Published online Oct 28, 2015. doi: 10.4329/wjr.v7.i10.306
Peer-review started: April 11, 2015
First decision: June 24, 2015
Revised: September 6, 2015
Accepted: October 1, 2015
Article in press: October 8, 2015
Published online: October 28, 2015
Processing time: 199 Days and 5 Hours
Hepatocellular carcinoma (HCC) is the sixth-most common type of cancer worldwide. The only definitive treatment modalities capable of achieving a cure are hepatic resection and hepatic transplantation. However, most patients are not candidates for these therapies. Overall, treatment options are driven by the stage of HCC. Early-stage disease is treated with ablative therapies, with radiofrequency ablation the ablative therapy of choice. Microwave ablation and irreversible electroporation are the other upcoming alternatives. Intermediate-stage disease is managed with transarterial chemoembolization (TACE), while advanced-stage disease is managed by sorafenib, with TACE and radioembolization as other alternatives.
Core tip: Treatment of hepatocellular carcinoma is dependent on the stage of disease. Early-stage disease is managed by resection. Radiofrequency ablation (RFA), is becoming an attractive alternative for very early-stage disease. Early-stage disease is treated with ablative therapies. RFA is the ablative therapy of choice. RFA, however, is not effective in all cases. Microwave ablation and irreversible electroporation are upcoming alternatives. Transarterial chemoembolization (TACE) is the modality of choice for intermediate-stage disease. TACE-based multimodal treatment is becoming acceptable. Advanced-stage disease is managed by sorafenib. However, TACE and radioembolization are other alternatives.
- Citation: Kalra N, Gupta P, Chawla Y, Khandelwal N. Locoregional treatment for hepatocellular carcinoma: The best is yet to come. World J Radiol 2015; 7(10): 306-318
- URL: https://www.wjgnet.com/1949-8470/full/v7/i10/306.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i10.306
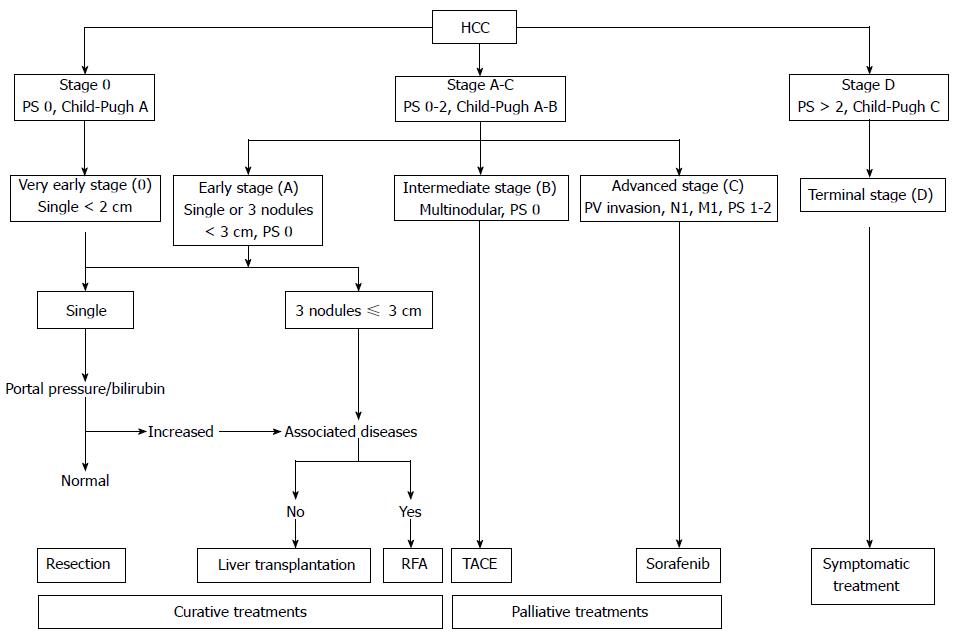
Hepatocellular carcinoma (HCC) is the sixth-most common type of cancer worldwide and the third-leading cause of cancer-related death[1]. The Barcelona Clinic Liver Cancer (BCLC) classification not only stages but also guides the clinical management of patients with HCC (Table 1 and Figure 1)[2]. An integral component of the BCLC staging is the Child-Pugh classification (Table 2)[3].
| BCLC classification | |
| Stage | Description |
| Very early | PS 0, Child-Pugh A, single HCC, 2 cm |
| Early | PS 0, Child-Pugh A–B, single HCC or 3 nodules, 3 cm |
| Intermediate | PS 0, Child-Pugh A–B, multinodular HCC |
| Advanced | PS 1–2, Child-Pugh A–B, portal neoplastic invasion, nodal metastases, distant metastases |
| Terminal | PS 2, Child-Pugh C |
| Child-pugh classification | |||
| Finding | 1 point | 2 points | 3 points |
| Encephalopathy grade | None | Mild | Severe |
| Ascites | Absent | Mild to moderate | Severe, refractory |
| Serum bilirubin (mg/dL) | 2 | 2-3 | > 3 |
| Serum albumin (g/dL) | > 3.5 | 2.8-3.5 | < 2.8 |
| INR | < 1.7 | 1.7-2.2 | > 2.2 |
The only definitive treatment modalities capable of achieving a cure are hepatic resection and hepatic transplantation. However, a limited number of patients (10% to 20%) are considered fit for these treatments. A large percentage of the remaining patients are managed by so-called liver-directed regional therapies. Among these, methods of tumor ablation (TA) have shown the most clinical promise. In addition to down-staging patients, TA may have a potential curative role, especially in very-early- and early-stage HCCs[4]. The various options for ablative therapies are listed in Table 3.
| Chemical ablation | PAI |
| PEI | |
| Cryoablation | Nitrous oxide |
| Liquid nitrogen | |
| Argon | |
| Thermal ablation | RFA |
| LITT | |
| HIFU | |
| MWA | |
| Electroporation | IRE |
There has been a significant evolution of TA therapies as an alternative option for patients with unresectable tumors. This has been rendered feasible by significant technical advancements and evidence from well-conducted studies that have suggested improved outcomes in patients treated with TA[5-7].
We will discuss the various treatment options for HCC based on the BCLC staging.
The standard treatment in this group is surgical resection. Such patients are unlikely to decompensate after resection and have an excellent 5-year survival rate (> 75%)[8]. The most commonly used surgical technique for this group is anatomic resection, which involves en-bloc removal of a liver segment (supplied by a major portal vein branch and the hepatic artery). This technique is preferred because it theoretically allows the eradication of intrahepatic metastases of HCC. However, a major consideration is treatment-related mortality, which is in the range of 1%-3%[9]. TA methods provide an alternative treatment option for nodules smaller than 2 cm in diameter. Percutaneous radiofrequency ablation (RFA) is the standard TA technique at most centers worldwide. Lesions that are not sub-capsular, perivascular, or peribiliary are ideal targets for percutaneous RF ablation. In centrally located tumors, RFA perhaps presents a challenge to the dominant position of surgical resection for treatment. A very high complete response rate (97%) and a 5-year survival rate of 68% has been reported with RFA in very-early-stage HCC[10]. Cho et al[11], in their recent decision-analysis study, concluded that RFA and hepatic resection should be considered equally effective for the treatment of very-early HCC. Takayama et al[12] performed a retrospective analysis of 2550 patients (RFA/resection = 1315/1235) with very early HCC, finding no statistically significant difference in overall survival (OS) rates in the RFA versus resection groups (95% vs 94%). In a recent meta-analysis, there was no significant difference in the overall 1- and 3-year survival and disease-free survival rates between the resection and RFA groups for tumors < 2 cm in diameter[13].
Based on these published data, it is recommended that RFA be considered a first-line treatment option for very-early-stage HCC, with surgical resection reserved for patients for whom individual variables preclude RFA. In certain patients who are not candidates for RFA (e.g., sub-optimal location) or surgery (e.g., increased bilirubin level or signs of portal hypertension), percutaneous ethanol injection (PEI) may be considered[14]. In a randomized trial by Giorgio et al[15], patients with a single HCC ≤ 3 cm were randomly assigned to receive PEI or RFA, and comparable 3-year (74% and 78%, respectively, for each treatment) and 5-year (68% for both treatments) survival rates were found.
Resection, liver transplantation, and percutaneous TA are the various treatment options for this large, heterogeneous group. Besides the percutaneous approach for ablation, other approaches for ablation have been described, including a laparoscopic route and an open surgical route. Advanced techniques are also described for lesions abutting the diaphragm and gastrointestinal tract. The surgical routes for ablation allow treatment of larger lesions, avoiding the hindrances posed by the tissues overlying the liver.
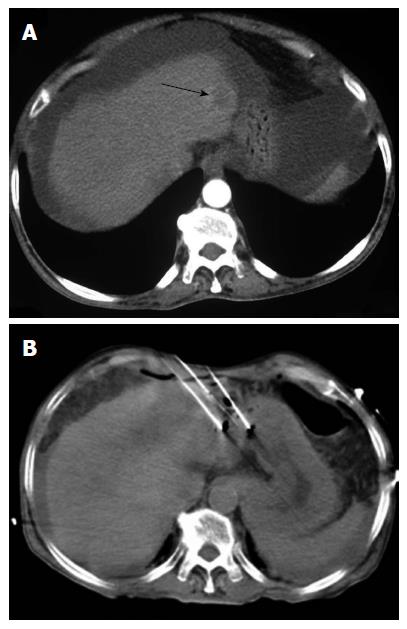
Among the different TA techniques, RFA is currently the favored treatment option for patients with early-stage HCC (Figure 2)[16]. Percutaneous chemical ablation is cost-effective, easy-to-perform, and has found worldwide acceptance for small lesions. Between PEI and percutaneous acetic-acid injection (PAI), multiple studies have failed to show any superiority of one over the other[17]. PEI and PAI are limited by the need for multiple sessions to achieve the clinically desired results. With the introduction of RFA, chemical ablation has decreased in popularity.
There are four types of RF electrodes commercially available: Two models of retractable-needle electrodes (model 70 and model 90 Star-burst XL needles, RITA Medical Systems, Mountain View, CA; LeVeen needle electrode, Boston Scientific, Boston, MA), an internally cooled electrode (Cool-Tip RF electrode; Radionics, Burlington, MA), and a separable clustered electrode (Octopus®; STARmed, Goyang, Korea)[6].
The RITA RF electrode consists of a 14-gauge, insulated outer needle that has nine retractable, curved electrodes of various lengths. When deployed, the device assumes the approximate configuration of a Christmas tree. The LeVeen RF electrode has retractable, curved electrodes and an insulated, 17-gauge outer needle that contains 10 solid, retractable, curved electrodes. It assumes the configuration of an umbrella upon deployment. The Cool-Tip RF device has an insulated, hollow, 17-gauge needle with an exposed needle tip of variable length. The needle shaft has two internal channels for perfusion with chilled water. In an attempt to increase the size of the possible ablation area, the manufacturer has placed three of the cooled needles in a parallel, triangular cluster with a common hub (a multi-tined electrode). In the Octopus®, RF energy is applied to two electrodes, while, simultaneously, RF energy is switched between a pair of electrodes. This can create a large ablative zone with a spherical shape that has better efficiency of RF energy delivery over a given treatment time.
In order to achieve therapeutic results similar to those achieved with traditional surgery, surgical margins of approximately 1 cm are required for successful resection by RFA. However, current RFA technology, with internally cooled or expandable electrodes, achieve a limited volume of coagulation necrosis, resulting in marginal recurrence rates up to 41%, especially with tumors greater than 3 cm in diameter. Several types of electrodes have been developed to overcome this limitation and achieve larger ablation zones, including perfused, clustered, saline-infused expandable, and multipolar electrodes. In addition, a multiple electrode-switching system can achieve substantially larger ablation volumes than techniques using conventional RF ablation with single electrodes having overlapping ablations[6].
Five randomized, controlled trials (RCTs) have compared RFA and PEI for the treatment of early-stage HCC. The results of three RCTs (Table 4) showed that RFA is more effective than PEI in terms of better local disease control[14,18-21]. However, in trials by Lencioni et al[14] and Brunello et al[21], the differences in overall 1- and 3-year survival rates between RFA and PEI were not statistically significant. Further, independent meta-analyses of early-stage HCC cases have confirmed the survival benefit conferred by RFA over PEI[22-25].
Recent studies regarding the long-term outcomes of RFA-treated patients have shown consistently high 5-year survival rates in early-stage HCC[26-29]. In studies by Lencioni et al[26] (n = 144), Tateishi et al[27] (n = 221), Choi et al[28] (n = 359), and N’Kontchou et al[29] (n = 67), 3- and 5-year survival rates were reported, respectively, as 76% and 51%, 83% and 63%, 78% and 64%, and 82% and 76% for Child A or BCLC resectable disease[26-29]. In a study by Kalra et al[30], 31 patients with 41 unresectable HCCs were treated with RFA. Over a follow-up period ranging from 3 mo to 6 years, ablation was successful at a rate of 80.5%. Eight patients had tumor recurrences. The survival rate at 1 year in patients who had completed at least 1 year of follow-up was 63.3%[20].
Thus, a question arises: Can RFA replace surgical resection as a first-line treatment for early-stage HCC? Several retrospective studies and a single RCT found no statistically significant difference in survival rates between surgical resection and RFA[31-37].
Chen et al[38] conducted an RCT on 180 patients with a solitary HCC ≤ 5 cm who received either percutaneous RFA or surgical resection. No significant difference was noted in the overall and disease-free survival rates between the RFA and resection groups in terms of their respective 1-year (95.8% and 93.3%) and 4-year (67.9% and 64.0%) OS rates. However, in the RCT by Huang et al[39], the 1-, 3-, and 5-year OS and recurrence-free survival rates in the surgical resection group were significantly higher than in the RFA group (P = 0.001, P = 0.017).
An important factor limiting the success of RFA is tumor size, as RFA may fail to ablate the entire tumor volume for tumors larger than 3 cm along the longest axis, particularly at their periphery. The results of RFA are also greatly affected by tumor location. The presence of a large vessel (3 mm or more) in the vicinity of the lesion reduces heat deposition by a “heat sink” effect[40]. Furthermore, lesions in the vicinity of vital structures like the gallbladder, bile ducts, or colon are difficult to treat due to the inherent risk of thermal damage.
Therefore, when RFA is not precluded by tumor location, it is proposed that solitary HCCs larger than 3 cm and smaller than 5 cm in size should be considered for combination therapy with RFA[41-45]. One of the forms of combination therapy is TACE-preceded RFA. The rationale behind lipiodol TACE-preceded RFA is as follows: a state of transient liver infarction is induced by the lipiodol, which regurgitates into the portal branches via the peribiliary venous plexus, thereby decreasing the heat sink effect, expanding the ablative area, and promoting the ablation of satellite lesions[46]. Several studies have reported significantly better survival rates with TACE-preceded RFA compared to RFA alone for intermediate-sized lesions[45,47-48]. In another administration method, TACE may follow RFA, with TACE expected to handle the peripheral part of a tumor where RFA achieves sub-optimal temperatures[45]. A phase III randomized, double-blinded, placebo-controlled study investigating the efficacy and safety of thermally sensitive liposomal doxorubicin in combination with RFA compared to RFA alone in the treatment of unresectable HCC is ongoing[49].
When RFA cannot be performed, largely in view of tumor location, TACE combined with drug eluting beads (DEB) is an alternative. Complete necrosis was reported in 77% patients undergoing DEB-TACE prior to transplantation[50].
HCCs larger than 5 cm comprise another critical group in early-stage HCC. These patients are precluded from transplantation, according to the Milan guidelines, yet surgical resection should be considered for these patients as ablative therapies are unlikely to be effective with lesions of this size[51]. Combination therapies are also expected to be inferior to surgical resection.
Other thermal ablative therapies-including laser-induced thermotherapy (LITT), high-intensity focused ultrasound (HIFU), and microwave ablation (MWA) and irreversible electroporation (IRE) are other alternative modes of therapy; however, these are still evolving technologies and there is too little data at present to put forth concrete recommendations. Of the above-mentioned alternatives, MWA and IRE appear promising and may even supplant RFA in the future.
LITT is based on the use of an Nd-YAG (neodymium: yttrium aluminum garnet) diode laser that allows the delivery of a precise amount of energy to a pre-defined region. Maximal tissue penetration and the desired therapeutic results are achieved by producing slow heating within its therapeutic window. Most studies with LITT have been reported in patients with liver metastases, with favorable survival rates and an acceptable complication profile[52,53]. A single trial of LITT for HCC evaluated the efficacy of a combination of TACE and LITT compared to TACE alone. TACE and LITT were performed in 54 patients, while TACE alone was administered to 51 patients. After a follow-up of 24 mo, survival rates were significantly better in the TACE and LITT (79.6%) than in the TACE alone (60.8%) group[54]. A multicenter study (involving 432 cirrhotic patients with a single tumor ≤ 4 cm or three or fewer nodules ≤ 3 cm each, comprising together 548 lesions), reported that the ideal candidates for laser ablation are younger (< 73 years) with normal serum albumin levels. Child-Pugh class A patients with tumor size ≤ 3 cm and a well-differentiated histologic pattern who achieved an initially complete ablation had median survival of 65 mo. Median time to recurrence was 24 mo, and median disease-free survival was 26 mo[55]. However, this form of treatment is not preferred, largely due to the availability of better alternative ablative therapies.
In HIFU, high-intensity ultrasound in the range of 100-10000 W/cm2 is delivered to a focal region. The absorption and subsequent intense acoustic energy generates temperatures above 60 °C in a short span of time, producing coagulative necrosis. Successful ablation of HCC has been reported by several studies[56-58]. In a recent study, Ng et al[59] presented data on 49 patients treated with HIFU for unresectable HCC. Complete clinical response in this series was 79.5%, and 1- and 3-year survival rates were 87.7% and 62.4%, respectively. In another series by Xu et al[55], 145 patients with HCC were treated with HIFU. Symptom improvement and pain relief was achieved in 84.8% of patients[56]. A two-year survival rate of 80% was reported for early-stage disease. Chan et al[57] compared HIFU to RFA in 103 patients with HCC. HIFU was associated with higher complication rates (skin burns and pleural effusion) than was RFA. There was no significant difference in the 3-year OS rate between HIFU and RFA[57]. A completely extracorporeal HIFU device for treatment of HCC, though clinically feasible, is capable of ablating only small-volume lesions unless a partial rib resection is performed. Moreover, this procedure is associated with attenuation and various complications, including skin burns and gastric lesions. For these reasons, an open procedure seems appropriate despite being more invasive. Besides allowing a better staging of malignancies, large areas of liver can be rapidly ablated in an open procedure. In a feasibility study by Dupré et al[60], this approach was found to be effective in patients with colorectal liver metastases. In 30 ablations performed in 15 patients, intra-operative HIFU was found to be safe, feasible, and without damage to neighboring tissues[60]. Recently, Gandini evaluated in an animal model the use of HIFU for assisting liver resection in an open procedure[61]. They found that HIFU-assisted liver resection is associated with reduced bleeding risk.
The basic principle of ablation in MWA is heat generation using dielectric hysteresis. High-frequency microwaves (typically 900 to 2500 MHz) lead to polarization and rapid oscillation of the intracellular water molecules. The resulting kinetic energy transfer produces heat, coagulation necrosis, and TA. The advantages of MWA over RFA are tabulated in Table 5. Shibata et al[62] compared the effectiveness of MWA with that of RFA. There was no statistically significant difference in the effectiveness of the two procedures. However, a trend favoring RFA was recognized in that study with respect to local recurrence and complication rates[62]. In another study by Yin et al[63] comparing the therapeutic efficacy of RFA and MWA in treating HCCs > 3 cm, both RFA and MWA were found to be effective and safe. Several studies have evaluated the efficacy of MWA when used alone in treating HCCs[63]. Sato et al[64], in one of the earliest studies, demonstrated the safety and efficacy of MWA in 19 patients with unresectable HCCs. MWA was potentially curative in 73.7% of patients[64]. The authors concluded that MWA is a safe and potentially curative treatment option in patients with HCCs having advanced liver cirrhosis and multifocal or central tumors. In another case series, 60 patients with initial HCC (n = 15) and recurrent HCC (n = 45) were treated with MWA. Three-year recurrence free survival rates for initial HCC and recurrent HCC were 36.7% and 8.8%, respectively; 5-year OS for all patients was 43.1%[65]. Several other case series also reported a favorable outcome with MWA[66-68]. A recent study evaluated the efficacy and safety of percutaneous MWA versus TACE for large HCC (5-7 cm). Sixty-four patients were divided into two groups and treated with either MWA or TACE. A higher rate of complete ablation (75%) was achieved with fewer sessions of MWA than of TACE, and MWA displayed a lower incidence of tumor recurrence, de novo lesions, or post-treatment ascites[69].
| Achieves higher temperatures and relatively larger ablative zones in a shorter time |
| Ablative zones are more consistent and uniform in character |
| Better safety profile |
| Less post procedural pain |
| Not affected by the heat sink effect |
| Multiple applicators can be used simultaneously |
Though several initial studies have shown favorable results with cryoablation, many recent studies have raised concerns regarding serious side effects and complications associated with this technology, including cryoshock, hypothermia, cracking of the ice ball, hemorrhage, biloma, abscess, pleural effusion, and death[70,71].
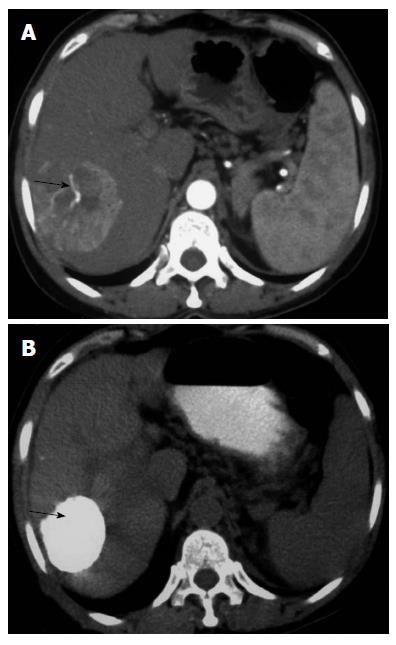
IRE is a non-thermal ablative technique that achieves cell death by creating pores in the lipid bilayer of cell membranes using an electric current. This is accomplished by micro- to millisecond electrical pulses (at 1000–3000 V) delivered via needle electrodes, causing loss of cellular homeostasis and eventually cell death (Figures 3 and 4). In contrast to thermal ablative methods that cause coagulative necrosis by gross heat damage to a cell, IRE acts at the level of cellular membranes and produces cell death by apoptosis[72]. As a result, it spares important structures like blood vessels, bile ducts, and tissue stroma[73]. The NanoKnife (AngioDynamics, New York) is the most commonly used commercial device. It utilizes a 2500 V generator system. Advantages of IRE over other ablative techniques are listed in Table 6. A comparison of various ablative techniques in terms of ease of ablation titration, cost, and tissue specificity is given in Table 7.
| Selective cellular target |
| Cell death via apoptosis |
| Sparing effect on important structures |
| Not affected by heat sink (compared to RFA) |
| Sharp boundary between the treated and untreated areas |
| RFA | MWA | IRE | |
| Principle | Thermal | Thermal | Non-thermal |
| Collateral damage | + | + | - |
| Ease of ablation titration | + | + | ++ |
| Cost | + | + | ++ |
| Duration of therapy | + | + | ++ |
| Tissue effect | |||
| Tumor | + | + | + |
| Nerve | + | + | - |
| Vessels | + | + | - |
| Hepatic architecture | + | + | - |
Early clinical experience with IRE regarding safety and efficacy during ablation of HCC is encouraging. However, most of the available data are short-term. In one of the initial reviews, by Charpentier, IRE was not only found to be safe but also potentially superior to other techniques for lesions abutting major vascular structures[73]. In another retrospective review, IRE-specific treatment outcomes, rates of recurrence, and complications were evaluated in 28 patients with tumor locations precluding other forms of ablation. IRE was found to be safe, with only 3% of patients suffering complications. At 6-mo follow-up, recurrence was reported in 3 patients (5.7%)[74]. Several prospective studies have also established the safety and efficacy of IRE. Cannon et al[75] reported a 100% initial success rate with IRE in 44 patients with HCC. Adverse events were noted in 11% of patients; however, all complications resolved within 30 d. Local recurrence-free survival rates of 97.4% and 59.5% were recorded at 3 and 12 mo, respectively. A multi-institutional study evaluated the learning curve associated with IRE. Over 2 years, 150 consecutive patients participated at seven institutions[76]. The authors found that treatments of larger lesions and lesions with a greater degree of vascular involvement could be performed safely and effectively with increased experience over a period of time. In a recent study, Narayanan et al[77] compared post-procedural pain and tolerance in patients treated with IRE to that related to RFA. A total of 43 patients (RFA 22, IRE 21) were included, and post-procedural pain was comparable in both groups[77]. In another recent trial, Niessen et al[78] evaluated the risk factors associated with short-term local recurrence after IRE. Twenty-five patients with 48 malignant liver lesions (HCC = 22; cholangiocarcinoma = 6; metastases = 20) underwent IRE. Fourteen of the 48 treated lesions (29.2%) showed early local recurrence after 6 mo. Factors predicting short-term local recurrence were tumor volume (> 5 cm3) and underlying disease type (HCC had a significantly favorable outcome compared to metastases and cholangiocarcinoma). However, distances to the surrounding vessels and bile ducts were not significantly associated with local recurrence.
Due to limited data regarding long-term safety and efficacy, the use of IRE on a widespread scale is not recommended at present. The best use of this technology, in the meantime, is for selected patients in whom other currently available ablative treatments are not feasible.
TACE is recommended as the standard of care for intermediate-stage HCC (Figure 5), based on improved survival demonstrated in a meta-analysis that compared TACE to the best available supportive care or to other, suboptimal therapies[79]. A limitation of this study was the considerable heterogeneity between the individual study designs as well as the study results. Only 2[80,81] of the 6 included RCTs reported a 2-year survival benefit over conservative management[80-85]. Additionally, a recently concluded Cochrane trial concluded that there is no firm evidence to support or refute either TACE or bland transarterial embolization (TAE) for patients with unresectable HCC. The group suggested that more adequately powered and bias-protected trials are needed[86].
Greater standardization of TACE protocols is needed. The basic principle guiding an ideal TACE protocol is to achieve a maximum and sustained concentration of the chemotherapeutic agent in the tumor bed. Systemic exposure, if necessary at all, should be minimized. In an attempt to achieve these goals, embolic microspheres (drug-eluting beads, or DEB) were introduced that are capable of sequestering doxorubicin hydro-chloride from solution through controlled release following selective administration. Compared with lipiodol-based regimens, these increase local drug concentration and, in turn, the efficacy[87]. The PRECISION V trial provides evidence for the efficacy and safety of TACE with DEB compared to conventional TACE[88]. The results from this trial demonstrated greater tolerance, with significant reductions in doxorubicin-related side effects and serious hepatotoxicity compared to conventional TACE. The objective response rate in the TACE with DEB group was significantly better than in the conventional TACE group[88]. An important observation from the trial was that high-dose doxorubicin treatment was successfully achieved in the whole DEB group.
A recent study compared the treatment of HCC with TACE using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded DEB[89]. A total of 158 patients were enrolled in this study. TACE with lipiodol-doxorubicin and gelatin sponges (group A), TACE with lipiodol-doxorubicin and microspheres (group B), and TACE with doxorubicin-loaded DEB (group C) were performed in 64, 41, and 53 patients, respectively. In group C, a significantly higher doxorubicin dosage was achieved and complete response rates were significantly higher[85].
Studies have investigated the ideal size of the microspheres for drug delivery in DEB-TACE. A recent study compared the safety and efficacy of 70-150 μm DEB to 100-300 μm DEBs[90]. A cohort of HCC patients who underwent TACE with two vials of 100-300 μm DEBs was compared to those treated with one vial of 70-150 μm DEBs followed by one vial of 100-300 μm DEBs. Though the short-term efficacy did not differ, TACE with smaller DEBs (70-150 μm) followed by larger DEBs (100-300 μm) was found to be more likely to cause hepatobiliary adverse events.
It remains to be thoroughly evaluated whether the addition of the chemotherapeutic agent to embolic microspheres improves treatment effectiveness. This issue has been addressed in a few studies. In one RCT, bland embolization was compared to embolization with beads loaded with doxorubicin[91]. The results demonstrated a significantly lower tumor-progression rate at 12 mo in the DEB group than in the bland embolization group[91]. Another study established the superiority of DEB-TACE compared to bland embolization[50]. The authors studied the degree of necrosis in explanted livers following TACE with epirubicin-loaded DEB and after bland embolization in patients on a transplant waiting list. Complete necrosis was achieved in 77% of tumors in the DEB group and only 27% of tumors in the bland embolization group, a statistically significant difference between the two groups[50].
A recent trend has been towards combination therapy in an attempt to achieve better tumor-free survival rates. Published research supports the advantage of various forms of combination therapies with TACE (with lipiodol or DEB). Ginsburg et al[92] compared retrospectively the outcomes and complications of transcatheter arterial chemoembolization using drug-eluting embolic agents combined with RFA or microwave MWA in the treatment of HCCs[92]. A total of 89 patients with HCCs were recruited for combination therapy, with TACE plus RFA (group A) administered to 38 patients and TACE plus MWA (group B) administered to 51 patients. Complete local-tumor response rates were 80.4% and 76.6% for groups A and B, respectively, with no statistically significant difference between the two groups. The median tumor PFS and overall PFS were also comparable between the two groups. The authors concluded that both combination therapies are effective treatments for HCC. Iezzi et al[93], in a recent prospective trial, evaluated the efficacy of single-step RFA and DEB-TACE in patients with single HCCs > 3 cm. The group treated with combined therapy showed significantly lower 2-year recurrence and significantly higher survival rates than did the group treated with chemoembolization alone. In the on-going SPACE trial (Sorafenib or placebo in combination with TACE for intermediate-stage HCC), the potential synergy between TACE (with DEB) and sorafenib is being investigated[94]. The basis for this trial is that hypoxia can lead to neoangiogenesis (a potential situation with TACE monotherapy). An anti-angiogenic agent might inhibit the post-TACE surge in VEGF-mediated signaling, preventing tumor growth. Moreover, systemic administration may suppress tumor foci distant from the TACE site. In phase II of the SPACE trial, 307 patients undergoing TACE were randomized to sorafenib (n = 154) or placebo (n = 153) groups. The results from phase II reported a hazard ratio (HR) for time-to-tumor progression (TTP) of 0.797 (95%CI: 0.588-1.080; P = 0.072), with median TTP (50th percentile) of 169 and 166 d in the sorafenib and placebo groups, respectively. The primary goal of improving TTP by using sorafenib-TACE with DEB was achieved in the SPACE study. The data from on-going phase III trials are awaited to confirm these favorable results.
Studies have also compared the survival of patients following TACE with the survival of patients following the resection of large HCCs. A recent meta-analysis (comprising 12 studies) reported a survival benefit of resection compared to TACE in patients with BCLC stage A and B HCC.
Radioembolization is another therapeutic option for intermediate-stage HCC. The most commonly employed radioembolization technique at present employs microspheres coated with a β-emitting isotope, yttrium 90 (90Y). Similar to TACE, intra-arterially injected microspheres are preferentially delivered to the HCC, with selective emission of high-energy, low-penetration radiation to the tumor. Several phase I and II clinical trials have documented the safety of radioembolisation[95-98]. The efficacy of radioembolization for the treatment of HCC has also been reported by a number of cohort studies and retrospective analyses. Retrospective studies report that patients with intermediate-stage HCCs may have similar survival following treatment with either conventional TACE or radioembolization. However, longer time-to-progression and decreased toxicity have been reported in patients receiving radioembolization[99].
According to the BCLC guidelines, systemic therapy with the multikinase inhibitor sorafenib is considered the standard choice for patients with advanced HCC[100].
Conventionally, TACE is contraindicated in advanced HCC patients who have portal-vein invasion, owing to the risk of hepatic insufficiency[100]. However, recent studies suggest that TACE can be safely performed even in this group[101-103]. Survival benefits in patients with advanced HCC have been suggested by various studies. Song et al[104] reported on the efficacy and safety of TACE-based multimodal treatment in patients with large HCCs (> 10 cm). Of the 146 consecutive patients recruited in the study, 119 patients with portal-vein thrombosis received TACE-based multi-modal treatments (including systemic chemotherapy = 46, radiotherapy = 25, RFA or PEI = 21, surgical resection = 13, and liver transplantation = 4). The remaining 27 received conservative management, comprising the control group. Objective tumor response and OS were significantly better in the TACE-based multimodal treatment groups. Kim et al[105] compared the efficacy of TACE with and without radiotherapy (RT) vs sorafenib for advanced HCC with portal vein tumor thrombosis (PVTT). Of the 557 patients with HCC with PVTT, 295 received TACE, 196 received TACE with RT, and 66 received sorafenib. The TACE plus RT group showed significantly better OS than did either the TACE-alone or the sorafenib groups.
Studies suggest that radioembolization might be an effective treatment option for patients with advanced HCCs. In a recent study by Salem et al[106], a cohort of 291 patients with HCC was treated with 90Y. Of all patients, 52% were BCLC class C. TTP for the entire cohort was 7.9 mo. Sub-group analyses revealed that TTP in the absence of portal vein thrombosis was 15.5 mo while TTP in the presence of portal vein thrombosis was 5.6 mo, suggesting that treatment with 90Y glass microspheres could represent an effective option, especially in patients with portal vein thrombosis for whom TACE is conventionally not thought suitable. Several other studies have also reported favorable results in advanced HCC[107-109]. However, major bodies worldwide have recommended further RCTs to evaluate the safety and efficacy of 90Y[110].
Though there are several emerging techniques for managing HCCs in different stages of disease, clarity regarding the application and safety of one method over another and about the use of combinations of different methods remains contentious. Well-planned RCTs covering all stages of HCCs are required before a standard of care can be adopted.
P- Reviewer: Asirvatham SJ, Baiocchi GL, Francica G S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] |
| 3. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 4. | Toso C, Mentha G, Kneteman NM, Majno P. The place of downstaging for hepatocellular carcinoma. J Hepatol. 2010;52:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3592] [Article Influence: 276.3] [Reference Citation Analysis (4)] |
| 6. | Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 561] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 7. | Karanicolas PJ, Jarnagin WR, Gonen M, Tuorto S, Allen PJ, DeMatteo RP, D’Angelica MI, Fong Y. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg. 2013;148:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [PubMed] |
| 9. | Chen J, Huang K, Wu J, Zhu H, Shi Y, Wang Y, Zhao G. Survival after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2011;56:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [PubMed] |
| 11. | Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:422-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:7252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [PubMed] |
| 15. | Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, Esposito V, Coppola C, Giorgio V. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res. 2011;31:2291-2295. [PubMed] |
| 16. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] |
| 17. | Schoppmeyer K, Weis S, Mössner J, Fleig WE. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;3:CD006745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-1723. [PubMed] |
| 19. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [PubMed] |
| 20. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [PubMed] |
| 21. | Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 24. | Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;12:CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [PubMed] |
| 27. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [PubMed] |
| 28. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [PubMed] |
| 29. | N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 30. | Kalra N, Kang M, Bhatia A, Duseja AK, Dhiman RK, Arya VK, Rajwanshi A, Chawla YK, Khandelwal N. Role of radiofrequency ablation in unresectable hepatocellular carcinoma: An Indian experience. Indian J Radiol Imaging. 2013;23:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102-107. [PubMed] |
| 32. | Ogihara M, Wong LL, Machi J. Radiofrequency ablation versus surgical resection for single nodule hepatocellular carcinoma: long-term outcomes. HPB (Oxford). 2005;7:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Wakai T, Shirai Y, Suda T, Yokoyama N, Sakata J, Cruz PV, Kawai H, Matsuda Y, Watanabe M, Aoyagi Y. Long-term outcomes of hepatectomy vs percutaneous ablation for treatment of hepatocellular carcinoma < or =4 cm. World J Gastroenterol. 2006;12:546-552. [PubMed] |
| 34. | Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192-198. [PubMed] |
| 35. | Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg. 2008;12:1521-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology. 2008;55:2171-2174. [PubMed] |
| 37. | Peng ZW, Chen MS, Liang HH, Gao HJ, Zhang YJ, Li JQ, Zhang YQ, Lau WY. A case-control study comparing percutaneous radiofrequency ablation alone or combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. Eur J Surg Oncol. 2010;36:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [PubMed] |
| 39. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 641] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 40. | Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954-960. [PubMed] |
| 41. | Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126. [PubMed] |
| 42. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353-2360. [PubMed] |
| 43. | Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006;16:661-669. [PubMed] |
| 44. | Helmberger T, Dogan S, Straub G, Schrader A, Jüngst C, Reiser M, Waggershauser T, Jakobs T, Hoffmann RT, Löhe F. Liver resection or combined chemoembolization and radiofrequency ablation improve survival in patients with hepatocellular carcinoma. Digestion. 2007;75:104-112. [PubMed] |
| 45. | Lencioni R, Crocetti L, Petruzzi P, Vignali C, Bozzi E, Della Pina C, Bargellini I, Cioni D, Oliveri F, De Simone P. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J Hepatol. 2008;49:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Kudo M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2004;39:205-214. [PubMed] |
| 47. | Wang YB, Chen MH, Yan K, Yang W, Dai Y, Yin SS. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16:389-397. [PubMed] |
| 48. | Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 49. | Morimoto M; Celsion. Phase 3 study of ThermoDox with radiofrequency ablation (RFA) in treatment of hepatocellular carcinoma (HCC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: http://www.clinicaltrial.gov/ct2/show/NCT00617981 NLM Identifier: NCT00617981. |
| 50. | Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Majno PE, Mentha G, Mazzaferro V. Partial hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: confirming the trial that will never be, and some comments on the indications for liver resection. Hepatology. 2010;51:1116-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Mack MG, Straub R, Eichler K, Engelmann K, Zangos S, Roggan A, Woitaschek D, Böttger M, Vogl TJ. Percutaneous MR imaging-guided laser-induced thermotherapy of hepatic metastases. Abdom Imaging. 2001;26:369-374. [PubMed] |
| 53. | Eickmeyer F, Schwarzmaier HJ, Müller FP, Nakic Z, Yang Q, Fiedler V. [Survival after laser-induced interstitial thermotherapy of colorectal liver metastases--a comparison of first clinical experiences with current therapy results]. Rofo. 2008;180:35-41. [PubMed] |
| 54. | Zhou ZJ, Xu RD, Li WK, Zhuang WX, Lu LG, Shao PJ, Chen XM, Luo PF. [Transarterial oily chemoembolization combined with interstitial laser thermotherapy for treatment of hepatocellular carcinoma]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1866-1868. [PubMed] |
| 55. | Xu G, Luo G, He L, Li J, Shan H, Zhang R, Li Y, Gao X, Lin S, Wang G. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Pacella CM, Francica G, Di Lascio FM, Arienti V, Antico E, Caspani B, Magnolfi F, Megna AS, Pretolani S, Regine R. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27:2615-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Chan AC, Cheung TT, Fan ST, Chok KS, Chan SC, Poon RT, Lo CM. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg. 2013;257:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Cheung TT, Fan ST, Chan SC, Chok KS, Chu FS, Jenkins CR, Lo RC, Fung JY, Chan AC, Sharr WW. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol. 2013;19:3083-3089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 59. | Ng KK, Poon RT, Chan SC, Chok KS, Cheung TT, Tung H, Chu F, Tso WK, Yu WC, Lo CM. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Dupré A, Melodelima D, Pérol D, Chen Y, Vincenot J, Chapelon JY, Rivoire M. First clinical experience of intra-operative high intensity focused ultrasound in patients with colorectal liver metastases: a phase I-IIa study. PLoS One. 2015;10:e0118212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Gandini A, Melodelima D, Schenone F, N’Djin AW, Chapelon JY, Rivoire M. High-intensity focused ultrasound (HIFU)-assisted hepatic resection in an animal model. Ann Surg Oncol. 2012;19 Suppl 3:S447-S454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [PubMed] |
| 63. | Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, Liu GJ, Liang JY, Lau WY. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Sato M, Watanabe Y, Ueda S, Iseki S, Abe Y, Sato N, Kimura S, Okubo K, Onji M. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110:1507-1514. [PubMed] |
| 65. | Itoh S, Ikeda Y, Kawanaka H, Okuyama T, Kawasaki K, Eguchi D, Korenaga D, Takenaka K. Efficacy of surgical microwave therapy in patients with unresectable hepatocellular carcinoma. Ann Surg Oncol. 2011;18:3650-3656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547-1555. [PubMed] |
| 67. | Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299-307. [PubMed] |
| 68. | Seki S, Sakaguchi H, Iwai S, Kadoya H, Kabayashi S, Kitada T, Fujii H, Tanaka T. Five-year survival of patients with hepatocellular carcinoma treated with laparoscopic microwave coagulation therapy. Endoscopy. 2005;37:1220-1225. [PubMed] |
| 69. | Abdelaziz AO, Nabeel MM, Elbaz TM, Shousha HI, Hassan EM, Mahmoud SH, Rashed NA, Ibrahim MM, Abdelmaksoud AH. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: prospective analysis. Scand J Gastroenterol. 2015;50:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109-113; discussion 113-114. [PubMed] |
| 71. | Jungraithmayr W, Burger D, Olschewski M, Eggstein S. Cryoablation of malignant liver tumors: results of a single center study. Hepatobiliary Pancreat Dis Int. 2005;4:554-560. [PubMed] |
| 72. | Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223-231. [PubMed] |
| 73. | Charpentier KP. Irreversible electroporation for the ablation of liver tumors: are we there yet? Arch Surg. 2012;147:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Kingham TP, Karkar AM, D’Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, Sofocleous CT, Solomon SB, Jarnagin WR, Fong Y. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 75. | Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 76. | Philips P, Hays D, Martin RC. Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: learning curve evaluation in the first 150 patients treated. PLoS One. 2013;8:e76260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Narayanan G, Froud T, Lo K, Barbery KJ, Perez-Rojas E, Yrizarry J. Pain analysis in patients with hepatocellular carcinoma: irreversible electroporation versus radiofrequency ablation-initial observations. Cardiovasc Intervent Radiol. 2013;36:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Niessen C, Igl J, Pregler B, Beyer L, Noeva E, Dollinger M, Schreyer AG, Jung EM, Stroszczynski C, Wiggermann P. Factors associated with short-term local recurrence of liver cancer after percutaneous ablation using irreversible electroporation: a prospective single-center study. J Vasc Interv Radiol. 2015;26:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] |
| 80. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] |
| 81. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [PubMed] |
| 82. | A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256-1261. [PubMed] |
| 83. | Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578-1583. [PubMed] |
| 84. | Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453-456. [PubMed] |
| 85. | Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129-134. [PubMed] |
| 86. | Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo) embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;3:CD004787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 87. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [PubMed] |
| 88. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1207] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 89. | Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK, Tsai HM, Chuang MT. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015;16:125-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Deipolyi AR, Oklu R, Al-Ansari S, Zhu AX, Goyal L, Ganguli S. Safety and efficacy of 70-150 μm and 100-300 μm drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2015;26:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 92. | Ginsburg M, Zivin SP, Wroblewski K, Doshi T, Vasnani RJ, Van Ha TG. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Iezzi R, Pompili M, La Torre MF, Campanale MC, Montagna M, Saviano A, Cesario V, Siciliano M, Annicchiarico E, Agnes S. Radiofrequency ablation plus drug-eluting beads transcatheter arterial chemoembolization for the treatment of single large hepatocellular carcinoma. Dig Liver Dis. 2015;47:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): phase II, randomized, double-blind SPACE trial. J Clin Oncol. 2012;30 Suppl 4:Abs LBA154. |
| 95. | Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, Van Buskirk M, Roberts CA, Goin JE. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194-S205. [PubMed] |
| 96. | Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, Sergie Z, Wong CY, Thurston KG. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627-1639. [PubMed] |
| 97. | Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, Panizo A, Gil B, Inarrairaegui M, Herrero I. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792-800. [PubMed] |
| 98. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 99. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1324] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 100. | Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087-2094. [PubMed] |
| 101. | Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, Lim YS, Lee HC, Chung YH, Lee YS. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 102. | Jang JW, Bae SH, Choi JY, Oh HJ, Kim MS, Lee SY, Kim CW, Chang UI, Nam SW, Cha SB. A combination therapy with transarterial chemo-lipiodolization and systemic chemo-infusion for large extensive hepatocellular carcinoma invading portal vein in comparison with conservative management. Cancer Chemother Pharmacol. 2007;59:9-15. [PubMed] |
| 103. | Yen FS, Wu JC, Kuo BI, Chiang JH, Chen TZ, Lee SD. Transcatheter arterial embolization for hepatocellular carcinoma with portal vein thrombosis. J Gastroenterol Hepatol. 1995;10:237-240. [PubMed] |
| 104. | Song do S, Nam SW, Bae SH, Kim JD, Jang JW, Song MJ, Lee SW, Kim HY, Lee YJ, Chun HJ. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2015;21:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, Ryoo BY, Kang YK, Lee D, Kim KM. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320-329.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 106. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 779] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 107. | Iñarrairaegui M, Martinez-Cuesta A, Rodríguez M, Bilbao JI, Arbizu J, Benito A, Alegre F, D’Avola D, Herrero JI, Quiroga J. Analysis of prognostic factors after yttrium-90 radioembolization of advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Iñarrairaegui M, Thurston KG, Bilbao JI, D’Avola D, Rodriguez M, Arbizu J, Martinez-Cuesta A, Sangro B. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2010;21:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 109. | Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 110. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers V. 2. 2010. [Accessed March15, 2015]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. |