INTRODUCTION
In the United States, about 2.7 million doctors’ office visits/year are related to patients complaining about finger, hand or wrist symptoms[1]. The diagnosis of these symptoms can include various types of nerve entrapments, tendon disorders, overuse of muscles or nonspecific pain syndromes[1]. The most common type among them is carpal tunnel syndrome (CTS), which accounts for 90% of all entrapment neuropathies[2,3] and is one of the most commonly diagnosed disorders of the upper extremities[3,4]. It is expected that 1 in 5 patients who complain of symptoms of pain, numbness and a tingling sensation in the hands will be diagnosed with CTS based on clinical examination and electrophysiological testing[3]. CTS is estimated to occur in 3.8% of the general population[3,5], with an incidence rate of 276:100000 per year[6], and happens more frequently in women than in men, with a prevalence rate of 9.2% in women and 6% in men[3,7]. It is most often seen bilaterally at a peak age range of 40 to 60 years old; however, it has been seen in patients as young as twenty and as old as eighty-seven years old[3,8].
The carpal tunnel (CT) is found at the base of the palm. It is bounded partly by the eight carpal bones and partly by a tough fibrous roof called the transverse carpal ligament (TCL). The tunnel gives passage to: (1) eight digital flexor tendons (two for each of the medial four fingers); (2) flexor pollicis longus (FPL) tendon for the thumb; (3) their flexor synovial sheaths; and (4) the median nerve (MN)[1]. CT is therefore quite tightly packed and any condition that might increase the volume of the structures inside it can cause compression of the MN. This in turn might lead to ischemia of the nerve which presents as pain and paresthesia[1,8].
The American Academy of Orthopedic Surgeons (AAOS) defines CTS as “a symptomatic compression neuropathy of the median nerve at the level of the wrist”[3,9]. MN gives sensory branches to the lateral three fingers and the lateral half of the ring finger so that when it is compressed, symptoms of CTS are manifested in those fingers[3]. The palm of the hand, however, remains unaffected by CTS as it is supplied by the sensory cutaneous branch of median nerve (PCBMN). This branch arises about 6 cm proximally to the TCL, then passes superficially to the ligament so it is not affected by the pressure changes within the CT[3].
Furthermore, the most common diagnosis in patients with symptoms of pain and numbness is idiopathic CTS with a tingling sensation along the MN distribution in the hands[10]. Although this syndrome is widely recognized, its etiology remains largely unclear. Recent biomechanical, MRI and histological studies have strongly suggested the close relationship of the dysfunction of neuronal vasculature, synovial tissue and flexor tendons within the CT and the development of idiopathic CTS[11,12].
CT is the fibro-osseous pathway on the palmar aspect of the wrist which connects the anterior compartment of the distal forearm with the mid-palmar space of the hand. On its bottom, the CT is made up of the carpal bones articulating together to form a backward convex bony arch, resulting in formation on the dorsal side and concave on the palmar side, forming a tunnel-like groove called the sulcus carpi. This osseous groove is topped volar by the tough flexor retinaculum (FR), which arches over the carpus, thus converting the sulcus carpi into the CT.
FR can be differentiated into three continuous segments: (1) a proximal thin segment called the volar carpal ligament. It is the thickened deep antebrachial fascia of the forearm; (2) the middle tough segment is the TCL; and (3) the distal segment is formed from an aponeurosis which extends distally between the thenar and hypothenar muscles. Therefore, it is recommended to have a more extensive surgical release instead of only resection of the middle segment of the FR[13].
The width of the CT is about 20 mm at the level of the hook of hamate, which is narrower compared to its proximal (24 mm) or distal (25 mm) end[13,14] counterparts. Moreover, the narrowest sectional area of the tunnel is located 1 cm beyond the midline of the distal row of the carpal bones where its sectional area is about 1.6 cm2[15].
In healthy individuals, the intra-CT pressure is about 3-5 mmHg when the wrist is in a neutral position[16,17]. MN blood flow was found to be impaired when the CT pressure approached or exceeded 20-30 mmHg. Common functional positions of the wrist, e.g., flexion, extension or even using a computer mouse, might result in an increase of tunnel compression pressures to levels high enough to impair MN blood flow[18]. For example, placing the hand on a computer mouse increase the CT pressure to 16-21 mmHg, while using the mouse to point and click increased the CT pressure up to 28 to 33 mmHg[19]. Interestingly, CT pressure was shown to increase to 63 mmHg with 40 degrees of wrist extension and 0 degrees of metacarpophalangeal flexion[20].
The position of adjacent muscular structures is thought to play a significant role in these positional increases in CT pressure[20]. In a study of the MN in fresh human cadavers, a significant distal bulk of the flexor digitorum superficialis (FDS) muscle was found to enter the proximal aspect of the tunnel during wrist extension[21]. Similarly, the lumbrical muscles were shown to enter the distal aspect of the tunnel during metacarpophalangeal flexion. Computer modeling suggests that when the metacarpophalangeal joints are flexed to 90 degrees, the lumbrical muscles remain in the CT, even if the wrist is kept extended[22].
A thorough knowledge of the complex anatomy of the CT and its surrounding structures in addition to an emphasis on its clinical applications is essential for a better understanding of the pathophysiology of CTS, along with its symptoms and signs. Such knowledge will enable surgeons to take the most appropriate and safest approach during open or endoscopic carpal tunnel release (ECTR) surgeries by accurately identifying structures at or near the CT in order to avoid or reduce its surgical complications and ensure optimal patient outcome. It is also important to be aware of the likely possible anatomical variations that might be the cause of MN compression or may be anticipated and more readily recognized by hand surgeons. This review aims to provide an overview of CTS by considering anatomy, pathophysiology, clinical manifestation, diagnostic modalities and management of this common condition, with an emphasis on its diagnostic imaging evaluation.
CLINICAL AND SURGICAL ANATOMY OF CT
Movements of the wrist joint have an effect on the shape and width of the CT. The width of the tunnel decreases considerably during the normal range of wrist motion and since the bony walls of the tunnel are not rigid, the carpal bones move relative to each other with every wrist movement. Both flexion and extension increase the CT pressure. The cross section of the proximal opening of the CT was found to be significantly decreased with a flexing wrist joint. This is likely due to the radial shifting of the TCL and the movement of the distal end of the capitates bone. In extreme extension, the lunate bone compresses the passage as it is pushed towards the interior of the tunnel[15].
TCL is the thick (2-4 mm) central segment of the FR. It is a strong fibrous band formed from interwoven bundles of fibrous connective tissues[13] and is short and broad (average width is 25 mm and length is 31 mm)[23,24]. It extends from the distal part of the radius to the distal segment of the base of the third metacarpal. The mean proximal limit of its central portion is 11 mm distal to the capitate-lunate joint and the mean distal limit of its distal portion is 10 mm distal to the carpometacarpal joint of the third metacarpal[13].
Regarding laminar configuration of the TCL, four basic laminae were identified: (1) strong distal transverse; (2) proximal transverse; (3) ulnar oblique; and (4) radial oblique. The most common pattern showed predominance of the distal transverse and the ulnar oblique laminae in every layer of the FCL. In half of the dissected hand samples, the distal transverse and ulnar oblique laminae dominated in the superficial layer, while the proximal transverse and the radial oblique laminae dominated in the deep layer. So, the strong distal transverse lamina is likely to be excised during the final step of ECTR because of its superficial localization. This could be a major cause for the frequent occurrence of incomplete release. Moreover, the almost universal superficial ulnar oblique lamina predisposes to scarring, which may cause radial shifting of the ulnar neurovascular bundle and may affect the PCBMN. It is concluded that the minor complications of ECTR depend partly on the variations in the laminar arrangement of the TCL[25]. In another study performed on eight dissected TCLs, the transverse fibers were the most prominent (> 60%), followed by the oblique fibers in the pisiform-trapezium direction (18%), the oblique fibers in the scaphoid-hamate direction (13%) and finally the longitudinal fibers (8%)[26].
Borders of the TCL
The TCL is attached medially to the pisiform bone and hook of the hamate, while laterally it splits into superficial and deep laminae. The superficial lamina is attached to the tubercle of the scaphoid and trapezium and the deep lamina is attached to the medial lip of the groove on the trapezium. Together with this groove, the two laminae form a tunnel, lined by a synovial sheath containing the tendon of flexor carpi radialis (FCR)[24].
Proximal border of the TCL
Proximally, the TCL is attached to the volar carpal ligament which extends from the radius to the ulna over the flexor tendons as they enter the wrist[24]. This border corresponds to the distal flexion wrist crease, which also crosses the proximal end of scaphoid and pisiform bones.
Distal border of the TCL
This border is attached to the central portion of the palmar aponeurosis (PA). As measured along the axis of the radial border of the ring finger, the average distance between this border and the superficial palmar arch ranges from 5.5-19 mm[27-31]. The mean distance from this distal border to the nearest aspect of the motor branch of MN is about 2.7-6.5 mm[23,32].
Immediately proximal to the distal end of the TCL and in line with the axis of ring finger, a palmar fat pad (fat drop sign) is visualized overlapping this border. It is a reliable anatomic landmark during CT release which must be retracted in order to visualize the distal end of the TCL[14]. Its proximal aspect lies at about 2 mm proximally to the distal edge of the TCL. The distance between the distal end of the TCL and the palmar fat pad decreases by flexing the fingers, but the distance between the TCL and the palmar arch or the PCBMN is not markedly affected. When dividing the TCL from proximal to distal, visualization of the proximal part of the fat pad is a useful indication that the distal edge of the TCL is within approximately 2 mm and indicates that distal dissection beyond this level is unnecessary in order to avoid injury of the superficial palmar arch or the PCBMN[32].
Surfaces of the TCL
Palmar (volar) surface: This surface gives partial origin to all the thenar and hypothenar muscles except the abductor digiti minimi muscle and it also receives partial insertion from the flexor carpi ulnaris (FCU) and palmaris longus (PL).
This surface is entirely hidden by the muscular attachments, which makes it appear much deeper than surgeons think. This might urge surgeons to make a longer incision for good exposure of the TCL and to complete its division[33]. The middle part of this surface is crossed by the PL tendon (if present), with a nerve on each of its sides; palmar cutaneous branch of ulnar nerve (medially) and PCBMN (laterally). The ulnar nerve and vessels cross the medial part of this surface through a special fascial tunnel called the Guyon tunnel[33].
The superficial branch of the radial artery arises from the radial artery just before the latter curves round the carpus. It passes through and occasionally over the thenar muscles, which it supplies. It sometimes anastomoses with the end of ulnar artery to complete the superficial palmar arch[24].
When present, it is a slender and flattened tendon, which passes superficially to the TCL and lies medially to the tendon of FCR. It is partially inserted into its central part of the TCL and extends distally to attach to the proximal part of PA. Frequently, it sends a tendinous slip to the thenar muscles. The MN lies deep to this tendon but when absent, the nerve becomes separated from the skin only by a thin subcutaneous fat and deep fascia[24].
PCBMN arises from the MN proximal to the TCL. It pierces the deep fascia and runs superficially to the TCL, just laterally to the PL tendon. It then divides into lateral branches supplying the thenar skin, communicating with the lateral cutaneous nerve of forearm. The medial branches supply the central palmar skin and communicate with the palmar cutaneous branch of ulnar nerve[24].
Injury to the PCBMN is the most common complication of CT surgery[34] and it has been suggested that the mini incision done between the superficial palmar arch and the most distal part of the PCBMN in the palmar region is the safe zone for CT surgery[34]. Decreased levels of discomfort in patients undergoing endoscopic and subcutaneous types of CT release may be in part due to the preservation of the crossing cutaneous nerves during these procedures[35].
Communicating sensory branches may be multiple and often arise in the proximal forearm and sometimes from the anterior interosseous branch. They pass medially between FDS and FDP and behind the ulnar artery to join the ulnar nerve. This communication is a factor in explaining anomalous muscular innervations in the hand[24]. In relation to an incision for CT release, PCBMB was found to cross the incision only in one specimen (of 25 fresh frozen cadaveric hands), while its terminal branches were identified at the margin of the incision in another two specimens[35].
It arises from the ulnar nerve near the middle of the forearm at about 4.9 cm proximally to the pisiform bone. It then runs distally just medially and parallel to the PL tendon. It enters the palm of hand superficially to the TCL. In 24 specimens, at least one, usually multiple, transverse palmar cutaneous branch was identified originating at about 3 mm distally to the pisiform within Guyon’s canal. In another 10 specimens (of 25 hands), a nerve of Henle arose at about 14.0 cm proximally to the pisiform, travelling with the ulnar neurovascular bundle to the wrist flexion crease[35].
They pass superficially to the FR and enter the hand by passing through a groove between the pisiform (medially) and the hook of hamate (laterally and more distally). The ulnar artery is radial to the nerve and can be easily felt on the ulnar side of the front of the wrist. They usually pass just over the ulnar to the superior portion of the hook of the hamate. Over the FR, they are kept in place by a fascial extension from the volar carpal ligament, forming the ulnar canal (Guyon’s canal). This extension is attached medially to the pisiform bone and blends laterally with the TCL[33]. They lie in the shelter of the lateral edge of the tendon of FCU[24]. Pisiform bone is palpated at the base of the hypothenar eminence and serves to mark the entry on its lateral side of the ulnar nerve and artery into the hand. The mean distance from the radial aspect of the pisiform to the radial border of Guyon’s canal and the ulnar edge of the PL tendon is about 10.3 mm and 16.1 mm respectively[23]. As the ulnar nerve passes between the pisiform and hook of hamate, it terminates by dividing into superficial and deep branches.
With the wrist in neutral position, a looped ulnar artery runs from 2-7 mm medially[27] to 1-4 mm laterally to the hook of the hamate[36]. It then continues to form the superficial palmar arch. With the wrist in radial deviation, the looped ulnar artery migrates to the ulnar side of Guyon’s canal (-2-2 mm radially to the hook of the hamate). During ulnar deviation of the wrist, the ulnar artery shifts more laterally beyond the hook of the hamate (2-7 mm). So, in order to minimize postoperative bleeding and avoid iatrogenic ulnar vascular and neural injury, it is recommended to: (1) transect the TCL over 4-5 mm apart from the lateral margin of the hook of the hamate without placing the edge of the scalpel toward the ulnar side; (2) not to transect the TCL in the ulnar deviation wrist position[27]; and (3) make the proximal portal just medial to the PL tendon in order to spare the ulnar neurovascular structures[36]. However, injury to the ulnar artery within Guyon’s canal has not been a problem during ECTR surgery[14].
Variations: (1) An anomaly of the ulnar nerve with an aberrant branch was observed to cross the CT incision[37]; and (2) A small arterial branch (average diameter, 0.7 mm) arising from the ulnar artery ran transversely just over the TCL in 6 (of the 24 specimens). This branch was consistently located within 15 mm proximally to the TCL distal margin[27].
The deep surface of the TCL: With the carpal bones, this surface forms the CT which is traversed by nine flexor tendons of the fingers, their flexor synovial sheathes and the median nerve.
The median nerve is the softest and most volar structure in the CT. Its average cross-sectional area is 6.19 mm[38]. It lies directly beneath the TCL and is superficial to the nine digital flexor tendons (Figure 1). Proximally to the TCL, the MN lies just laterally to the tendons of FDS and between the tendons of FCR and PL (Figure 2). Its location extends an average of 11 mm radially to the hook of hamate[27]. Distally to the TCL, it enlarges and flattens and usually divides into five or six branches: (1) the recurrent motor branch; (2) three proper digital nerves (two to the thumb and one to the radial side of index finger); and (3) two common digital nerves (one to index/ middle and one to middle/ ring)[24]. Trapped or pinched nerves have a useful electrical property for the diagnosis in that the speed of its conduction slows at the site of trouble due to demyelination.
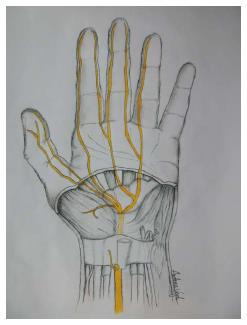
Figure 1 Sketch of the palm, showing specific details of the inner structures of the carpal tunnel (inside the wrist).
The median nerve and its branches after the wrist are marked in yellow.
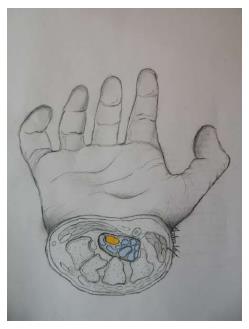
Figure 2 Sketch of the cross-section of the carpal tunnel on a hand.
Median nerve is shown in yellow and the nine flexor tendons are marked in blue.
Anomalies of the median nerve: Variations of the MN at the wrist were reported in about 11% of the examined specimens. Neural variations arising from the medial aspect of the MN were common and could be a cause of iatrogenic injury during endoscopic or open release[39].
In a study performed on 246 carpal tunnels at operation, four groups of variations were described: (1) variations in the course of MN were found in 12%; (2) accessory branches at the distal portion of the CT in 7%; (3) high divisions of the MN in 3%; and (4) accessory branches proximal to the carpal canal in 1.5%. These findings emphasize the importance of approaching the MN from the ulnar side when opening the CT[40].
High bifurcation of the MN
Persistent median artery: the median artery is a transitory vessel that represents the embryological axial artery of the forearm. It normally regresses in the second fetal month[41,42]. Its persistence in the human adult has been documented as two different types: as a large, long vessel which reaches the hand (palmar type); or as a small and short vessel which ends before reaching the wrist joint (antebrachial type)[43,44]. It occurred in about 3.4%-20% of a 646 population sample of hands[37,45]. It is more frequent in females than in males, occurring unilaterally more often than bilaterally and slightly more frequently on the right than on the left. Most frequently, it arises from the caudal angle between the ulnar artery and its common interosseous trunk (59%). Other origins may be from the ulnar artery or from the common interosseous trunk. It ends as the 1st, 2nd or 1st and 2nd common digital arteries (65%) or joins the superficial palmar arch (35%). It pierces the MN in the upper third of the forearm in 41% of cases with the palmar type[45]. The median artery in its palmar type passes under the FR, running in the CT together with the MN and flexor tendons. This relationship has been considered an etiological factor in CTS[46].
An aberrant sensory branch arising from the ulnar side of the MN and piercing the ulnar margin of the TCL was found in 3% of hands (of 110 in operations)[39].
Martin-Gruber anastomosis is a motor communicating nerve, which may cross over from the median to ulnar nerve in the forearm (motor not sensory connections). It occurs in two patterns: either from the MN in the proximal forearm to the ulnar nerve in the middle to distal third of the forearm; or from the anterior interosseous nerve to the ulnar nerve[47].
Other motor anastomoses between the MN and ulnar nerve include: (1) motor branch of the MN to superficial head of flexor pollicis brevis (FPB) and ulnar nerve to the deep head of the FPB; (2) anastomosis, of the MN and ulnar motor branches through first lumbrical or through innervation of the adductor pollicis muscle; (3) branch of the MN to third lumbrical joining neural branch to this muscle from deep branch of ulnar nerve; (4) the MN may also form anastomoses with branch of radial nerve close to abductor pollicis brevis which has the radial nerve innervating this muscle; and (5) first dorsal interosseous, adductor pollicis or even abductor digiti minimi may be innervated by the MN[47].
Motor branch (recurrent or thenar branch)
It is a short and thick branch commonly arising from the radial side of the MN. It may however, arise from the volar or the ulnar side of the MN[14]. It may be the first palmar branch or a terminal branch which arises level with the digital branches of MN. It runs laterally, just distal to the TCL, with a slight recurrent curve beneath the part of the PA covering the thenar muscles. It runs around the distal border of the TCL to lie superficially to the FPB, which it usually supplies, and continues either superficially to the muscle or through it. It gives a branch to the abductor pollicis brevis, which enters the medial edge of the muscle and then passes deep to it to supply the opponens pollicis, piercing its medial edge. Its terminal part occasionally gives a branch to the 1st dorsal interosseous, which may be its sole or partial innervation. It may arise in the CT and pierces the TCL, a point of surgical importance[24]. The position of the motor branch (in 30 hands) was extraligamentous in 46%-60%, subligamentous in 31%-34% and transligamentous in 6%-23% of 116 fresh frozen cadaveric hands[34,40]. So, the most common pattern of the motor branch is extraligamentous and recurrent. The mean distance between the distal edge of the TCL and this branch is about 2.7-6.5 mm[23,32].
The flexor tendons
The flexor tendons are the four tendons of the FDS, four tendons of the FDP and the tendon of the FPL. The superficialis tendons are all separate and the tendons for the middle and ring fingers lie superficially to those for the index and little fingers. The MN lies superficially to the tendons of FDS. The profundus tendons are still deeper to the FDS tendons. Only the slip to the index finger is separate; the other three are still fused and lie medially to the index slip[33]. The FPL tendon passes radially through a special canal between the two laminae of the TCL and the groove of trapezium. It is surrounded by a separate synovial sheath called the “radial bursa” which extends along the thumb as far as the insertion of the tendon at the base of the distal phalanx. Proximally, the radial bursa extends to a point 2.5 cm above the wrist joint/TC. It is sometimes connected to the base of the second metacarpal or may be absent[24].
MECHANICS OF FLEXOR TENDONS AND THE MN WITH FINGER AND WRIST MOVEMENTS
Along their course, the long flexor tendons pass through a flexor pulley system which includes the TCL, PA and the digital pulleys, where the lubricant effect of synovial fluid maintains low friction between these tendon and the pulleys. In vivo and during active flexion and extension of the wrist and fingers, measurements revealed that longitudinal tendon excursion is about 24-50 mm[48,49], while MN excursion was found to range from 11-28 mm during wrist and elbow movement[50,51].
It is highly suggested that non-inflammatory fibrosis and thickening of the synovium is a leading cause for MN compression[52]. These synovial changes also alter the gliding characteristic of the subsynovial connective tissue (SSCT), where it moves en bloc with the tendons and MN, which may play a role in the etiology of CTS[53,54]. About 90% of the synovial specimens resected from patients with idiopathic CTS did not exhibit inflammatory changes, but mostly edema or fibrosis[55,56]. Other findings of chronic synovial degeneration were reported as indicated by the increase in fibroblast density, collagen fiber size and vascular proliferation[57].
Additionally, the flexor tendons move upwards (volar displacement) from the floor of the CT during active finger movement[58-60]. This movement causes a force of compression/ reaction between the tendons and the TCL. Almost the same amount of force of the flexor tendon could be applied to the TCL during finger movement[61].
Because the SSCT and tendon are physically connected, a decrease in SSCT motion (due to fibrosis) relative to the tendon would increase the shear strain on the SSCT with tendon motion. Thus, this result suggests that the SSCT may be predisposed to maximum shear injury from activity done in 60 degrees of wrist flexion more than the motion in all other wrist positions[62]. During hand and finger motions, friction between the FDS tendon and the MN is thought to play a role in the development of cumulative trauma disorders[62]. Also, the ratio of MN excursion to tendon excursion was much lower in finger-only motions compared to wrist motions with or without finger motion[63]. High velocity tendon motion was reported to predispose to SSCT shear injury[64].
A step forward damage in the SSCT in the CT was observed to follow repeated stretch tests within the physiological range of tendon excursion[12,62]. Similarly, repetitive hand activities caused thickening of the synovial lining of the tendons that share the CT with the MN[20,65].
Furthermore, shear tension and injury of the SSCT in CTS patients is significantly higher than that in normal subjects[66] and the excursion of the MN is markedly reduced[50,51]. This finding may be consistent with the fact that fibrosis of the synovial tissue within the CT is often observed in CTS patients.
PALMAR APPONEUROSIS (STRUCTURE AND FUNCTION)
The deep fascia of the palm of hand (palmar fascia) is thin over the thenar and hypothenar eminences, but its central portion, the PA, is triangular in shape. It has great strength and thickness. Its apex is continuous proximally with the distal border of TCL and receives the expanded tendon of the PL. Its base divides below into four slips, one for each finger[33].
The PA covers the central compartment of the hand which contains the long flexor tendons and their synovial sheaths, the lumbricals, the superficial palmar arch and branches of the median and ulnar nerves with their digital nerves and vessels. Between the flexor tendons and the fascia covering the deep palmar muscles lies the medial central palmar (mid-palmar) space which is continuous with the space of at distal forearm in front of pronator quadratus (Space of Parona) via the CT[24].
The deeper part of each slip subdivides into two processes, which are inserted into the fibrous sheaths of the flexor tendons. At the points of division into the slips, numerous strong transverse fascicular fibers of the PA are positioned at the proximal margin of the flexor tendon sheath. They bind the separate processes together and are attached by vertical septa to the underlying transverse metacarpal ligament, thus forming a tunnel around the flexor tendon and a PA pulley for the flexor tendons in conjunction with the first and second annular pulleys of the digital flexor mechanism[67,68].
The PA pulley might be considered as important as the annular and cruciate flexor tendon pulleys. The PA decreases the tendency to bowstring around the metacarpophalangeal joint with a combination of proximal annular pulleys[69].
The PA forms a fibrotendinous complex that functions as the tendinous extension of the PL when present and as a strong stabilizing structure for the palmar skin of the hand. It has a deeper transverse portion that crosses the palm at the proximal end of the metacarpal bones.
Aponeurosis provides firm attachment to overlying skin, helps to form the ridges in the palm, which in turn help to increase friction so that we can grasp objects firmly, protects underlying structures and provides attachment to muscles. The transverse fascicular fibers of the PA at the proximal margin of the flexor tendon sheath appear to function as a pulley[67].
CLINICAL DIAGNOSIS OF CTS
The stages of CTS symptoms and signs can be categorized into three stages. In the first stage, the patient will awaken from sleep with a feeling of a numb or swollen hand, with no actual swelling visible. They may feel severe pain coming from their wrist emanating to their shoulder, with a tingling in their hand and fingers known as brachialgia paresthetica nocturna. Patients will note that shaking or flicking of their hand will stop the pain and that their hand may feel stiff in the morning. The second stage involves the symptoms being felt during the day. These may be felt especially when the patient performs repeated hand or wrist movements or if they remain in the same position for a long time. Patients may also notice clumsiness when using their hands to grip objects, resulting in the objects falling. The third and final stage occurs when there is hypotrophy or atrophy of the thenar eminence. When this stage is reached, sensory symptoms may no longer be felt at all[70].
When diagnosing a patient with CTS, it is important to create a case history relevant to the characteristic signs of CTS. The patient must be questioned about whether their symptoms occur mainly at night or during the day, whether certain positions or repeated movements provoke their symptoms, if they use any vibratory instruments for work, whether their symptoms are felt in the hand, wrist or shoulder (and where in the areas symptoms are felt), what patients may do to alleviate symptoms (shaking, flicking, etc.), or if the patient may have a predisposing factor[70]. Many factors may in fact be connected to CTS. They can include inflammatory arthritis, diabetes mellitus, pregnancy, hypothyroidism, Colles’ fracture, acromegaly, amyloidosis, adiposity, myxedema, chronic polyarthritis or the use of corticosteroids and estrogens[1,70].
A proper physical examination of the patient’s hand and wrist is an important first step towards the diagnosis of CTS as certain physical findings may suggest the presence of other conditions. Abrasions or ecchymosis on the wrist and hands may indicate that there has been injury to the tissue, which could also include injury to the median nerve. If bony abnormalities like the boutonniere deformity, the swan neck deformity or the ulnar deviation of the wrist are found, it could be concluded that the patient suffers from rheumatoid arthritis. If bossing on the carpal or distal phalanx is observed, osteoarthritis may be the cause. Other neuropathy syndromes or carpometacarpal arthritis may be suspected if thenar atrophy is seen as this condition usually happens only with severe and chronic CTS, which is not as common[71].
Since patient history and physical examination have only limited diagnostic value and do not reveal the specific areas of symptom occurrence, patients can additionally be asked to fill out a self-diagnosis questionnaire known as the Katz Hand Diagram. A Katz Hand Diagram allows the patient to specify where they are experiencing symptoms and to classify the symptoms as numbness, pain, tingling or hypoesthesia. The completed symptom diagram can then be classified into one of three patterns of CTS.
Classical pattern: symptoms experienced by at least two of either the first, second or third fingers. Symptoms may also involve the fourth and fifth fingers, as well as wrist pain and radiation of pain proximally to the wrist however should not involve the palm or dorsum of the hand is not allowed. Probable/possible pattern: includes the same symptoms as in the classical pattern, however the palmar symptoms should only be limited to the median side. Possible pattern: symptoms involving only one of the first, second or third finger. Unlikely pattern: no symptoms are present at all in the first, second or third finger[70].
A classical or probable diagram indicates the presence of CTS (sensitivity = 64%; specificity = 73%)[70-73]. An additional subjective test is referred to as the flick sign (sensitivity = 93%; specificity = 96%)[71,73] where the patient is simply asked whether or not they relieve the symptoms which awaken them at night with flicking or shaking of their hands. If the patient reports that this does happen to them, this may be indicative of CTS[74].
Additionally, traditional tests known as provocative tests can be easily conducted by the physician on the patient to determine the possibility of CTS. One such test is a wrist flexion test known as Phalen’s test (sensitivity = 57%-91%; specificity = 33%-86%)[71,73,74] that involves the patient placing their elbows on a flat surface, maintaining their forearms vertically and allowing their wrists to fall into flexion for up to one minute.
The “reverse Phalen’s test” (sensitivity = 57%; specificity = 78%)[74], also known as the wrist extension test or “Wormser’s test”[75], is also possible and involves the patient actively extending their fingers and wrist for two minutes. Another well known test is Tinel’s sign (sensitivity = 23%-60%; specificity = 64%-87%)[71,73,74], where the physician taps along the patient’s median nerve near the carpal tunnel. Durkan’s test (sensitivity = 64%; specificity = 83%)[74], or carpal compression, is a test where the physician presses on the proximal edge of the carpal ligament with their thumb, compressing the median nerve. The hand elevation test (sensitivity = 75.5%; specificity = 98.5%)[76] is done by asking the patient to raise both of their arms, along with their elbows and shoulders, and holding the position for up to two minutes. The tourniquet test (sensitivity = 21%-59%; specificity = 36%-87%)[73,74], or Gillet test, is performed by having the physician raise a blood pressure cuff placed on the patient’s arm to the level of their systolic blood pressure. For all of the above noted tests, if paresthesia develops or increases in the median nerve distribution within one minute or less, then the test is deemed to have a positive result and CTS in the patient may be suspected[1,74].
It is important to note that, although provocative tests and physical examination are simple and low cost methods to test for reproduction of the patient’s symptoms and to determine if CTS should be suspected, provocative tests have scarce or no diagnostic value[1,70,71] and physical examination has inadequate predictive value if the likelihood of CTS is low[77]. There have been no trends identified between testing positive for various provocative tests and the severity of CTS[78] and therefore proper diagnostic conclusions based on these tests cannot be made.
Nerve conduction studies
Due to this lack of diagnostic value in the mentioned tests, if CTS is suspected in a patient, adjunctive electrodiagnostic tests can be performed for a better diagnosis as they quantify and stratify the severity of CTS[71]. These tests include nerve conduction studies and electromyography which may help with the future decision of treatment options and they have a sensitivity of 56% to 85% and a specificity of at least 94%[71]. Because of their high specificity and sensitivity percentages, nerve conduction studies are considered to be the gold standard for CTS diagnosis[3,72]. Nerve conduction studies provide insight on the median nerve’s true physiological health on a quantitative basis by comparing the latency and the amplitude of the nerve across the carpal tunnel to another nerve segment not passing through the carpal tunnel (e.g., the radial or ulnar nerve)[3]. This is done by transcutaneously stimulating the nerve to create an action potential through an electrical pulse and having the depolarization wave detected by a recording electrode, that has been placed proximally or distally, and defining the response time of the median nerve as the “sensory conduction velocity” (SCV)[3,70,79].
It is necessary to compare the response of the median nerve to another nerve not within the carpal tunnel due to the fact that there are different factors like gender, age, obesity, temperature, finger diameter or concurrent systemic disease that can influence the latency or amplitude of the median nerve[3,80,81]. Using these controls increases the accuracy and sensitivity of diagnosis, with a sensitivity of 80%-92% and a specificity of 80%-99%[3,79]. Additional data may also be obtained by studying the distal motor latency (DML) in the median and ulnar nerves in the same hand[3].
Diagnostic criteria for CTS in nerve conduction studies include the median nerve showing extended amounts of sensory and motor latencies as well as delayed or diminished sensory and motor conduction velocities[3]. Nerve conduction studies focus on defining whether there has been damage to the median nerve inside the carpal tunnel to quantify the severity of this nerve damage using a scale and to define the physiology of this injury as a conduction block, demyelination or axonal degeneration[3,9,70]. The electrophysiological classification of the severity of CTS has been defined by the American Association of Electrodiagnostic Medicine (AAEM) and is as follows: (1) Negative CTS: normal findings on all tests (including comparative and segmental studies); (2) Minimal CTS: abnormal findings only on comparative or segmental tests; (3) Mild CTS: SCV is slowed in the finger-wrist tract with normal DML; (4) Moderate CTS: SCV is slowed in the finger-wrist tract with increased DML; (5) Severe CTS: absence of sensory response is seen in the finger-wrist tract with increased DML; and (6) Extreme CTS: complete absence of a thenar motor response[3,70,82].
Nerve conduction studies can be paired with electromyography in order to tell the difference between muscle weakness that has been created by neurological disorders and primary muscle conditions[71]. Adjunctive tests are, however, best used for patients who present with untypical symptoms and examination or if they possess an intermediate probability of having CTS. Although nerve conduction studies are a more accurate way of diagnosing CTS, they cannot be used for every patient showing symptoms of CTS as this would be expensive and inefficient[3,72]. Although nerve conduction studies are the most sensitive and accurate way to diagnose CTS, false positives and false negatives are still possible and account for 16%-34% of “clinically defined CTS” going undiagnosed[3,83].
When diagnosing CTS, it is important to remember that many other conditions can produce similar symptoms to CTS[71]. A thorough physical examination along with an accurate patient history is an important first step for a correct diagnosis and the following list include some of the conditions that CTS must be differentiated from, along with the physical findings they are associated with: Wrist arthritis: seen in patients experiencing limited motion at the wrist or if there are radiological findings of arthritis; Carpometacarpal arthritis of thumb: characterized by joint line pain, pain experienced during motion or arthritis in radiological findings; Cervical radiculopathy (C6-C7): symptoms can include neck pain and numbness in the thumb and index finger only; Flexor carpi radialis tenosynovitis: can be suspected if there is tenderness near the base of the thumb; Ulnar or cubital tunnel syndrome: signs can include first dorsal interosseous weakness or tingling in the fourth and fifth digit; Median nerve compression at elbow: if there is tenderness at the proximal forearm; Raynaud’s phenomenon: if the patient has a history of symptoms related to cold exposure; Vibration white finger: seen in patients who use vibrating hand tools at work; Volar radial ganglion: if a mass near the base of thumb is found above the wrist flexion crease; Brachial plexopathy (in particular of the upper trunk); Thoracic outlet syndrome and CNS disorders (multiple sclerosis, small cerebral infarction)[3,70,71].
Electrodiagnostic testing can be helpful with a differential diagnosis by being able to identify other hand dysesthesia conditions like cervical radiculopathy, polyneuropathy or median nerve entrapment syndromes[73,84].Additionally, needle electromyography specifically may be helpful with disorders that are proximal to the median nerve as well as to rule out a radiculopathy[70].
Quantitative sensory testing is also used for sensory or motor tests to give quantitative results when diagnosing CTS. These tests include testing for touch threshold using Semmes-Weinstein Monofilaments (SWMF) (sensitivity = 59%-72%; specificity = 59%-62%)[73,74], also referred to as Weinstein Enhances Sensory Test (WEST), where a five piece SWMF/WEST set is used and the filaments are applied onto digit pulps. Positive results of this test are deemed as a threshold greater than 2.83 in digits D1-D3 (an abnormal result); usually D2 or D3 are also assessed and comparison with D5 can improve the specificity as it eliminates thresholds that are larger than 2.83 due to aged or calloused skin. Another quantitative test is the two-point discrimination test (sensitivity = 6%-32%; specificity = 64%-99%)[73,74] where the patient is asked to differentiate between the touch of prongs as they are applied until the skin blanches. Positive results (an abnormal finding) of this test are > 5 mm on pulps. Testing for the vibration threshold, measuring with either a tuning fork (sensitivity = 55%; specificity = 81%)[74] or vibrometer (sensitivity = 50%; specificity = 73%)[74], is also possible. These tests include applying a tuning fork (at 256 cps) tangentially to the fingertip pulp D1-D3 after hitting it to the affected side and comparative site or by applying a vibration stimulus to the digital pulp with a vibrometer and observing if the patient’s feeling is different compared with the normal site (D5) or alternate site or, in the case of the vibrometer, if the thresholds are greater than norms for positive results. Current perception threshold (sensitivity = 80%; specificity = 61%)[74] is tested for by delivering different frequencies of current and touching the patient with the equipment delivering this current. This stimulates the sensory nerves and positive results can be identified by comparing the patient’s thresholds and frequency ratios with established norms in a computer software analysis. Thenar weakness or thenar atrophy (sensitivity = 4%-28%; specificity = 82%-99%)[71,73] can also be tested for by visually inspecting the abductor pollicis brevis to look for loss of muscle bulk (positive result for thenar atrophy) or to use Oxford grading for the abductor pollicis brevis and observing a grade less than 5 (for thenar weakness). Thenar muscles are innervated by the median nerve, so the impairment of these muscles is indicative of the compromise of the motor fibers[74].
All in all, debate and disagreement still exist on the proper and accurate diagnosis of CTS. However, most experts can agree that the combination of nerve conduction studies (currently deemed the gold standard for diagnosis) and subjective symptoms allow for the most accurate way of diagnosing CTS[78].
Computed tomography and conventional X-ray
Plain radiography has a limited role in diagnosing primary CTS as it cannot reveal the soft tissue part of the carpal tunnel. However, it might be useful in cases associated with bony stenosis, fracture and soft tissue calcification[85]. Therefore, it should not be indicated unless there is a history of trauma to the hand or limitation in the range of wrist movement. CT scanning might provide a better alternative than plain radiography to clearly visualize the bony part of the carpal tunnel (Figure 3).
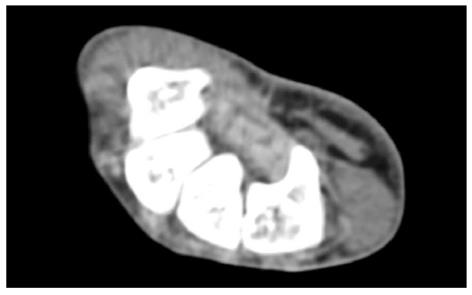
Figure 3 Axial computed tomography scan shows bony part of carpal tunnel at the level of outlet.
Bony structures from left to right are HAMATE, CAPITATE, TRAPEZOID, TRAPEZIUM. FR (arrow) b and flexor tendons can be detected by computed tomography scan.
It can easily reveal the unusual bony structures and the structure occupying the space within the carpal tunnel that are not discovered by external examination[86]. However, compression of the nerve cannot be clearly visualized unless it is due to the bony condition. In addition, several other reports have demonstrated the minor contribution of this approach in cases of primary CTS[87,88]. In summary, plain radiograph and CT scan play minor roles in CTS diagnosis and they are difficult to standardize due to their inherent limitations in evaluating soft tissue changes[85]. Therefore, CT scans and plain radiography should not be used as routine CTS diagnostic tools unless hard tissue related changes such as bone fracture, osseous carpal stenosis and calcifying of soft tissue are suspected[85].
Ultrasonography
Due to recent advances at increasing resolution of sonographic pictures, it is possible to acquire a high quality image of peripheral nerves and fascia. Ultrasonography (US) is also able to identify changes in the flexor retinaculum, perineural and intraneural vascularization of the median nerve in idiopathic carpal tunnel syndrome (Figures 4-7). It can also identify the causes for secondary CTS. A study by Nakamichi and Tachibana included 414 symptomatic wrists and 408 control wrists[89]. Both sonography and nerve conduction studies were performed and the results were compared. The cross-sectional area of the median nerve was measured at the distal edge of the flexor retinaculum, the hook of hamate and the pisiform. Clear differences between the symptomatic patients and control patients were observed at all three levels. Nakamichi and Tachibana proposed cut off values of the CSA for each level ranging from an average of 12 mm2 to 13, 11 and 14 mm2 at all three levels. Specificity was found to be greater than 95% with sensitivity ranging from 43%-57%[89].
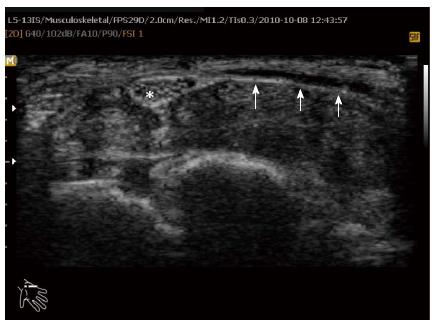
Figure 4 Axial ultrasound image shows flexor retinaculum bowing as an echogenic line (arrow) in carpal tunnel and cross sectional area of median nerve (stellate) in a patient with carpal tunnel syndrome.
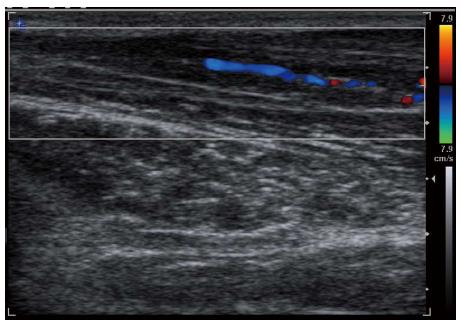
Figure 5 Longitudinal color Doppler sonogram in a 40-year-old woman with severe carpal tunnel syndrome shows intraneural hypervascularity in the median nerve.
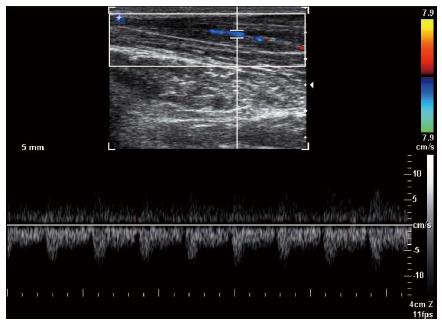
Figure 6 Spectral Doppler waveform of the median nerve shows low resistance hypervascularity of affected median nerve in a 40-year-old woman with severe carpal tunnel syndrome.
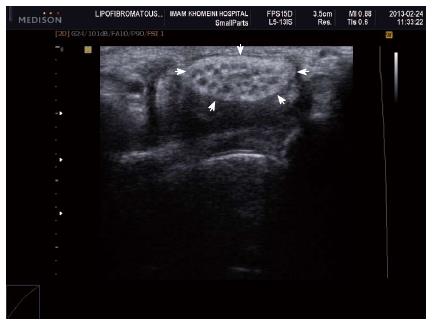
Figure 7 Axial ultrasound image shows hypoechoic cable like neural fascicle (arrows) separated by substratum hyperechoic fat in a patient with secondary carpal tunnel syndrome due to lipofibromatous hamartoma of the median nerve.
Along the same lines, in a study carried out by El Miedany et al[90], sensitivity and specificity were determined by measuring the CSA at the carpal tunnel inlet. Sensitivity and specificity measurements were found to be much higher than those presented by Nakamichi and Tachibana[89]. The study included 96 symptomatic wrists and 156 control wrists, all of which were analyzed using both ultrasound and nerve conduction studies. Cut off for mild disease was 10 mm2, moderate at 13 mm2 and severe at 15 mm2. Sensitivities and specifics for mild, moderate and severe disease were measured to be 98% and 100%, 98% and 97%, and 97% and 99%.
Theoretically, nerve enlargement results from a series of factors including inflammation, fibrosis, new axonal growth, endoneurial edema, demyelination, remyelination, etc. These indicators of increased CSA are all visible on US. Recent studies have shown that US is effective in confirming the diagnosis of CTS. One advantage that US has over NCS is that other lesions which display symptoms similar to CTS can be excluded from examination; these include tenosynovitis, mass lesions and anatomic defects. Moreover, US is low cost, readily available, noninvasive and total examination time is short. It can be recommended to use US as a replacement for clinical findings and first-line therapy in the diagnosis of CTS. However, in more complicated cases where diagnosis of CTS may require some confirmation, US would be a great tool as it is more sensitive and less invasive than nerve conduction studies.
It remains the case that there is not yet a reliable diagnostic standard for CTS. However, many of the studies in this review were able to rule out CTS if the CSA of the median nerve in the carpal tunnel inlet was below 10 mm2. Flexor retinaculum bowing, flattening of the median nerve and decreased longitudinal excursion on dynamic assessment are other measurements offered in addition to the CSA of the median nerve in the US assessment of CTS but are not as reliable. Limitations of US include axial and lateral resolution of the transducer that restrict the sonographic measurements as well as the challenge in differentiating the MN from the surrounding structures, especially in the distal CT. Ultrasonography can be implemented universally when a standardized protocol is used because the measurements are found to be reproducible.
Magnetic resonance imaging
Magnetic resonance (MR) imaging allows for good imaging of soft tissue. This makes MR imaging the optimal choice when studying CT in detail (Figure 8).
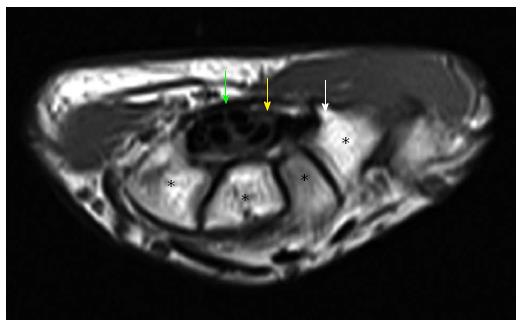
Figure 8 Axial T1W image of carpal tunnel at the level of tunnel outlet shows bony part of carpal tunnel as intermediate signal intensity composed from left to right hamate, capitates, trapezoid, trapezium.
White arrow shows hook of hamate, yellow arrow shows median nerve, green arrow shows flexor retinaculum. Asterisks indicate carpal bones.
The drawbacks to MR imaging are that it is very costly, time consuming and not readily available. For these reasons, it is not advisable for MR imaging to be used in the diagnosis of CTS. The use of MR imaging is advisable in cases where the patient is resistant to therapy and/or for research. MR imaging does not add enough information of value to justify its use in the routine diagnosis of CTS in place of clinical and electrophysiological evaluation. Use of MR imaging can be justified in various circumstances, such as acute severe CTS following blunt trauma, arthritis, lack of evidence of nerve compression, surgical failure, long lasting CTS, detection of fibrous tissue and scars surrounding the MN and other anomalies of CT[91]. MRI can be used to determine the exact point of nerve entrapment, for diagnosis in the case of ambiguous symptoms and to identify space-occupying lesions[92].
MR imaging is useful for revealing the cause of nerve compression or elongation. Studies of MRI on CTS have shed tremendous light on the pathophysiology of idiopathic CTS. Typical indicators of idiopathic CTS are proximal enlargement of the cross-sectional area (CSA) of the median nerve of the carpal tunnel, greater signal intensity over the MN and palmar bowing of the TCL[93]. The degree to which these findings are evident depends on the stage of the disease as enlargement of the CSA and increased signal intensity become more pronounced as the disease progresses. On T2-weighted MRI scans, high-signal intensity over the median nerve can indicate any of the following: accumulation of the axonal transportation, myelin sheath degeneration or edema[94]. Enlargement of the structures within the carpal tunnel may be indicated by palmar bowing of the TCL. The severity of nerve compression can be determined accurately by sagittal images[95]. It must be noted that no parameter has been developed that is able to be used to define CTS clearly. However, MRI provides the greatest diagnostic sensitivity for idiopathic CTS[96]. MRI can be used to predict surgical outcomes in patients with CTS irrespective of NCSs. Moreover, patients prefer MRI to NCSs[96]. MRI studies have indicated that increased T2-signal intensity in the median nerve and bowing of the flexor retinaculum seem to be the most sensitive MR signs of CTS. Increases in CSA area, flattening of the MN and peritendon pathology are other potential indicators of CTS on MRI[97].
Previous reviews on MRI have pointed to the complications also encountered in this review. Original studies provide unsatisfactory descriptions of the reference diagnosis and only recruit asymptomatic referents[93]. Consensus criteria of CTS include symptoms, clinical findings and electrophysiological criteria[16,98]. The lack of consistency in diagnostic criteria provides for variations in patient spectra which takes a toll on the test accuracy[99]. A possible remedy for this in the future would be to include more detailed descriptions of the symptoms of the referents before the original studies.
Bak et al[100] studied twenty patients with CTS to determine if various MRI parameters correlated with nerve conduction test results. Results of the nerve conduction tests did not affect inclusion in the study. While no correlation was found, it should be noted that the patients did not show enlargement of the cross sectional area of the MN. The nerves seemed round rather than flat. Such a finding begs the question of diverse pathophysiological mechanisms behind CTS and how the diverse stages of chronicity of the disease affect the imaging outcome.
Unsatisfactory documentation of clinical characteristics has been shown to lead to overestimation of test accuracy. On the other hand, unsatisfactory documentation of reference diagnosis leads to underestimating test accuracy[99]. Additionally, insufficient blinding and a poor description of results may also lend to the overestimation of test accuracy.
Two studies that used referents with contralateral symptom-free hands did not find the cross sectional area of the median nerve to be enlarged[100,101], which is an observation made in other studies in which healthy volunteers were the referents. This finding can be attributed to other factors besides CTS. So long as there is a gold standard for the diagnosis of CTS, any other disease that causes similar symptoms may prove to hinder future studies.
TREATMENT OF CARPAL TUNNEL SYNDROME
Treatment of CTS can be classified as surgical and non-surgical. Surgical treatments include standard open carpal tunnel release, endoscopic carpal tunnel release, open carpal tunnel release combined with procedures and open carpal tunnel release using various incision techniques[102]. Non-surgical treatments, also referred to as conservative treatments, include a wider range of options such as splinting, cortical steroid injections, non-steroidal anti-inflammatory drugs, B6 vitamin, diuretics, ultrasound therapy, ergonomic positioning, manual therapy intervention, lidocaine patches and acupuncture[103-105]. Treatment decisions on carpal tunnel syndrome are based on the severity of the symptoms. Non-surgical treatments are recommended for patients with mild symptoms of CTS. Patients with moderate to severe symptoms are recommended for surgical evaluation.
Non-surgical treatment
Non-surgical treatment of CTS is recommended for patients that show mild to moderate symptoms of CTS. There is a variety of treatments that do not involve surgical procedures; however, splinting and steroids are most commonly used and supported by evidence[102]. The most common splinting method is the neutral splint, which involves the immobilization of the wrist in a neutral position. This neutralizes flexion and extension of the wrist, thus increasing carpal tunnel pressure[106](Gerritsen, 2001 #73). Neutral splinting is generally recommended for use for a period of 6 wk during night time, however studies have shown better improvement in the full time use of the splints[107]. Long term studies have shown considerable results of treatment success of night wrist splints at 3 mo of treatment[108] and after 12 mo of additional treatment after 6 wk treatment with night splints[109]. Other splinting methods include soft hand splints[106,110], volar wrist cock-up and modified ulnar gutter splints[111]. Soft hand splinting and neutral wrist splinting have been seen to show no significant differences within 3 mo of treatment[112]; however, soft hand splinting can be considered an alternative to neutral wrist splinting[113]. Treatment using steroids is done by local injection of corticosteroids directly into the patient’s carpal tunnel. There is a risk associated with the injections and the possible decompression of the median nerve[114], thus different approaches have been used regarding the injection site of the steroid. Injection through the wrist crease has been done using a distal or proximal approach to the wrist crease, with the distal approach resulting in a more comfortable and alternative approach[115]. Other methods include intercarpal injections of steroids, shown to be a safe approach. Several studies have been conducted using an ulnar approach to the palmaris longus tendon[114,116] and have shown the treatment to be effective and risk free. Overall, treatments involving local steroid injections are effective in relieving symptoms associated with CTS for a short time, thus they are considered an effective short-term treatment[110,117,118]. Local anesthetic injections such as procaine hydrochloride have been shown to be effective additions to steroid injections; however, studies have shown that procaine hydrochloride injections are as effective as steroid injections in short term treatment of CTS[119,120].
A different approach to steroidal treatments is oral steroids such as prednisolone, proven effective for short-term treatment[121]. Studies involving several types of nonsteroidal anti-inflammatory drugs have concluded that NSAIDs have no effect in pain relief and treatment of CTS and their effect has been compared to that of a placebo[71,122]. However, other studies have shown effectiveness of NSAIDs drugs such as Naproxen in short term pain relief for CTS[123]. Several studies have concluded that pyridoxine and diuretics, such as trichlormethiazide, have no more effect than a placebo in the treatment of CTS[71,122]. Ergonomic positioning has been tested for possible effects on CTS and, despite the presence of pain relief after a period of 12 wk under treatment, the evidence was not enough to prove the effect of ergonomic positioning on CTS[124]. Heated lidocaine patches have also been identified as possible alternative short-term treatments for CTS, showing significant pain reduction following a two week study[105]. Acupuncture is another treatment approach for CTS. Studies have shown significant pain reduction in patients with CTS using proximal and distal acupoints, as well as improvement of overall subjective symptoms using sham acupoints[125,126]. Acupuncture has been shown to be as effective as night splints in the treatment of CTS[105]. Ultrasound therapy is another alternative conservative treatment that has been shown to have positive effects in short term treatments of CTS in patients showing mild to moderate symptoms[127,128]. Manual therapy intervention studies have concluded improved signs and symptoms for CTS[104]. It is important to note the role of sonography in assessing the possible effects on different types of treatments for CTS, including corticosteroid injection and splinting treatments[106].
Surgical treatment
Surgical treatment of CTS consists of the division of the transverse carpal ligament which reduces the pressure on the median nerve by increasing the space in the carpal tunnel[2]. Surgery is recommended for most patients with moderate to severe CTS. There are two different categories of methods used for surgical treatment of CTS: open release and endoscopic release. Open carpal tunnel release consists of the standard method of open release, as well as several modified methods. Modifications to the standard open carpal tunnel release (OCTR) include new incision techniques, such as the mini-open release, and addition of other procedures such as epineurotomy[102,129]. The standard open carpal tunnel release consists of a longitudinal incision at the base of the hand and in line with this incision, the incision of the subcutaneous tissue, the superficial palmar fascia and the muscle of the palmaris brevis[129]. The mini-open carpal tunnel release is a relatively new technique that consists of a longitudinal incision that varies from 1.5-3.0 cm, placed in line with the radial border of the ring finger[129]. Different tools have been used for the mini-open carpal tunnel release, such as the Indiana Tome, the Knifelight, the Safeguard System and PSU retractor[129]. Epineurotomy has been used as an additional procedure to the OCTR, with the prospective of minimizing median nerve compression occurring after standard OCTR[72]. Endoscopic carpal tunnel release (ECTR) is another new technique that was developed by Okutsu and colleagues since 1986[131]. The two most commonly used methods of endoscopic carpal tunnel release are the single-portal and dual-portal technique; techniques that differ based on the number of ports used to access the carpal tunnel[130]. The single portal technique consists of the release of the transverse carpal ligament by using a single incision at the wrist. The double-portal technique consists of two incisions, one at the wrist and one at the palm of the hand. Several studies have tried to compare the efficiency and outcomes of the techniques involving carpal tunnel release procedures. Open carpal tunnel release and endoscopic carpal tunnel release have been shown to have no significant differences in outcomes within 12 wk of surgery[132] and within 1 and up to 5 years of surgery[129]. Mini-open carpal tunnel release and standard open carpal tunnel release have shown no significant differences within 4 mo of surgery[133] and within 6 mo of surgery[134]; however, mini-open carpal tunnel release has been shown to have better outcomes in earlier stages after surgery[134]. ECTR release is sometimes favored over OCTR as dividing the skin from below preserves the muscle and overlying skin, thus facilitating return to work; however, it has an increased risk of nerve or artery injury because of limitations in visualization[129]. ECTR has been shown to have better outcomes in muscle strength within 12 wk of surgery[132] and better outcomes compared to both standard open and mini-open release within 4 wk of surgery[133]. Additional epineurotomy procedures have been shown to have no significant difference in electrophysiological effects or nerve volume compared to the standard OCTR within 180 d after surgery[135].