Published online Jun 28, 2013. doi: 10.4329/wjr.v5.i6.241
Revised: May 3, 2013
Accepted: May 18, 2013
Published online: June 28, 2013
Processing time: 124 Days and 4.9 Hours
AIM: To evaluate the response to treatment in patients with neuroendocrine tumor liver metastases following yttrium-90 (90Y) radioembolotherapy, as a function of image patterns at presentation for 90Y radioembolotherapy.
METHODS: The study cohort consisted of patients with hepatic metastatic neuroendocrine tumors treated with 90Y at our institution during a two-year time period. Hepatic metastases were evaluated on a pre-therapy study assessing relative arterial enhancement compared to liver, lesion size, necrosis of the lesion, and associated tumor burden in the liver. We used six response criteria: Response Evaluation Criteria in Solid Tumors (RECIST) size, World Health Organization (WHO) size, European Association for the Study of the Liver (EASL) necrosis guidelines, Choi size, Choi necrosis and combination of Choi size and necrosis.
RESULTS: About 65 lesions in 17 patients met study criteria and formed the cohort. Statistically significant response was found for lesions < 5 cm vs those ≥ 5 cm with RECIST (P = 0.04), WHO (P = 0.002) and combined Choi criteria (P = 0.02). Hyperenhancing lesions demonstrated greater response only with the Choi size criteria (P = 0.04). Lesions with ≤ 50% necrosis on the pre-scan had statistically significant greater response with the Choi necrosis criteria (P = 0.01). There was no statistical significance for response comparing lesions < 2 cm vs≥ 2 cm or in comparing the degrees of tumor burden.
CONCLUSION: Based on our findings in this study, it is suggested that initial imaging findings, as listed above, are not a good predictor of response to 90Y radioembolization.
Core tip: Treatment modalities for neuroendocrine tumor liver metastases (NETLM) include systemic chemotherapy, surgery, ablation, chemoembolization, and yttrium-90 (90Y) radioembolization. Radioembolotherapy is aimed at delivering high dose of radiation via intra-arterial administration. There are reports of good response rates of NETLM following radioembolization. There are a few reports on the selection criteria based on imaging features. To our knowledge, there are no reports that have evaluated multiple imaging features as selection criteria for 90Y radioembolization. Our goal is to evaluate for a specific imaging feature at presentation that may predict response to treatment. The response will be evaluated using various criteria of tumor response.
- Citation: Neperud J, Mahvash A, Garg N, Murthy R, Szklaruk J. Can imaging patterns of neuroendocrine hepatic metastases predict response yttruim-90 radioembolotherapy? World J Radiol 2013; 5(6): 241-247
- URL: https://www.wjgnet.com/1949-8470/full/v5/i6/241.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i6.241
Patients with neuroendocrine tumors (NET) often have clinical symptomatology that is most commonly due to hormone secretion by the tumor, which warrants treatment. The most common site of metastatic of disease of NET from pancreatic or gastrointestinal origin is to the liver. Thirty percent to eighty percent of patients have liver metastases which is associated with a worse prognosis[1,2]. The treatment of neuroendocrine tumor liver metastases (NETLM) is aimed at alleviating clinical symptoms in patients with non-resectable tumors. In addition, treatment of NETLM is also guided by the hope of improving survival, reducing tumor burden, and preventing progression of disease[1-3].
Treatment modalities for NETLM include medical (somatostatin analogues octreotide (Sandostatin; Novartis Pharmaceuticals, East Hanover, NJ) streptozocin (Zanosar; Sicor Pharmaceuticals, Irvine, CA), doxorubicin (Pharmacia and Upjohn), 5-fluorouracil (5-FU; Adrucil; Teva Parenteral Medicines, Irvine, CA) cisplatin (Baxter) Mitomycin C (Ben Venue Laboratories, Bedford, OH), and bevacizumab (Avastin; Genetech, South San Francisco, CA), surgical (resection and transplantation), ablation, and embolotherapeutic methods [transcatheter arterial embolization (TAE), chemoembolization (TACE), and yttrium-90 (90Y) radioembolization][2,3]. Radioembolotherapy is aimed at delivering high dose of radiation to the tumor via intra-arterial administration of 90Y. Several studies have reported good radiographic response rates of NETLM following 90Y radioembolization ranging from 39%-69%[4-9].
The most common presentation on computerized tomography (CT) and magnetic resonance (MR) of NETLM is a hypervascular mass that enhances during the late arterial phase and is hypodense to liver on the delayed phase of contrast administration. However, the CT and MR imaging of NETLM that are going to be treated with 90Y may be variable at presentation for 90Y radioembolotherapy. This may be due in part to the fact that 90Y is not always performed as the first line of treatment and the imaging features may be variable due to prior therapy. The CT and MR imaging features of these tumors may be large or small in size, may have small or large tumor burden in the liver, may have a large necrotic component, or may have variable enhancement relative to the liver parenchyma[10-12]. There are a few reports on the selection criteria based on imaging features for patients with NETLM to be treated with 90Y radiotherapy[8,9,13]. However, to our knowledge, there are no reports that have evaluated multiple imaging variables as selection criteria for 90Y radioembolization.
Response Evaluation Criteria in Solid Tumors (RECIST) is a commonly used criterion to evaluate response to treatment of liver metastases. This criterion is based on changes in size (one measurement, Table 1)[14]. Similarly, World Health Organization (WHO) guidelines are also based on size changes (two measurements, Table 1)[15]. Recent advances in therapy of liver metastases, such as anti-angiogenic therapy and ablation, have required that in addition to tumor measurement criteria, other criteria should be used to evaluate response to treatment. These criteria are based on size, tumor density, and tumor necrosis. For example, Miller et al[16] showed that a combination of size and necrosis criteria is a more accurate representation of response to treatment compared with solely evaluating size in patients treated with 90Y. These newer guidelines include the European Association for the Study of the Liver (EASL), which is based on evaluating necrosis[17]. The Choi criterion has been recently validated for the treatment of liver metastases of gastrointestinal stromal tumor with anti-angiogenic therapy. This criterion includes size and density criteria[18].
| Size | Necrosis | ||||
| RECIST | WHO | Choi | EASL | Choi | |
| PR | > 30% reduction in unidim-ensional diameter | > 50% reduction in product of greatest bidimensional (perpen- dicular) diameters | > 10% reduction in unidime-nsional diameter | > 50% increase in necrosis | > 15% decrease in tumor density |
Our goal is to evaluate if there are specific imaging features of NETLM at presentation for 90Y therapy that may predict response to treatment. The response will be evaluated using various criteria of tumor response listed in Table 1. In particular, our goal is to evaluate whether the size of tumor, the degree of liver tumor burden, the necrosis of the lesions, and the relative enhancement of the lesion to liver can be used as selection criteria in determining if a patient will respond to 90Y radiotherapy.
The institutional review board approved this retrospective analysis. Medical records were retrospectively reviewed to identify patients that had neuroendocrine hepatic metastases who had received treatment with 90Y microspheres at our institution. Broad inclusion criteria for our retrospective cohort were as follows: (1) radiologically proven liver metastases from a neuroendocrine tumor source treated with 90Y microspheres at our facility in the given time period; (2) pre-radioembolotherapy imaging to include either contrast-enhanced CT or MR; and (3) follow-up imaging obtained a minimum of 18 wk post-radioembolization. More specific inclusion and exclusion criteria were established for our study. Patients who had undergone treatment in the interim between 90Y treatment and the follow-up imaging, to include TAE/TACE, ablation, new systemic chemotherapy regimen, or surgical resection, were excluded from our analysis. Patients were not excluded, however, if they had any such therapies prior to 90Y as long as it occurred prior to the initial imaging. Follow-up imaging assessment was only recorded up to the point when a new treatment was begun. Patients with prior surgical resections were not excluded from this study given that we only analyzed lesions in the distribution of the treated vessels. For patients who had prior ablation for liver metastases, the information and prior imaging was available so that we were able to confidently avoid including previously treated lesions in our study. A majority of our patients were on octreotide therapy prior to the treatment and were continued on this following 90Y. We did not exclude patients based on this criterion, but if that was commenced as a new therapy after the 90Y treatment, any imaging following the start date was not assessed. Deceased patients were still included if they had the minimum imaging follow-up of 18 wk that we required for inclusion. Patients that have been on several different chemotherapeutic regimens or had participated in clinical trials were not exclude from our study as long as they were no longer receiving the therapy during the time of evaluation and we had imaging between cessation of those therapies and the date of radioembolization so that we could adequately assess that the response in the lesions were a result of the 90Y.
Selection of patients was made from a two-year period dating 1/1/2008 through 12/31/2009. A total of 111 patients presented for evaluation for treatment with 90Y. Of these 111 patients, 47 were patients with NETLM. Eighteen of 47 patients were not treated for various reasons to include uncorrectable excessive pulmonary shunting, other treatment planning (i.e., surgery or bland embolization), the decision for the patient to proceed with hospice, or death.
A total of 29 patients with NETLM were treated in the 2008-2009 calendar years. Four patients were excluded from our study due to lack of follow-up imaging at our institution. A fifth patient was excluded who had multiple prior ablations and wedge resections making the liver lesions too inconspicuous to be adequately measured. Six more patients were excluded who did not have the required follow-up of at least 18 wk following 90Y treatment. Another patient was excluded due to lack of intravenous contrast on one of the included imaging studies. This brought the total to 17 patients (11 females, 6 males; age range of 32-78 years) who were included in our cohort. Four lesions were measured for each 90Y treatment, with the exception of one patient who had a solo liver metastasis, to give a total of 65 lesions selected for data analysis.
Imaging consisted of either CT or MR with administration of intravenous contrast. Routine follow-up CT and MR examinations were performed at scheduled intervals using standard imaging protocols. The imaging studies selected for data collection for each patient were the baseline exam prior to 90Y treatment and the first exam performed beyond the required 18 wk minimum period, allowing imaging obtained in the range of 18-40 wk following treatment for our data collection.
All patients had a pre-treatment contrast-enhanced cross-sectional imaging study to assess the volume of metastatic disease to aid in dose qualification of the treatment. The 90Y therapy planning arteriography is described in detail elsewhere[3]. In brief, 90Y planning angiogram was performed to identify any aberrant hepatic anatomy and, at this time, 99 m technetium-labeled macroaggregated albumin (99mTc-MAA) was injected, followed by nuclear medicine planar and single photon emission computed tomography (SPECT) imaging to assess the radiotracer distribution. The planar imaging was used to determine shunt fraction and the SPECT scan was used to evaluate for extrahepatic MAA distribution. This information was used for dosing calculations and prescriptions for activity of 90Y to be used for treatment. Patients with more than 20% shunting were not allowed to proceed.
The median activity of yttrium administered for our cohort of patients was 48.7 mCi (1.8019 GBq), ranging from 27.0 mCi (0.999 GBq) to 75.5 mCi (2.7935 GBq). A total of twenty 90Y sessions were performed on the seventeen study patients. Sixteen whole liver and four right lobe treatments were performed. Three patients had two separate treatment sessions with two of those patients having whole liver treatments and the third patient having right lobe treatment followed by whole liver treatment.
Extent of metastases within the liver ranged from 10%-80%. For our study, we separated these predetermined tumor burdens into two categories: those with 50% or less and those with greater than 50% of metastatic disease in the liver.
Lesion selection was made by two board certified radiologists during analysis of imaging for each treatment session. Note was made of the vessels through which the radiotherapeutic agent was administered and lesions were only selected in the distribution of those arteries. Measurements of the selected lesions were made separately by each physician. Measurements were obtained on the same phase of imaging throughout all pre and post treatment scans on each patient, which was either based on availability within the cumulative exams or by discretion of the radiologists based on the best phase or sequence to measure the lesions consistently.
The 65 lesions were assessed for the initial enhancement pattern relative to the liver and degree of necrosis of the lesions as well as the changes in necrosis or density of the lesions identified on follow-up exams. Two other board certified radiologists gave their independent analyses. Any discrepancies in opinion were reassessed jointly by the four radiologists and the group came to a consensus for those cases.
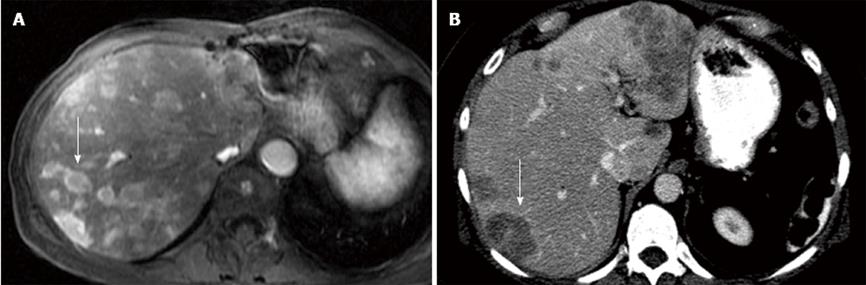
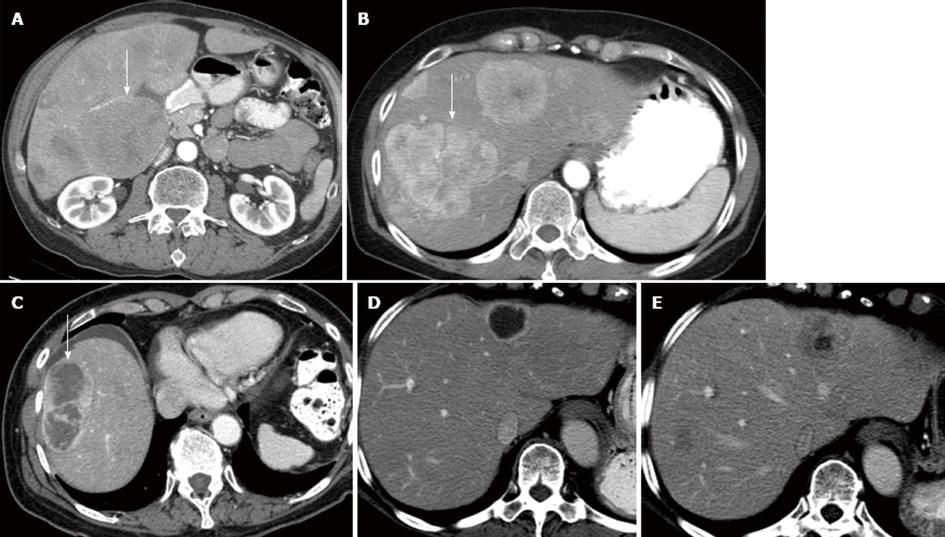
Each metastatic lesion was evaluated on the pre-therapy imaging study and was placed into five separate categories: (1) relative arterial enhancement compared to the liver with two categories representing those hyperenhancing relative to the liver (Figure 1A) vs those which were hypoenhancing (Figure 1B) or isoenhancing (Figure 2A) in relation to the liver; (2-3) two different size characteristics: (2) < 2 cm vs≥ 2 cm and (3) ≥ 5 cm vs < 5 cm; (4) the percent necrosis of the lesion, separated into two categories lesion with ≤ 50% (Figure 2B) vs > 50% necrosis (Figure 2C); and (5) the percent of tumor burden on the liver separated in two categories, patients with ≤ 50% or > 50% tumor burden in the liver. The tumor burden used for each lesion was based on the percentage calculated from the SPECT scan performed during treatment planning.
Indicators for response were based on two measurements (size and necrosis): Size was evaluated with (1) RECIST; (2) WHO; and (3) Choi criteria. There were no complete responders (CR) based on any criteria. The results were categorized into partial response (PR) or no PR. Based on the RECIST criteria, a PR is defined as a ≥ 30% decrease in the (sum of) longest one-dimensional diameter[14]. We evaluated the lesions individually rather than as a sum for RECIST criteria, still applying the same guidelines. WHO defines PR as ≥ 50% decrease in the tumor area (product of the longest diameter and the greatest perpendicular diameter)[15]. Choi size criterion classifies a response by a 10% decrease in tumor size[18].
The EASL is often referenced for evaluating of necrosis as a measure of tumor response to therapy[17]. While this was based on management of HCC, several studies have incorporated this criterion into their analysis[16,19,20]. Based on EASL guidelines a positive response was considered when there was an increase of greater than 50% in lesion necrosis following treatment (Figure 2 D, E) . This value was stricter than the value of 30% used by Keppke et al[19] and Miller et al[16] and smaller than the 65% used by Duke et al[20]. Choi criterion for necrosis has been described as > 15% decrease in tumor density following therapy[18]. The Choi criteria of size and necrosis can be combined. This combination has been reported to be more accurate prediction of overall survival than RECIST[18]. The Choi criterion for size and necrosis was originally established on the evaluation of gastrointestinal stromal tumors in response to imatinib, it is felt that this may apply to other tumor types and therapies[18].
The relationships between pre-treatment imaging pattern and response were evaluated using the Fisher’s exact statistical analysis. GraphPad Software© was used to create the contingency tables. P values were calculated using a two-tailed test with significance level set at P = 0.05.
Sixty five lesions were analyzed. Each lesion was placed into categories based on relative enhancement, size, degree of necrosis and the level of associated tumor burden. Response was determined for each lesion based on size and necrosis using the six separate response criteria. The results of the analysis are detailed in Table 2.
| Variable | Size | Necrosis | Size and necrosis | |||||||||
| n | RECIST | P value | WHO | P value | Choi | P value | EASL | P value | Choi | P value | Combined Choi | |
| Total lesions | 65 | |||||||||||
| Enhancement | 1.00 | 1.00 | 0.04 | 0.18 | 0.22 | |||||||
| Hypo/iso | 14 | 2 (14) | 2 (14) | 4 (29) | 0 (0) | 3 (21) | 0 (0) | |||||
| Hyper | 51 | 9 (18) | 10 (20) | 31 (61) | 8 (16) | 22 (43) | 12 (24) | |||||
| Necrosis | 1.00 | 0.67 | 0.32 | 0.34 | 0.01 | |||||||
| > 50% | 10 | 1 (10) | 1 (10) | 7 (70) | 0 (0) | 0 (0) | 0 (0) | |||||
| < 50% | 55 | 10 (18) | 11 (20) | 28 (51) | 8 (15) | 25 (45) | 12 (22) | |||||
| Size A (cm) | 0.35 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| < 2 | 10 | 3 (30) | 2 (20) | 5 (50) | 1 (10) | 4 (40) | 1 (10) | |||||
| > 2 | 55 | 8 (15) | 10 (18) | 30 (55) | 7 (13) | 21 (38) | 11 (20) | |||||
| Size B (cm) | 0.04 | 0.002 | 0.31 | 0.47 | 0.07 | |||||||
| > 5 | 25 | 1 (4) | 0 (0) | 11 (44) | 2 (8) | 6 (24) | 1 (4) | |||||
| < 5 | 40 | 10 (25) | 12 (30) | 24 (60) | 6 (15) | 19 (48) | 11 (28) | |||||
| Tumor burden | 1.00 | 0.74 | 0.42 | 1.00 | 0.79 | |||||||
| < 50% | 45 | 8 (18) | 9 (20) | 26 (58) | 6 (13) | 18 (40) | 8 (18) | |||||
| > 50% | 20 | 3 (15) | 3 (15) | 9 (45) | 2 (10) | 7 (35) | 4 (20) | |||||
The first column lists the various imaging patterns at presentation prior to 90Y treatment. This includes enhancement relative to liver (hypervascular vs iso/hypovascular), necrosis of the lesion (< 50%), size (< 2 cm, A), size (< 5 cm, B), and the degree of tumor burden in the liver (< 50%)(Table 2). For example, 51 lesions were hypervascular compared to liver following the administration of intravenous contrast.
The other columns list the number of lesions that met the criteria for PR for each response criteria [RECIST, WHO, Choi (size), EASL, Choi (necrosis), Choi (combined sized and necrosis)]. For example, nine hypervascular lesions met the criteria for PR using RECIST criteria (18%, P = 1.0), but this was not statistically different. Only the Choi response criteria for size and Choi response criteria for combined were statistically significantly differently. This was seen only on the degree of lesion enhancement: The hypervascular lesions were more likely to show PR than isointense or hypovascular lesions.
Radioembolization with 90Y is a treatment option that is increasingly being utilized for patients with neuroendocrine metastases that have failed other treatments. No definite imaging criteria have been established for selecting patients to receive this treatment. In our study, we looked at several specific imaging characteristics of the lesions prior to treatment to assess if there was a correlation between the imaging features prior to treatment and the response to treatment. This could be very useful in the selection process for patients with NETLM being considered for treatment with 90Y. There are multiple reported criteria for the evaluation of response to treatment of various liver metastases. The best response criterion for evaluation of NETLM following 90Y treatment has not been defined. Criteria available to evaluate tumor response include RECIST, WHO, Choi (size, necrosis, and combined), and EASL. Using these available criteria, all the imaging characteristics at presentation that were evaluated were not found to be reliable predictors of response. The only partial exception to this conclusion was tumor size prior to treatment. Our results suggest that lesions that were larger than 5 cm compared to lesions that were equal or smaller than 5 cm had statistical difference in response based RECIST, WHO and the combined Choi criteria (not on all criteria). The lesions that were smaller than < 5 cm had better response. Based on single criteria, such as Choi (size), the lesions that enhanced more than the liver prior to treatment showed better response. Also based on single criteria, Choi (necrosis), lesions with less than 50% necrosis prior to treatment had better response. But these results are insufficient to exclude patients from 90Y treatment based on imaging characteristics prior to treatment.
A few studies have been previously conducted that looked at variables as prognostic indicators affecting response and survival following 90Y radioembolization for NETLM. Saxena et al[8] found three factors that were associated with better response: female gender, well-differentiated primary tumors and low hepatic burden. Of those, and amongst the other variables tested, the only imaging characteristic we evaluated was the degree of hepatic tumor burden. Cao et al[9] evaluated prognostic factors for the impact on survival where they found a correlation between radiographic response and survival. They looked at response in relation to many factors, one of which was extent of tumor burden, which was found to have a significant effect on survival. No other specific characteristics of the lesions were evaluated for this study either. Kalinowski et al[4] and Kennedy et al[6] both report that response rates were high, even with extensive tumor burden. We did not find any correlation with response to treatment and tumor burden.
Neuroendocrine tumors are often hypervascular and therefore, one might theorize that these lesions might respond better to intra-arterial therapies, such as 90Y radioembolization. A study by Sato et al[13] evaluated vascularity of lesions and found there was no correlation with this and survival and, therefore, concluded that lesions that are hypovascular should not be considered a contraindication while assessing patients for 90Y radioembolotherapy. We did not find any correlation with response to treatment and tumor vascularity. To our knowledge, no other studies have been done assessing the size or relative necrosis of lesions prior to 90Y therapy as a predictor of response.
A limitation of our study is the retrospective nature of the study. Another limiting factor is that the number of lesions analyzed. Based on the RECIST criteria, our study had an overall response rate of 17% (11/65). This is lower than other reported response rates, which ranged from 39% to 69%, in studies that also used RECIST[7-9]. This difference may be due to our low numbers. Another limitation of our study was that our method to define the vascularity of the lesions and the degree of necrosis was based on consensus evaluation. More objective methods of analyzing the lesions may alter the categories in which each particular lesion was placed. Other imaging techniques such as PET or MRI with diffusion weighted imaging were not employed for our study since these modalities were not available in each patient included in our cohort.
Many prior studies have demonstrated that 90Y radioembolization is a promising treatment for patients with NETLM. More studies still need to be performed to further identify significant factors that may facilitate the patient selection process. Based on the findings in this study, it is suggested that the initial imaging findings should not exclude patients with NETLM from 90Y radioembolization therapy. Additional studies with larger number of lesions would need to be performed to confirm these findings.
The radioembolization therapy with yttrium-90 (90Y) has become a treatment modality for neuroendocrine liver metastases. The response to 90Y treatment of neuroendocrine liver metastases is varied. A potential indicator for response to treatment may be the imaging features of the liver metastases prior to 90Y treatment. These metastases may be differentiated by size, enhancement pattern, degree of necrosis and percentage of liver tumor burden can be evaluated. It is possible that the varied response may be due to one of these imaging patterns.
There are few publications that have evaluated factors that may affect the response to treatment by 90Y of modalities for neuroendocrine tumor liver metastases (NETLM). These publications have addressed prognostic indicators such as tumor burden and differentiation of the tumor. To authors’ knowledge, there has been no evaluation of the response to treatment that combines size, necrosis, hypervascularity, and tumor burden. The response to treatment was evaluated not only by size criteria (World Health Organization and Response Evaluation Criteria in Solid Tumors) but other criteria were used (European Association for the Study of the Liver and CHOI).
There are few publications that have evaluated factors that may affect the response to treatment by 90Y of NETLM. These results did not indicate that an imaging pattern prior to treatment would have predicted response to treatment.
The selection of patients for 90Y treatment of NETLM cannot be based on size, tumor burden, necrosis, or enhancement of the liver metastases prior to treatment.
Apart from some important limitations like rather low patient numbers and the retrospective nature of the reported study, this article provides an interesting evaluation of imaging findings as a predictor of response to 90Y radioembolotherapy in patients with neuroendocrine liver metastases.
P- Reviewers Cerwenka HR, Dettmer AM, George T, Mesa H S- Editor Wen LL L- Editor A E- Editor Yan JL
| 1. | Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 415] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Frilling A, Sotiropoulos GC, Li J, Kornasiewicz O, Plöckinger U. Multimodal management of neuroendocrine liver metastases. HPB (Oxford). 2010;12:361-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17:1571-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Kalinowski M, Dressler M, König A, El-Sheik M, Rinke A, Höffken H, Gress TM, Arnold R, Klose KJ, Wagner HJ. Selective internal radiotherapy with Yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single center study. Digestion. 2009;79:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | King J, Quinn R, Glenn DM, Janssen J, Tong D, Liaw W, Morris DL. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RR, Liu D. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Rhee TK, Lewandowski RJ, Liu DM, Mulcahy MF, Takahashi G, Hansen PD, Benson AB, Kennedy AS, Omary RA, Salem R. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Saxena A, Chua TC, Bester L, Kokandi A, Morris DL. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251:910-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Cao CQ, Yan TD, Bester L, Liauw W, Morris DL. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg. 2010;97:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Paulson EK, McDermott VG, Keogan MT, DeLong DM, Frederick MG, Nelson RC. Carcinoid metastases to the liver: role of triple-phase helical CT. Radiology. 1998;206:143-150. [PubMed] |
| 11. | Dromain C, de Baere T, Baudin E, Galline J, Ducreux M, Boige V, Duvillard P, Laplanche A, Caillet H, Lasser P. MR imaging of hepatic metastases caused by neuroendocrine tumors: comparing four techniques. AJR Am J Roentgenol. 2003;180:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Bader TR, Semelka RC, Chiu VC, Armao DM, Woosley JT. MRI of carcinoid tumors: spectrum of appearances in the gastrointestinal tract and liver. J Magn Reson Imaging. 2001;14:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Sato KT, Omary RA, Takehana C, Ibrahim S, Lewandowski RJ, Ryu RK, Salem R. The role of tumor vascularity in predicting survival after yttrium-90 radioembolization for liver metastases. J Vasc Interv Radiol. 2009;20:1564-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21336] [Article Influence: 1333.5] [Reference Citation Analysis (1)] |
| 15. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [PubMed] |
| 16. | Miller FH, Keppke AL, Reddy D, Huang J, Jin J, Mulcahy MF, Salem R. Response of liver metastases after treatment with yttrium-90 microspheres: role of size, necrosis, and PET. AJR Am J Roentgenol. 2007;188:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3234] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 18. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1115] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 19. | Keppke AL, Salem R, Reddy D, Huang J, Jin J, Larson AC, Miller FH. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 2007;188:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Duke E, Deng J, Ibrahim SM, Lewandowski RJ, Ryu RK, Sato KT, Miller FH, Kulik L, Mulcahy MF, Larson AC. Agreement between competing imaging measures of response of hepatocellular carcinoma to yttrium-90 radioembolization. J Vasc Interv Radiol. 2010;21:515-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |