INTRODUCTION
Ductal adenocarcinoma (ADK) is the most common primary malignancy of the pancreas, representing about 80% of pancreatic tumors[1-3]. The majority of the lesions shows locally advanced or metastatic disease at the time of diagnosis[1,4]. The mortality rate is therefore significantly high[1]. As reported in the literature, the detection of a solid lesion of the pancreas, hypoenhanced after the administration of contrast medium, has to be considered an ADK until proved otherwise[3]. Moreover, some tumors surgically removed show distant metastases in the earliest months after treatment owing to their poor differentiation[5]. Hence, an accurate characterization and the differential diagnosis from other solid tumors of the pancreas are paramount to select the best treatment strategy.
The roles of diagnostic imaging methods are to correctly detect, characterize and stage the lesion. In European countries, conventional ultrasound (US) is usually the first imaging modality of choice for the investigation of patients with suspected pathologies of the pancreas since it is inexpensive, easy to perform and widely available[3,6,7]. The introduction of microbubble contrast agents significantly improved the diagnostic capabilities of the first examination, providing a real-time evaluation of the microvascular network of the lesion[8-11]. The dynamic study of the enhancement features allows obtaining an accurate characterization of the lesion and yields to determine the following examinations to achieve a faster and precise diagnosis[3]. In clinical practice, the operator judges the degree of enhancement and thus the evaluation consists of a subjective and therefore limited estimation[12-14]. In the recent past, new imaging techniques have been developed to perform a perfusion analysis of the tumor vascularization[15]. In the last years, some dedicated software for US systems has been developed to provide a perfusion study of the lesions based on the assessment of either raw data or video-intensity analysis. In a nutshell, the subsequent evaluation of a lesion studied at CEUS can allow a perfusion analysis. Therefore, the aim of our study was to determine whether the perfusion analysis after CEUS can allow a quantitative characterization of the enhancement in ductal adenocarcinoma of the pancreas. To our knowledge, no similar previous data have been reported in the literature.
RESEARCH
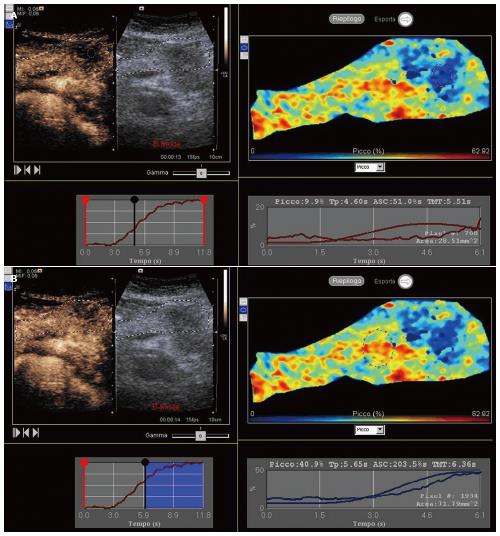
A total of 10 consecutive patients (8 males and 2 females; mean age: 59 years; range: 49-70 years) with suspected ductal adenocarcinoma of the pancreas underwent CEUS and were included in this prospective study. All lesions were pathologically proved. The examinations were performed on an Acuson S2000 US system (Siemens, Erlangen, Germany). After conventional US, a 2.4-mL bolus of microbubble contrast material (SonoVue®, Bracco, Milan, Italy) was hand-injected iv and immediately followed by a 5-mL saline flush. A dynamic evaluation of the enhancement of the pancreatic lesion was performed. A clip showing the lesion along its maximum diameter and covering the whole arterial phase (about 15-20 s after injection) was recorded. During the post-processing analysis, the operator manually drew a region of interest (ROI) covering the tissue that had to be studied and a color map was automatically generated by the system. Then, further and smaller ROIs of similar size were hand-drawn on the color map as follows: within the tumor, being careful not to comprise the central necrosis potentially present or the fibrotic boundary of the lesion or any main surrounding vessel; and within the surrounding parenchyma, avoiding comprising any main vessel or the main pancreatic duct, often dilated upstream. Finally, the peak of enhancement(%), time to peak(s) and ascending curve(%s) for each ROI were automatically measured by the system. Therefore, potential differences in perfusion parameters between the tumor and the adjacent pancreatic parenchyma were observed in real-time and calculated by using the Student’s t test. A P value < 0.05 was considered statistically significant.
In all patients, CEUS and the following quantitative perfusion analysis were performed (Figure 1). No adverse effects were observed. No patients were excluded from the study. Of the tumors, 5 were in the head and 5 in the body-tail of the pancreas. The mean diameter of the lesions was 3 cm, ranging from 2.5 to 4.8 cm. Within the tumor, the mean peak of enhancement, time to peak and ascending curve were 17.19%, 7.97s and 159.52%s respectively, whereas 33.57%, 8.89 s and 355.29%s resulted in the surrounding parenchyma.. A statistical significant difference between tumor and parenchyma was found in peak of enhancement (P < 0.0001) and ascending curve (P = 0.0075). On the other hand, the mean time to peak observed in the tumor and within the surrounding parenchyma did not show a significant difference (P = 0.1027).
Figure 1 Contrast-enhanced ultrasound.
Quantitative perfusion analysis of a ductal adenocarcinoma of the pancreatic body (A) in respect to the adjacent parenchyma (B).
DISCUSSION
In the present study, ten patients with ductal adenocarcinoma of the pancreas underwent CEUS followed by the quantitative perfusion analysis of the lesion. The perfusion parameters obtained within the tumor were compared with those observed within the adjacent parenchyma and a significant difference was found between the peak of enhancement and the ascending curve. Thus, the quantitative perfusion analysis after CEUS provided an objective quantification of the enhancement.
In the recent past, several studies were developed focused on the quantitative evaluation of the enhancement in focal pancreatic lesions[1-3]. computed tomography (CT) perfusion represents the imaging modality most often performed to achieve the perfusion analysis of the lesions, owing to its primary role in characterizing and staging pancreatic tumors[4,15]. The usefulness and importance of measuring pancreatic parenchymal perfusion in various diseases is well known. Although several imaging techniques have been proposed for perfusion measurement of the pancreas, perfusion CT may be considered the best choice, thanks to its linear correlation between contrast media concentration and density, which is essential for quantitative measurements[16]. Perfusion CT is a type of functional imaging that reflects the hemodynamic changes of tissues, such as blood perfusion and capillary permeability[17]. Nowadays, it represents the most important tool available and several papers focused on the application of perfusion CT in the study of different tumors have been published in the recent literature[15,18]. In particular, Miles for the first time measured the perfusion parameters of normal pancreas and some pancreatic diseases[19]. Similarly, perfusion MRI provides a method of measuring perfusion changes in a tissue, placing regions of interest (ROI) over the tissue of interest, and observing the first pass of administered magnetic resonance (MR) contrast medium. Previous investigators of dynamic contrast enhancement MR imaging have assessed pancreatic perfusion by performing a semiquantitative analysis of gadolinium enhancement parameters[20]. Quantitative analysis of regional blood perfusion by using dynamic contrast-enhanced MR imaging is based on the pharmacokinetics of tracer transport between compartments[20]. Further studies described the potential quantitative evaluation of the enhancement, derived from the offline evaluation of different pancreatic tumors studied at CEUS[21-23]. However, US is still the first imaging examination usually performed in patients with suspected pancreatic pathologies[6,7]. The iv administration of microbubbles therefore represents the immediate subsequent examination usually achieved[3]. The new software available could allow an objective quantification of the enhancement providing perfusion parameters similar to those obtained on perfusion CT. As a result, an immediate injection of microbubbles to better characterize a pancreatic mass already detected on US could allow a quantitative perfusion analysis of ductal adenocarcinoma of the pancreas.
The present study has some limitations due to the small number of patients and the manual injection of contrast agent. However, the amount of contrast material used is very small and the drawback of the manual injection is limited and further reduced by the relative measurement of both the pancreatic tumor and the surrounding pancreatic parenchyma.
Moreover, the limit arising from the analysis of the time-intensity curve not using the raw data has to be considered but there is, however, the advantage of having this software on site in the ultrasound scanner allowing immediate evaluation after examination, minimizing the requested time.
CONCLUSION
CEUS quantitative perfusion analysis of ductal adenocarcinoma of the pancreas is possible to give quantitative evaluation of the enhancement, allowing tumor objective characterization.
P- Reviewers: Cerwenka HR, Sartori S, Yazdi HR, Ying X S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Wu HL