INTRODUCTION
The goal in brain tumor surgery is to remove the maximum achievable amount of the tumor, preventing damage to “eloquent” brain regions, as the amount of brain tumor resection is one of the prognostic factors for time to tumor progression and median survival[1,2]. Preoperatively acquired magnetic resonance (MR) images can nicely delineate the tumor extent and adjacent anatomical structures. The application of an operating microscope in the late 1950s[3,4] revolutionized neurosurgery. Further advances included computer-assisted devices for surgical navigation[4,5] and, more recently, intraoperative imaging[6] to incorporate and correct for brain shift during the resection of the lesion. However, surgically induced contrast enhancement along the rim of the resection cavity[7] hampers interpretation of these intraoperatively acquired MR images (Figure 1). To overcome this uncertainty, perfusion techniques have been introduced. Dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) is a T2*-weighted technique that enables calculation of regional cerebral blood flow (rCBF) and regional cerebral blood volume (rCBV) maps. The measurement takes only 1 min, 20 s and does not extend the overall scanning procedure. It can be applied various times as it is independent of T1-effects after saturation, has proven to be as reliable as preoperatively performed DSC-MRI[8,9] and can distinguish residual tumor form surgically induced artefacts. Dynamic contrast-enhanced T1-weighted perfusion (DCE-MRI) has alternatively been used intraoperatively[10]. The beauty of this approach that requires more time than DSC-MRI (also at 3T) is that there is a by-product with the acquired T1-weighted images as the slope of contrast enhancement can easily be analyzed without the need for additional software. A quickly climbing slope depicts residual tumor tissue. However, the following still needs to be proven: can the DCE-MRI be repeatedly applied, is analysis unaffected by the commonly used absorbable hemostats (such as surgical®, Ethicon 360), and can it reliably differentiate other sources of contrast enhancement over time, such as bleedings. Both techniques, however, can differentiate residual tumor from surgically induced changes at the rim of the resection cavity and thus overcome the remaining uncertainty of intraoperative MRI in high grade brain tumor resection.
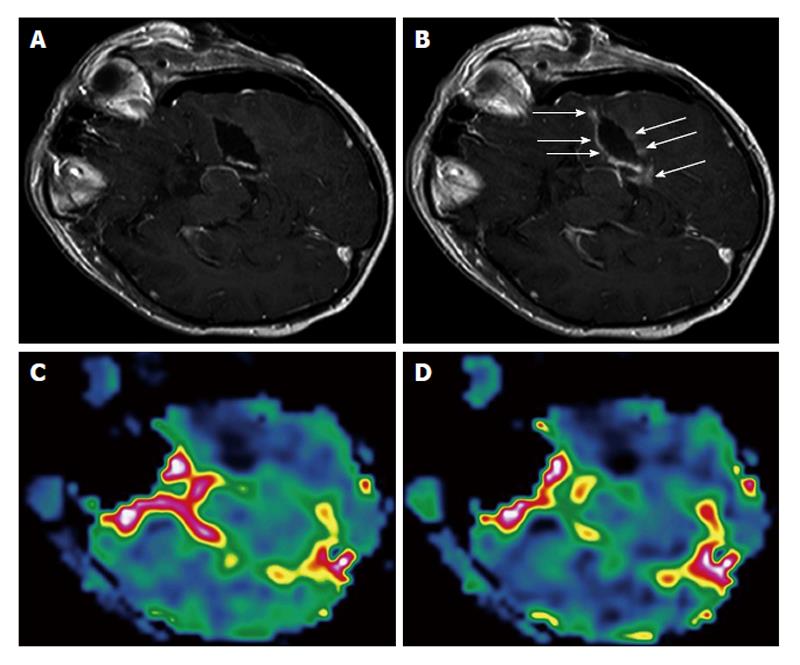
Figure 1 Surgically induced intraoperative contrast leakage.
Reprinted from NeuroImage with permission[9]. A: T1-weighted magnetic resonance (MR) image of the initial resection control. Residual tumor was depicted (not shown), neuronavigation was updated and the residual tumor was removed; B: T1-weighted MR image in identical orientation as in (A) of the second intraoperative resection control. At the border of the resection cavity there is contrast enhancement of previously non-enhancing tissue (arrows), which is caused by the neurosurgical resection leading to a leakage phenomenon. Perfusion maps of rCBV (C) and rCBF (D) at the second resection control demonstrate no elevated values in areas of contrast enhancement but complete resection of the tumor. rCBV: Regional cerebral blood volume; rCBF: Regional cerebral blood flow.
The extent of tumor resection is one of the prognostic factors for time to tumor progression and median survival for patients with both high and low grade gliomas[1,2]. Various attempts have been undertaken to achieve the maximum resection of a lesion. Most of them are imaging-based. Beginning in 1980, intraoperative ultrasound was the first imaging modality guiding neurosurgical procedures[3]. Preoperatively acquired images were integrated in computer-assisted devices for surgical navigation beginning in 1986[4,5]. Prior to resection of a lesion, an “image-to-patient” registration is necessary to align the MRI coordinates with the patient’s head position in the OR. However, due to brain shift during the resection of the lesion, or leakage of CSF, these preoperatively acquired images were progressively imprecise over time. The double-donut was the first intraoperative MRI system at the Brigham and Women’s hospital in Boston in 1997[6]. This enabled an update of the underlying anatomy at any time during the resection of a lesion, including an update of the navigation system. One major drawback, besides the costs of such a system, is that there is a surgically induced contrast enhancement along the resection cavity margin in intraoperatively acquired T1-weighted images after contrast administration[7] that can hamper judgment concerning the differentiation between residual solid tumor or just surgically induced artefact, especially taken together with the well known brain shift that may also preclude the comparison with preoperatively acquired images. Another approach that found wide acceptance was the use of intravenous administration of 5-aminolevulinic acid[11], leading to tumor fluorescence intraoperatively that also resulted in more complete tumor removal. However, lack of visible fluorescence in the adjacent tissue is not as highly predictive of normal tissue as biopsy proven[12]. Thus, some centers (where available) use both complementary methods. However, the contrast enhancement in these intraoperative images remains a challenge with conventional imaging only.
INTRAOPERATIVE SETUP
Instead of using the double-donut setup, which requires that the surgeon is within the magnetic field thus requiring non-magnetic tools[6], “twin operating theatres” have been proposed for both low[13,14] and more recently high field systems, with the need to transfer the patient between the imaging and surgical site. This has become the setup of choice in most institutions[8,15,16], although some move the magnet towards the surgical site[17]. Lower field systems with permanent magnets are also in use[18], which is a compromise but does not allow advanced imaging (see below). To use the conventional neurosurgical setup, including neuronavigation, microscope and conventional ferromagnetic instruments, the patient has to be outside the 0.5 mT or 5-Gauss line of the magnet during the surgical procedures to avoid a pull of ferromagnetic objects into the bore of the scanner, especially when the patient is positioned for scanning. Also, these objects interfere with imaging and can hamper image quality, causing artefacts. Dynamic sequences like DSC-MRI require a high field scanner (1.5 T or more), which come in “closed-bore” designs. Head fixation devices need to be MR-compatible. Whenever the surgeon wants an update, MRI can be performed after removing all ferromagnetic objects and sterile coverage of the craniotomy.
ADVANCED INTRAOPERATIVE MRI
To define residual tumor, various approaches have been performed using advanced imaging techniques. It has been demonstrated that parts of brain tumors do not enhance as biopsy proven[19] which can be depicted by MR spectroscopy (MRS), a technique that is very susceptible to artefacts and also time consuming. However, it helps to delineate typical changes associated with brain tumors and to define the real extent of a lesion more precisely than the use of conventional MR imaging only. Recently, this had been used intraoperatively to identify residual tumor with a sensitivity of 85.7% and specificity of 100%[20]. However, air filled (resection) cavities might preclude MRS, or small residual tumor areas might be missed, and it is impossible to map the complete rim of a resection cavity intraoperatively due to time restraints, thus the area to be monitored has to be defined.
Perfusion imaging in clinical routine is most commonly performed as DSC-MRI-weighted perfusion, which is T2*-weighted or as DCE-MRI. Both techniques are most commonly used for stroke imaging but also in neuro-oncology and intraoperatively.
DSC-MRI
DSC-MRI enables calculation of regional maps for relative blood volume and flow by administering conventional MR contrast agents while T2*-weighted images (i.e., 40 images/slice) are being acquired. In areas of blood-brain barrier breakdown (such as brain tumors), distinct zones with increased cerebral blood flow and volume can be depicted, which correspond to neovascularization and active metabolism within the tumor[21-28]. Prior to the DSC measurement, 2 cc of contrast agent are injected for reduction of the T1 effect (saturation). For perfusion imaging, the contrast agent is administered as a bolus, followed by a saline flush with a flow rate of 5 cc/s during a dynamic susceptibility-dependent T2*-weighted GE EPI sequence (i.e., TR/TE = 17/8 ms; FOV 240 mm; matrix 128 × 128; EPI factor = 17, number of slices 30 with slice thickness of 3.5 mm, duration: 1 min 20 s[8,9]). These data are then transferred to a workstation to create maps of the rCBF and rCBV and to measure the mean transit time of the contrast agent passing through the brain[29] based on the tissue dilution theorem. As absolute quantification is not yet possible, ratios to the unaffected hemisphere or adjacent tissue are created to judge the perfusion data. As T2*-weighted images are susceptible to artefacts caused by air fluid levels (such as resection cavities) or air filled spaces (like sinus), its intraoperative application required a multistage approach. Initially, a phantom study was performed using a model with a rigorous air water level that showed only moderate artefacts. In a second step, a model with continuous laminar flow was used that showed susceptibility artefacts close to the tubes (overestimation of perfusion adjacent to vessels[8]). In patients, residual tumor was depicted intraoperatively by DSC-MRI[8], which is independent of brain shift and surgically induced disruption of the blood brain barrier and was proven by histology. In a third step, the reliability of intraoperatively acquired data was demonstrated in a series of patients with high grade gliomas who had undergone pre- and intraoperative DSC-MRI with some residual tumor in the early intraoperative resection control[8,9]. Ratios of identical areas within the tumor tissue did not differ significantly between pre- and intraoperatively acquired data. Furthermore, there was a high correlation of the analyzed rCBV and rCBF ratios between pre- and intraoperative MRI exams. Intraoperatively, flexible two-channel surface coils were used, whereby one part was placed below the patient’s head at the beginning of the operation and the second part adjusted prior to intraoperative scanning on the craniotomy defect, both draped in a sterile fashion. DSC-MRI was performed in 1 min 20 s, which did not extend overall intraoperative MR imaging. Intraoperative sedation (such as propofol anesthesia) reduces the absolute values of CBF and CBV; however, the ratios between tumor and unaffected contralateral tissue remain constant[30]. DSC-MRI can be repeated various times as previously used contrast agent leads to a desired saturation of T1 effects but does not influence T2*-weighted images.
DCE-MRI
Dynamic contrast-enhanced T1-weighted perfusion MR imaging (DCE-MRI)[31-33] is the other commonly used perfusion technique. In DCE-MRI, k-trans is analyzed, which is the transfer coefficient (endothelial permeability surface product). DCE-MRI requires various sampling points over time and usually takes much longer than DSC-MRI. T1 is reduced by clinically used contrast agents, leading to a signal intensity increase in T1-weighted images. Thus, DCE-MRI measures contrast agent concentration as a function of time. Very recently, DCE-MRI was used intraoperatively at a 3 T MR scanner[10]. The used setup took 3 min and 45 s for the perfusion sequence. In addition to a pharmacokinetic modelling, the authors analyzed the slope of the signal intensity increase in these T1-weighted images. Residual solid tumor could be distinguished from surgically induced contrast enhancement at the rim of the resection border by a quickly climbing slope in tumors, compared to a low-amplitude undulating curve in brain tissue as proven by histology. This may have great potential as it is obviously much easier to apply and analysis of the slope of contrast enhancement does not require any additional software as such programs come with the scanner software. However, it still has to be proven whether or not DCE-MRI can be repeatedly applied in the unlikely event of multiple resection controls, whether analysis is affected by commonly used absorbable hemostats (such as surgical®, Ethicon 360), and also if it can reliably differentiate other sources of contrast enhancement over time, such as bleedings.
CONCLUSION
The goal in brain tumor surgery is to remove the maximum achievable amount of the tumor, preventing damage to “eloquent” brain regions, as the amount of brain tumor resection is one of the prognostic factors for time to tumor progression and median survival[1,2]. Preoperatively acquired MR images can nicely delineate the tumor extent and adjacent anatomical structures. The application of an operating microscope in the late 1950s[34] revolutionized neurosurgery. Further advances included computer-assisted devices for surgical navigation[4,5] and, more recently, intraoperative imaging[6] to incorporate and correct for brain shift during the resection of the lesion. However, surgically induced contrast enhancement along the rim of the resection cavity[7] hampers interpretation of these intraoperatively acquired MR images. To overcome this uncertainty, perfusion techniques have been introduced. DSC-MRI is a T2*-weighted technique that enables calculation of rCBF and rCBV maps. The measurement takes only 1 min 20 s and therefore does not extend the overall scanning procedure. It can be applied various times as it is independent of T1-effects after saturation, has proven to be as reliable as preoperatively performed DSC-MRI[8,9], and can distinguish residual tumor from surgically induced artefacts. DCE-MRI has also been used intraoperatively as an alternative[10]. The beauty of this approach that requires more time than DSC-MRI (also at 3 T) is that there is a by-product with the acquired T1-weighted images as the slope of contrast enhancement can easily be analyzed without the need for additional software. A quickly climbing slope depicts residual tumor tissue. However, it still has to be proven that DCE-MRI can be repeatedly applied, that analysis is unaffected by commonly used absorbable hemostats (such as surgical®, Ethicon 360), and that it can also reliably differentiate other sources of contrast enhancement over time, such as bleedings. Both techniques can differentiate residual tumor from surgically induced chances at the rim of the resection cavity and thus overcome the remaining uncertainty of intraoperative MRI in high grade brain tumor resection.
P- Reviewer: Sivak S S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Liu SQ