jang SK, Kim JH, Joo I, Jeon JH, Shin KS, Han JK, Choi BI. Differential diagnosis of pancreatic cancer from other solid tumours arising from the periampullary area on MDCT. Eur Radiol. 2015;25:2880-2888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Putzer D, Jaschke W. Radiological evaluation of focal pancreatic lesions. Dig Dis. 2015;33:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Heyn C, Sue-Chue-Lam D, Jhaveri K, Haider MA. MRI of the pancreas: problem solving tool. J Magn Reson Imaging. 2012;36:1037-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | de la Santa LG, Retortillo JA, Miguel AC, Klein LM. Radiology of pancreatic neoplasms: An update. World J Gastrointest Oncol. 2014;6:330-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | O’Neill E, Hammond N, Miller FH. MR imaging of the pancreas. Radiol Clin North Am. 2014;52:757-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Poot DH, den Dekker AJ, Achten E, Verhoye M, Sijbers J. Optimal experimental design for diffusion kurtosis imaging. IEEE Trans Med Imaging. 2010;29:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Yao X, Kuang T, Wu L, Feng H, Liu H, Cheng W, Rao S, Wang H, Zeng M. Optimization of MR diffusion-weighted imaging acquisitions for pancreatic cancer at 3.0T. Magn Reson Imaging. 2014;32:875-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Barral M, Taouli B, Guiu B, Koh DM, Luciani A, Manfredi R, Vilgrain V, Hoeffel C, Kanematsu M, Soyer P. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology. 2015;274:45-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Moseley M, Bammer R, Illes J. Diffusion-tensor imaging of cognitive performance. Brain Cogn. 2002;50:396-413. [PubMed] |
| 18. | Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N. Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol. 2009;19:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Ma C, Li YJ, Pan CS, Wang H, Wang J, Chen SY, Lu JP. High resolution diffusion weighted magnetic resonance imaging of the pancreas using reduced field of view single-shot echo-planar imaging at 3 T. Magn Reson Imaging. 2014;32:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [PubMed] |
| 21. | Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011;196:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 426] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 23. | Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2627] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 24. | Robertson RL, Glasier CM. Diffusion-weighted imaging of the brain in infants and children. Pediatr Radiol. 2007;37:749-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Morgan VA, Kyriazi S, Ashley SE, DeSouza NM. Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 2007;48:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Bittencourt LK, Hausmann D, Sabaneeff N, Gasparetto EL, Barentsz JO. Multiparametric magnetic resonance imaging of the prostate: current concepts. Radiol Bras. 2014;47:292-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Bruegel M, Holzapfel K, Gaa J, Woertler K, Waldt S, Kiefer B, Stemmer A, Ganter C, Rummeny EJ. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol. 2008;18:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Szafer A, Zhong J, Gore JC. Theoretical model for water diffusion in tissues. Magn Reson Med. 1995;33:697-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 411] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 30. | Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, Fujii H. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007;188:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, Masuda D, Arisaka Y, Takaori K, Tanigawa N. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Takeuchi M, Matsuzaki K, Kubo H, Nishitani H. High-b-value diffusion-weighted magnetic resonance imaging of pancreatic cancer and mass-forming chronic pancreatitis: preliminary results. Acta Radiol. 2008;49:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Jang KM, Kim SH, Min JH, Lee SJ, Kang TW, Lim S, Choi D. Value of diffusion-weighted MRI for differentiating malignant from benign intraductal papillary mucinous neoplasms of the pancreas. AJR Am J Roentgenol. 2014;203:992-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Lee NK, Kim S, Kim DU, Seo HI, Kim HS, Jo HJ, Kim TU. Diffusion-weighted magnetic resonance imaging for non-neoplastic conditions in the hepatobiliary and pancreatic regions: pearls and potential pitfalls in imaging interpretation. Abdom Imaging. 2015;40:643-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Park MJ, Kim YK, Choi SY, Rhim H, Lee WJ, Choi D. Preoperative detection of small pancreatic carcinoma: value of adding diffusion-weighted imaging to conventional MR imaging for improving confidence level. Radiology. 2014;273:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Birchard KR, Semelka RC, Hyslop WB, Brown A, Armao D, Firat Z, Vaidean G. Suspected pancreatic cancer: evaluation by dynamic gadolinium-enhanced 3D gradient-echo MRI. AJR Am J Roentgenol. 2005;185:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Fletcher JG, Wiersema MJ, Farrell MA, Fidler JL, Burgart LJ, Koyama T, Johnson CD, Stephens DH, Ward EM, Harmsen WS. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, Eikman EA, Malafa M. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 41. | Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32:2-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | Klauss M, Gaida MM, Lemke A, Grünberg K, Simon D, Wente MN, Delorme S, Kauczor HU, Grenacher L, Stieltjes B. Fibrosis and pancreatic lesions: counterintuitive behavior of the diffusion imaging-derived structural diffusion coefficient d. Invest Radiol. 2013;48:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Schnapauff D, Zeile M, Niederhagen MB, Fleige B, Tunn PU, Hamm B, Dudeck O. Diffusion-weighted echo-planar magnetic resonance imaging for the assessment of tumor cellularity in patients with soft-tissue sarcomas. J Magn Reson Imaging. 2009;29:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Herrmann J, Schoennagel BP, Roesch M, Busch JD, Derlin T, Doh LK, Petersen KU, Graessner J, Adam G, Habermann CR. Diffusion-weighted imaging of the healthy pancreas: ADC values are age and gender dependent. J Magn Reson Imaging. 2013;37:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, Kim JK, Lee MG. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28:928-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Fattahi R, Balci NC, Perman WH, Hsueh EC, Alkaade S, Havlioglu N, Burton FR. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009;29:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, Tateyama A, Nakajo M. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology. 2012;263:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, Schad LR, Stieltjes B. Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Invest Radiol. 2009;44:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 49. | Concia M, Sprinkart AM, Penner AH, Brossart P, Gieseke J, Schild HH, Willinek WA, Mürtz P. Diffusion-weighted magnetic resonance imaging of the pancreas: diagnostic benefit from an intravoxel incoherent motion model-based 3 b-value analysis. Invest Radiol. 2014;49:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Klauss M, Lemke A, Grünberg K, Simon D, Re TJ, Wente MN, Laun FB, Kauczor HU, Delorme S, Grenacher L. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol. 2011;46:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Kang KM, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging for characterization of focal pancreatic lesions. Radiology. 2014;270:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 52. | Muraoka N, Uematsu H, Kimura H, Imamura Y, Fujiwara Y, Murakami M, Yamaguchi A, Itoh H. Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations. J Magn Reson Imaging. 2008;27:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology. 1991;180:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 139] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Wang Y, Chen ZE, Nikolaidis P, McCarthy RJ, Merrick L, Sternick LA, Horowitz JM, Yaghmai V, Miller FH. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging. 2011;33:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1550] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 56. | Wang Y, Chen ZE, Yaghmai V, Nikolaidis P, McCarthy RJ, Merrick L, Miller FH. Diffusion-weighted MR imaging in pancreatic endocrine tumors correlated with histopathologic characteristics. J Magn Reson Imaging. 2011;33:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Nissan N, Golan T, Furman-Haran E, Apter S, Inbar Y, Ariche A, Bar-Zakay B, Goldes Y, Schvimer M, Grobgeld D. Diffusion tensor magnetic resonance imaging of the pancreas. PLoS One. 2014;9:e115783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 59. | Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, Hammond NA, Yaghmai V, Nikolaidis P. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics. 2011;31:E47-E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 60. | Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, Ruel L, Laurent A, Deux JF, Brugieres P, Rahmouni A. Liver cirrhosis: intravoxel incoherent motion MR imaging--pilot study. Radiology. 2008;249:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 517] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 61. | Zhang JL, Sigmund EE, Chandarana H, Rusinek H, Chen Q, Vivier PH, Taouli B, Lee VS. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology. 2010;254:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 62. | Grünberg K, Grenacher L, Klauss M. Diffusion-weighted imaging of the pancreas. Radiologe. 2011;51:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med. 2010;64:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 64. | Re TJ, Lemke A, Klauss M, Laun FB, Simon D, Grünberg K, Delorme S, Grenacher L, Manfredi R, Mucelli RP. Enhancing pancreatic adenocarcinoma delineation in diffusion derived intravoxel incoherent motion f-maps through automatic vessel and duct segmentation. Magn Reson Med. 2011;66:1327-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Hwang EJ, Lee JM, Yoon JH, Kim JH, Han JK, Choi BI, Lee KB, Jang JY, Kim SW, Nickel MD. Intravoxel incoherent motion diffusion-weighted imaging of pancreatic neuroendocrine tumors: prediction of the histologic grade using pure diffusion coefficient and tumor size. Invest Radiol. 2014;49:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev. 2006;30:762-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Fukuda Y, Ohashi I, Hanafusa K, Nakagawa T, Ohtani S, An-naka Y, Hayashi T, Shibuya H. Anisotropic diffusion in kidney: apparent diffusion coefficient measurements for clinical use. J Magn Reson Imaging. 2000;11:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 3065] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 69. | Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2356] [Cited by in RCA: 2164] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 70. | Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26 Suppl 1:S205-S223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 469] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 71. | Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4249] [Cited by in RCA: 3974] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 72. | Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36:1532-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 73. | Kuswanto CN, Teh I, Lee TS, Sim K. Diffusion tensor imaging findings of white matter changes in first episode schizophrenia: a systematic review. Clin Psychopharmacol Neurosci. 2012;10:13-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Wu MT, Tseng WY, Su MY, Liu CP, Chiou KR, Wedeen VJ, Reese TG, Yang CF. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: correlation with viability and wall motion. Circulation. 2006;114:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 75. | Uribe CF, Jones EC, Chang SD, Goldenberg SL, Reinsberg SA, Kozlowski P. In vivo 3T and ex vivo 7T diffusion tensor imaging of prostate cancer: Correlation with histology. Magn Reson Imaging. 2015;33:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Li L, Margolis DJ, Deng M, Cai J, Yuan L, Feng Z, Min X, Hu Z, Hu D, Liu J. Correlation of gleason scores with magnetic resonance diffusion tensor imaging in peripheral zone prostate cancer. J Magn Reson Imaging. 2015;42:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Sigmund EE, Vivier PH, Sui D, Lamparello NA, Tantillo K, Mikheev A, Rusinek H, Babb JS, Storey P, Lee VS. Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology. 2012;263:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 78. | Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 79. | Nissan N, Furman-Haran E, Shapiro-Feinberg M, Grobgeld D, Degani H. Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology. 2014;271:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Cauley KA, Filippi CG. Diffusion-tensor imaging of small nerve bundles: cranial nerves, peripheral nerves, distal spinal cord, and lumbar nerve roots--clinical applications. AJR Am J Roentgenol. 2013;201:W326-W335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Jambawalikar S, Baum J, Button T, Li H, Geronimo V, Gould ES. Diffusion tensor imaging of peripheral nerves. Skeletal Radiol. 2010;39:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Lee JW, Kim JH, Kang HS, Lee JS, Choi JY, Yeom JS, Kim HJ, Chung HW. Optimization of acquisition parameters of diffusion-tensor magnetic resonance imaging in the spinal cord. Invest Radiol. 2006;41:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Masutani Y, Aoki S, Abe O, Hayashi N, Otomo K. MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol. 2003;46:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 84. | Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. J Magn Reson. 2011;213:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 300] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 85. | Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 86. | Huang Y, Chen X, Zhang Z, Yan L, Pan D, Liang C, Liu Z. MRI quantification of non-Gaussian water diffusion in normal human kidney: a diffusional kurtosis imaging study. NMR Biomed. 2015;28:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1789] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 88. | Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006;19:236-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 325] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 89. | Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage. 2009;45:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 90. | Rosenkrantz AB, Sigmund EE, Johnson G, Babb JS, Mussi TC, Melamed J, Taneja SS, Lee VS, Jensen JH. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology. 2012;264:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 91. | Dietrich O, Reiser MF, Schoenberg SO. Artifacts in 3-T MRI: physical background and reduction strategies. Eur J Radiol. 2008;65:29-35. [PubMed] |
| 92. | Riffel P, Michaely HJ, Morelli JN, Pfeuffer J, Attenberger UI, Schoenberg SO, Haneder S. Zoomed EPI-DWI of the pancreas using two-dimensional spatially-selective radiofrequency excitation pulses. PLoS One. 2014;9:e89468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Dietrich O, Biffar A, Baur-Melnyk A, Reiser MF. Technical aspects of MR diffusion imaging of the body. Eur J Radiol. 2010;76:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 94. | Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 95. | Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 409] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 96. | Tsushima Y, Takano A, Taketomi-Takahashi A, Endo K. Body diffusion-weighted MR imaging using high b-value for malignant tumor screening: usefulness and necessity of referring to T2-weighted images and creating fusion images. Acad Radiol. 2007;14:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Huang WC, Sheng J, Chen SY, Lu JP. Differentiation between pancreatic carcinoma and mass-forming chronic pancreatitis: usefulness of high b value diffusion-weighted imaging. J Dig Dis. 2011;12:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Sandrasegaran K. Functional MR imaging of the abdomen. Radiol Clin North Am. 2014;52:883-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |