INTRODUCTION
Advancements in imaging technology have led to the increased detection of adrenal lesions. Adrenal abnormalities are detected in 5%-8% of patients who undergo computed tomography (CT)[1]. This incidence increases to 9%-13% in patients with known malignancies and the incidence of metastases in these adrenal lesions is 26%-36%[2-4]. Adequate characterization of adrenal lesions is crucial for patient care. In this article, we review the current adrenal imaging techniques in CT, magnetic resonance imaging (MRI) and positron emission tomography (PET) and how they are used to characterize adrenal lesions. If these modalities are not sufficient for characterizing lesions, adrenal biopsy should be considered.
Some basic features of adrenal lesions, such as their size, can help in their characterization[2]. Small (less than 4 cm), well-marginated and homogenously low-density lesions which have non-contrast Hounsfield Units (HU) less than 10 are usually benign and no further imaging is required[5,6]. Adrenal lesions that are large (more than 4 cm) with no classic benign features have a higher probability of malignancy[7]. Another feature of malignancy is rapid growth over a short interval, after excluding adrenal hyperplasia. Conversely, lesions that are stable for a long period of time are more likely to be benign. Morphological characteristics, including irregular margins, are not good predictors of malignancy[2].
CT
CT is one of the main modalities for characterizing adrenal lesions, using non-contrast lipid content and dual-energy imaging, triphasic and quadriphasic enhancement patterns, histogram analysis and perfusion analysis.
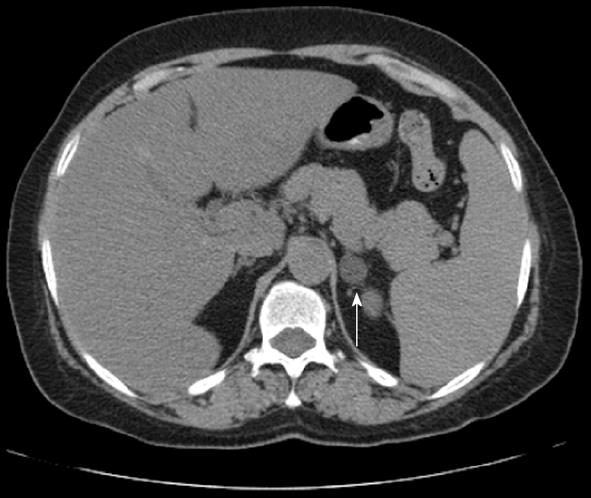
Most adenomas contain a large amount of intracellular fat, yielding low HU. Approximately 70% of adenomas contain intracellular lipid, consisting of cholesterol, fatty acids and neutral fat[8]. A meta-analysis of 10 studies demonstrated that a 10 HU threshold is associated with a sensitivity of 71% and specificity of 98%[2,9]. In another study, a threshold of 10 non-contrast HU yielded a sensitivity of 89% and specificity of 100%[2,10]. Blake et al[11] determined that a non-contrast HU of 10 or lower is specific for adenoma (Figure 1).
Figure 1 Axial non-contrast computed tomography demonstrates a left adrenal classic lipid-rich adenoma (arrow) with Hounsfield Units of -1.
Dual-energy, non-contrast CT has been used to differentiate adenomas from non-adenomas. The dual-energy composition analysis is based on the principle that different adrenal lesions possess unique attenuation properties at different voltage settings. In one study, 31 nodules were measured at 140 kVp and 80 kVp; a decrease in lesion attenuation between 140 kVp and 80 kVp had 100% specificity for adenomas by reflecting the adenomas’ intracellular lipid content. The sensitivity was only 50%; therefore, this technique is not widely used, but it may be useful if contrast-enhanced scans are not available. When contrast examinations are performed and the lesions have non-contrast HU more than 10, an additional evaluation with contrast wash-out patterns is performed[12].
Lipid-poor adenomas have an attenuation of more than 10 non-contrast HU and can be further characterized by their contrast enhancement and wash-out patterns. Only 30% of adenomas are lipid poor[1,13], which renders further characterization with contrast necessary. Adenomas tend to rapidly enhance after contrast administration and rapidly wash-out. Malignant lesions and pheochromocytomas also enhance rapidly but tend to exhibit slower wash-out patterns. Two methods have been developed to calculate wash-out patterns: the percentage of absolute contrast enhancement wash-out (ACEW) and the relative contrast enhancement wash-out (RCEW).
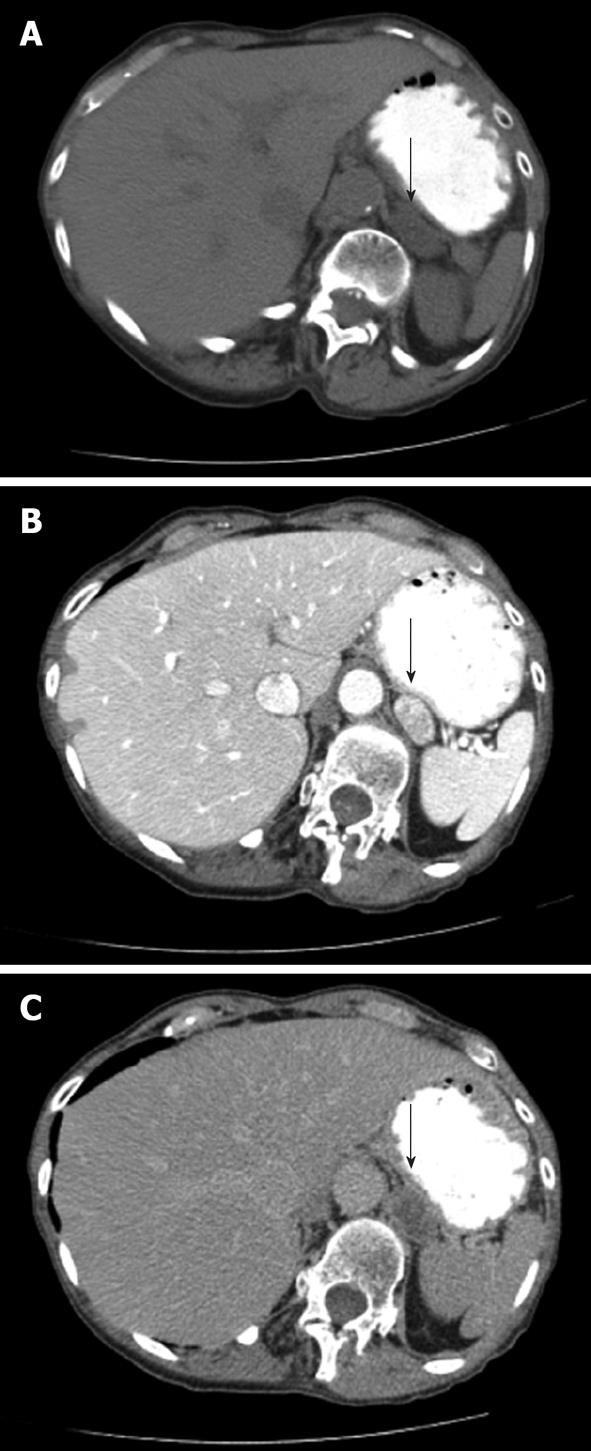
For the ACEW pattern, a non-contrast measurement, 60 s post-contrast measurement, and 10-15 min post-contrast delayed measurement are obtained, in which at least half the mass is included in the measurement field. The percentage of ACEW is: [(contrast-enhanced HU at 60 s - delayed contrast HU)/(contrast enhanced HU at 60 s - non-contrast HU)] × 100. If a 10 min delay is used, an ACEW of 52% or more has 100% sensitivity and 98% specificity[14]. If a 15 min delay protocol is used, an ACEW value of 60% or higher has approximately 87% sensitivity and 94% specificity[15,16]. Note that ACEW measurements require a non-contrast HU measurement and in everyday practice, this is not always obtained. In this patient population, the percentage of RCEW can be calculated as: % RCEW = [(contrast-enhanced HU at 60 s - delayed contrast HU)/(contrast-enhanced HU at 60 s)] × 100. If a 10 min delay parameter is used, a RCEW of 50% or higher has a sensitivity of 98% and specificity of 100% for characterizing adenomas[10]. If a 15 min delay parameter is used, a RCEW of 40% or more has a sensitivity of 96% and specificity of 100%[16]. Figure 2 shows ACEW and RCEW calculations from an indeterminate left adrenal mass. Quadriphasic CT has been suggested for differentiating adenomas from non-adenomas. In one study, unenhanced, arterial phase, venous phase and 5 min delayed scans were performed. Measurements, including absolute wash-out, relative percentage wash-out and percentage enhancement wash-out, had a diagnostic accuracy of 97.1%. RPWO was the most accurate wash-out parameter. The quadriphasic method of adrenal imaging is relatively new and promising and may allow for faster workflow because of its 5 min delayed scan vs a 10 or 15 min delayed scan[17].
Figure 2 A patient presents with an indeterminate left adrenal nodule (arrows) on computed tomography.
Non-contrast (A), arterial phase (B) and 15 min delayed (C) images of through the left adrenal gland demonstrate Hounsfield Units values of 19, 164 and 48, respectively. Absolute contrast enhancement wash-out (ACEW) percentage and the relative contrast enhancement wash-out (RCEW) percentage were calculated. The ACEW was [(164 - 48)/(164 - 19)] × 100 = 80%, which is greater than 60% and consistent with an adenoma. The RCEW was [(164 - 48)/164] × 100 = 70.7%, which is greater than 40% and also consistent with an adenoma.
Another technique used to differentiate adenomas from non-adenomas is calculating a relative wash-in ratio on early biphasic CT. It may be useful for further differentiating adenomas from metastases when non-contrast CT is not available. One study determined the difference in the relative percentage wash-in ratio from arterial and portal venous phases and found that many adenomas had faster enhancement, whereas most metastatic lesions had slower enhancement. Benign lesions may have patent vessels that allow for easy contrast passage and exhibit fast wash-in and wash-out from the arterial to portal phases. Malignant lesions have a high cellular density and densely packed vessels, causing higher resistance to contrast flow and slow wash-in and wash-out patterns[18]. The drawback is that there is some overlap in contrast-enhancement patterns between adenomas and non-adenomas; therefore, wash-in patterns are not always reliable[19]. In terms of wash-in techniques, venous phase post-contrast imaging has been shown to be useful in distinguishing adenomas from pheochromocytomas, in which the latter generally enhance more than adenomas and have overall higher levels of enhancement[19,20].
Another CT technique used to characterize adrenal lesions is the histogram analysis method, in which a cursor is placed over at least two-thirds of an adrenal mass, excluding areas of necrosis. The individual attenuation values of all the pixels in the region of interest (ROI) are plotted against their frequencies. With this technique, the mean, range and percentage of pixel values in the selected ROI are calculated. Generally, the number of negative pixels (less than 0 HU) in the ROI is directly correlated with the lipid content of the mass. Early studies revealed that nearly 97% of adenomas contain negative pixels and that metastases have no negative pixels[2,21]. Later studies demonstrated variability in negative pixel content in both adenomas and non-adenomas. Therefore, it is used as a supplemental tool to non-contrast CT. A more than 10% negative pixel content or non-contrast HU of less than 10 characterizes approximately 91% of adenomas[22,23]. The histogram analysis is not a useful supplement for enhanced CT scans because the sensitivity is only 12%[2,24], despite a specificity of nearly 100%. Another drawback of histogram analysis is that its results vary on the basis of the scanning technique and scanner used[8].
Another CT technique being investigated for adrenal characterization is adrenal gland perfusional analysis, in which the blood flow perfusion of adrenal masses of varying histological types is evaluated using deconvolution algorithm-based CT perfusion software. The perfusion parameters of adenomas and non-adenomas are investigated, included blood flow, blood volume, mean transit time and permeability surface-area production, which reflect tumor angiogenesis. Adenomas and non-adenomas differ, particularly with the blood volume and surface-area production parameters. When the blood volume was ≥ 9.325 mL/min per 100 g (P < 0.05), adenomas were found to have a sensitivity of 76.9% and specificity of 73.2%. In addition, adenomas had a higher permeability surface-area production value than non-adenomas[25].
MRI
MRI is used to characterize adrenal lesions by characterizing the tissue signal. Several MRI techniques for characterizing adrenal lesions involve spin-echo imaging, contrast-enhancement, chemical shift imaging (CSI), diffusion-weighted imaging (DWI) and MR spectroscopy.
A normal adrenal gland has a low to intermediate signal on both T1- and T2-weighted sequences. On T2-spin-echo imaging, adenomas are generally isointense or hypointense compared with a normal adrenal gland. Sometimes, adenomas contain small foci of altered intensity that represent vascularity, cystic change or hemorrhage. Malignant lesions contain more fluid and generally have a higher T2 signal than adenomas and a higher T2 signal than a normal adrenal gland. However, this is a generalization and not always accurate, with a large overlap in appearance between adenomas and metastases[26].
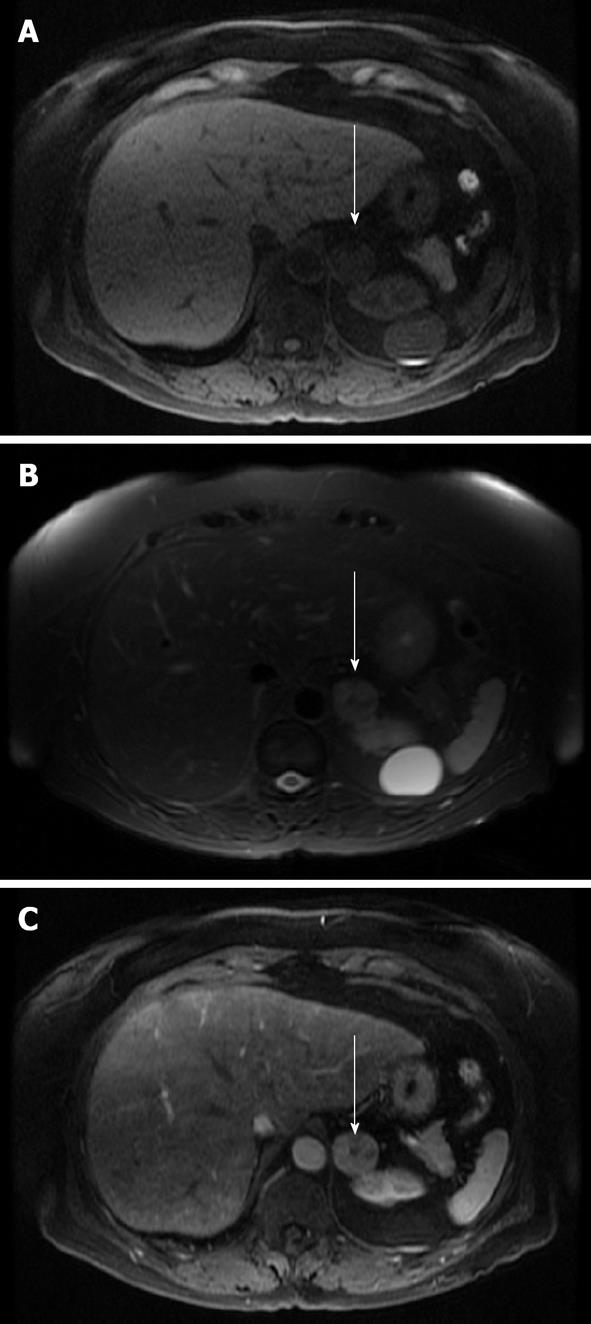
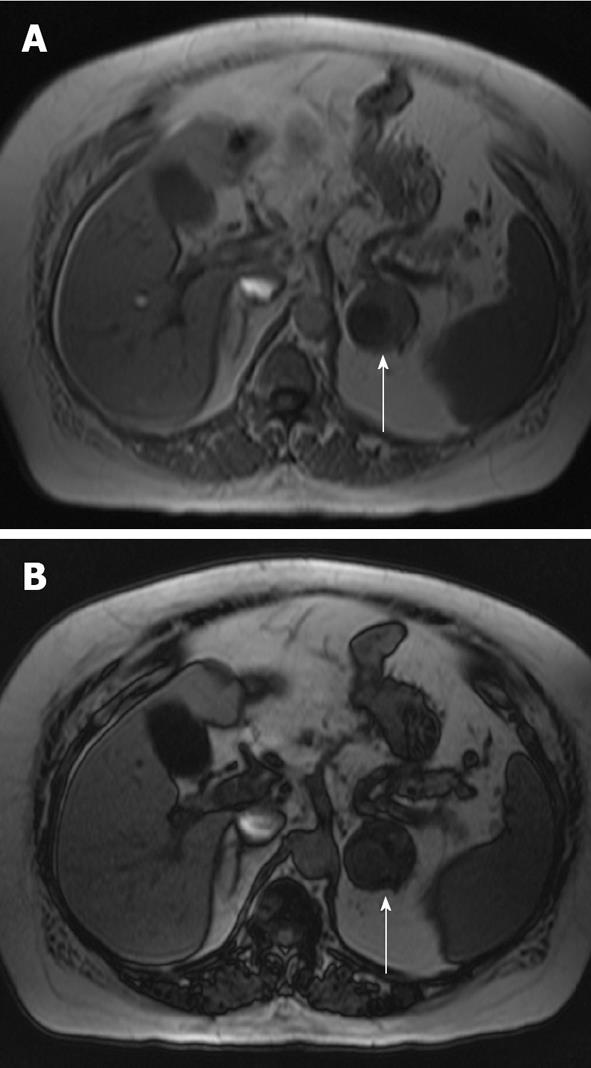
Contrast enhancement with MRI has also been used to characterize adrenal lesions. After administration of gadolinium on dynamic imaging, the time to reach peak enhancement is the best indicator for differentiating adenomas from adrenal malignancies. Typically, adenomas reached peak enhancement at 40 s and malignant lesions reached peak enhancement at 65 s; the actual peak value was not statistically significant[27]. Using a threshold of 53 s, the sensitivity was 87.5% and specificity was 80% for adenoma detection[27]. Another characteristic used is enhancement pattern: approximately 90% of adenomas exhibit a homogeneous or ring enhancement pattern and 60% of malignant lesions have a heterogeneous pattern. Some have described an early homogenous capillary blush at 18 s in about 70% of adenomas[28]. Significant overlap in contrast enhancement is present between adenomas and malignancies; thus, additional sequences, particularly CSI, are more useful in these cases. Figure 3 shows an adrenal adenoma and Figure 4 shows an adrenal metastasis with T1, T2 and post-contrast imaging.
Figure 3 Magnetic resonance imaging axial images of a left adrenal adenoma (arrows).
A: T1 non-contrast image which demonstrates low T1 signal; B: A T2 image which demonstrates intermediate high T2 signal; C: A post-contrast image which demonstrates heterogeneous enhancement.
Figure 4 Magnetic resonance imaging axial images of a right adrenal metastasis (arrows) from a primary non-small cell lung cancer.
A: A non-contrast T1 image that demonstrates low T1 signal; B: A T2 image which demonstrates intermediate high T2 signal; C: A post-contrast image which demonstrates peripheral enhancement and a degree of internal signal wash-out.
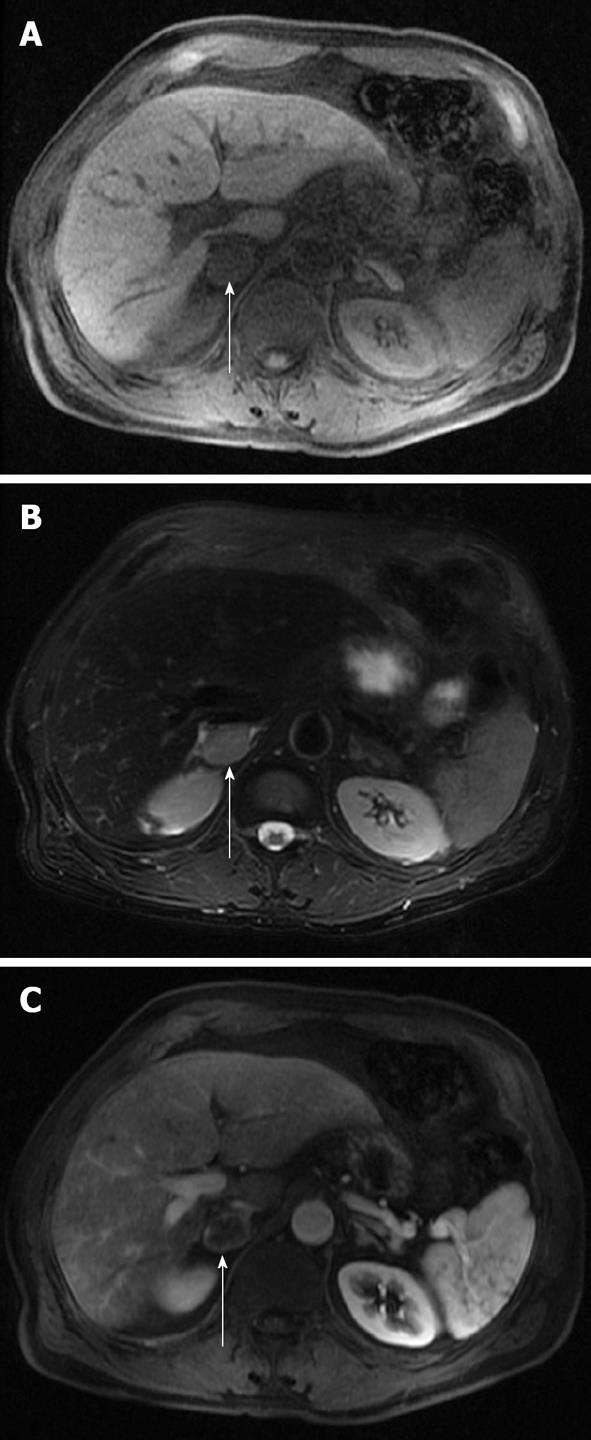
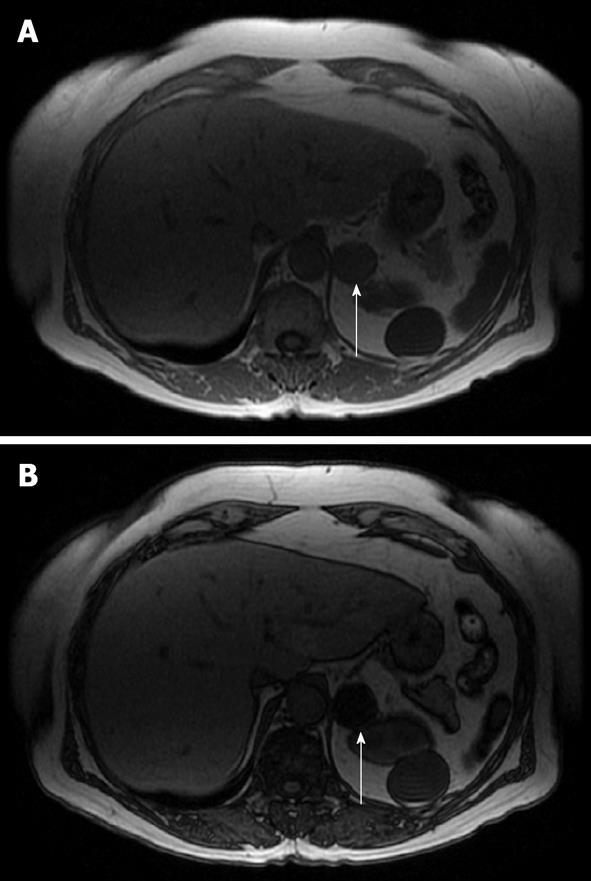
CSI is the mainstay for MRI of solid adrenal masses. CSI is based on the principle that protons in water molecules precess at a different frequency than protons in lipid molecules in a magnetic field. Sequences are created in which fat and water protons cycle in and out of phase with one another. In-phase imaging results from the additive effect of water and fat in the same voxel. Out-of-phase imaging results from the subtractive effect of water and fat in the same voxel. Adenomas exhibit signal loss on out-of-phase imaging compared with on in-phase imaging because fat protons precess at a lower frequency than water protons and thus cancel one another out on out-of-phase imaging. A lipid-rich adenoma has almost equal concentrations of fat and water in the same voxel and thus exhibits nearly complete signal loss on out-of-phase imaging. Note that some malignant lesions can exhibit signal loss on out-of-phase imaging, including clear cell carcinoma, pheochromocytoma and adrenal cortical cancer; in these cases, other sequences and features should be used to help in the differentiation, such as size and the presence of hemorrhage. Lipid-poor adenomas contain a low lipid-to-water proton ratio and thus do not drop signal on out-of-phase images and remain indeterminate on CSI[29,30]. Typically, pheochromocytomas, malignant lesions and some lipid-poor adenomas do not demonstrate a change in signal because of the lack of sufficiently detectable intracellular lipid. Figure 5 illustrates a lipid-rich adenoma and Figure 6 illustrates a lipid-poor adenoma with CSI.
Figure 5 Magnetic resonance imaging axial in-phase (A) and out-of-phase (B) images of a left lipid-rich adenoma (arrows).
Note the near complete loss of signal on the out-of-phase image due to almost equal concentrations of fat and water in the same voxel.
Figure 6 Magnetic resonance imaging axial in-phase (A) and out-of-phase (B) imaging of a biopsy-proven lipid poor adenoma (red arrow).
Note that the adenoma exhibits heterogeneous dropout on the out-of-phase imaging due to a low lipid-to-water proton ratio.
CSI has a sensitivity of 81%-100% and specificity of 94%-100%, similar to those of non-contrast CT[8,31]. No significant difference has been found between CT and MRI for lipid-rich adenomas. CSI has been shown to be superior to non-contrast CT in evaluating lipid-poor adenomas[32]. One study demonstrated that CSI is useful only when the non-contrast CT HU is less than 30[33]. In cases in which lipid-poor adenomas have a HU of 10 to 30, a lipid-poor adenoma was characterized with 89% sensitivity[2].
Other MRI adrenal chemical-shift characterization techniques include qualitative visual analysis and quantitative analysis using the signal intensity index (SII) and the adrenal/spleen ratio. SII is based on in- and out-of-phase imaging and is calculated as SII: [(lesion SI in-phase - lesion SI out-of-phase)/lesion SI in-phase] × 100. Typically, adenomas have an SII more than 5% and metastases exhibit indices lower than 5%. An SII more than 16.5% is indicative of a lipid-rich adenoma[34]. Some studies have used a threshold of 1%-30% to characterize adenomas on the basis of variability in imaging parameters[2,29,35]. The SII has proven useful as it has been found to have an accuracy of 100% at distinguishing adenomas from metastases[36].
Another quantitative analysis method uses the adrenal/spleen ratio (ASR), in which a comparison of adrenal mass intensity and extra-adrenal organ (such as the spleen) or paraspinal musculature intensity is used with ROI measurements. The liver is not typically used for comparison because of possible fatty infiltration or iron deposition[2]. The ASR measures the percentage of signal dropout of the adrenal lesion compared with that of the spleen and is calculated as: ASR = [(SI adrenal out-of-phase/spleen out-of-phase)/(SI adrenal in-phase/SI spleen in-phase] × 100. An ASR ratio of ≤ 70 has 78% sensitivity and 100% specificity for detecting adenomas[37]. SII has been found to be more reliable than ASR[2].
Another method of CSI quantitative analysis is subtraction CSI, in which out-of-phase images are subtracted from in-phase images and analyzed qualitatively or quantitatively. Qualitatively, adenomas typically exhibit higher intensities than metastases. Quantitatively, an ROI is obtained of the difference in mean signal intensity between out-of-phase and in-phase images. One study[38] found an accuracy of 100% in adenomas with a calculated value of more than 106 and in metastases with a calculated value of less than 36.
DWI has been investigated as an MRI tool for further characterizing adrenal lesions. DWI measures tissue cellularity and cell membrane integrity by Brownian motion, which is a heat-induced random motion in biological tissues. DWI has been found to be helpful in detecting tumors using diffusion effects with apparent diffusion coefficient (ADC) measurements. Theoretically, malignant lesions have increased cellularity, leading to restriction of water diffusion; therefore, they should have lower ADC values. A few studies have investigated the role of DWI in the adrenal glands. The results demonstrated that because of significant overlap between ADC values in benign and malignant lesions, DWI is not useful for distinguishing lesions[39,40]. In addition, DWI does not distinguish between lipid-rich and lipid-poor adenomas or between lipid-poor adenomas and other non-fatty lesions of the adrenal gland[39]. One study found that pheochromocytomas have significantly higher ADC values than adenomas and metastases[36]. Another study found that although DWI sequences alone may lead to misdiagnosis because of significant overlapping of benign and malignant ADC values, they can differentiate between the 2 when used in conjunction with additional conventional sequences[41].
MR spectroscopy has shown promise as a method for characterizing adrenal lesions. Proton (¹H) spectroscopy measures the biochemical nature of living tissues and yields data on tumor metabolism. Specific pattern changes in metabolite concentrations have been demonstrated in tumors using choline, creatine and sometimes lipid peaks. Choline is used as a tumor marker because it participates in cell membrane synthesis and breakdown. Creatine is used as a standard metabolite that yields information on cell energy status. Lipid peaks have been used but are associated with benign cellular processes such as intracellular lipids[42]. The percentage of lipid content is significantly lower in carcinomas than in adenomas[42,43]. One MR spectroscopy study demonstrated that on visual and qualitative analyses, only the presence of a positive choline peak was useful for distinguishing between benign and malignant masses. However, with the quantitative ¹H MR spectroscopy metabolite ratio, the analysis was more useful because adenomas and pheochromocytomas could be distinguished from carcinomas and metastases using a cut-off value of 1.20 for the choline-creatine ratio (92% sensitivity, 96% specificity), 0.38 for the choline-lipid ratio (92% sensitivity, 96% specificity) and 2.10 for the lipid-creatine ratio (45% sensitivity, 100% specificity). In addition, pheochromocytomas and carcinomas could be differentiated from adenomas and metastases with a 4.0-4.3 ppm/creatine ratio of more than 1.50 (87% sensitivity, 98% specificity). Comparing the 4.0-4.3 ppm/creatine ratio and the choline-creatine ratio provided the best distinction between adenomas, pheochromocytomas, carcinomas and metastases in 54 of 60 adrenal lesions[42]. Another study demonstrated that respiratory-triggered MR spectroscopy is useful for characterizing pheochromocytomas. Adrenal pheochromocytomas demonstrate a unique spectral signature of 6.8 ppm resonance that is not demonstrated in adenomas and may be due to the presence of catecholamine components in pheochromocytomas[44]. The results of these studies demonstrate that MR spectroscopy is a promising method for further characterizing adrenal lesions.
PET-CT
Another modality widely used to characterize adrenal masses is PET, which is a non-invasive whole-body imaging modality that uses the agent fluorine-18 fluorodeoxyglucose (18F-FDG), a radiolabeled glucose analog that is accumulated via cellular glucose transport mechanisms and trapped by intracellular phosphorylation. Increased glucose metabolism is demonstrated in malignant tumors and secreting adrenal lesions. FDG-PET reveals information about the biochemical activity of the lesion of interest (in this case, the adrenal gland) that precedes macroscopic anatomic changes[45].
Note that FDG is relatively widely used for PET imaging, but there are other non-FDG radiopharmaceuticals that have limited roles, including C11-MTO, which helps determine adrenocortical vs non-cortical origin. 18F-DA, 18F-DOPA and 68Ga-DOTA-peptide can be used to determine whether the lesion is of neuroendocrine origin. These non-FDG agents currently have limited clinical roles; thus, the focus in this section is on 18F-FDG-PET examinations.
PET-CT combines the anatomic cross-sectional data of CT with the functional data of PET. One study found that only 5% of normal adrenal glands can be visualized on PET alone but 68% can be identified with integrated PET-CT[46]. PET-CT has been used to distinguish benign from malignant masses, characterize the origin of the mass as adrenal vs non-adrenal and stage malignancies[5].
A normal adrenal gland usually has mild uptake of 0.95 to 2.46 peak standardized uptake values (SUVmax), which is visually lower than that of the background of liver[46]. Although quantitative analyses with SUV have been shown to be useful, visual quantitative analyses are as effective[47]. One reason is that SUV measurements are subject to many sources of variability, including scanning acquisition technique and volume averaging[45]. Lesions that have visual uptakes and SUVs lower than those of the liver are benign, with a specificity of 100%[2]. Figure 7 shows a PET-CT image of an adenoma with lower uptake than that of the liver. A study found that when an SUVmax cut-off value of 3.1 was used, the sensitivity was 98.5% and the specificity was 92%[48]. However, adrenal lesions with visual and quantitative uptake equal to or higher than those of the liver are problematic, with a specificity of 88% to 94%[47,49]. In addition, although the SUVmax for adenomas tends to be lower than that for metastases, there remains overlap; thus, another quantification method of the ratio of SUV of the adrenal mass to liver was created to eliminate background tracer uptake in the adrenal lesion[2]. One study used a threshold ratio of 1.45, with a 100% sensitivity and 88% specificity[50].
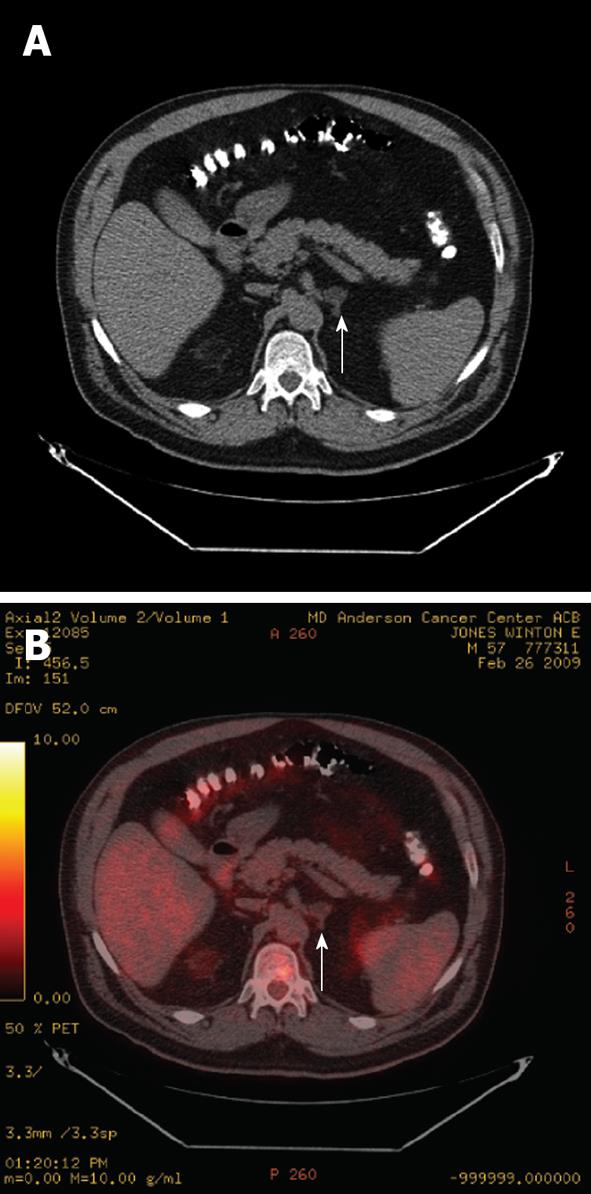
Figure 7 Axial non-contrast computed tomography image (A) and fused positron emission tomography/computed tomography image (B) of a patient with esophageal cancer with a left adrenal adenoma (arrows).
The adenoma has an Hounsfield Units of -10 on the non-contrast computed tomography (A) and demonstrates fluorodeoxyglucose uptake less than the liver (B), and is therefore not consistent with a metastasis.
Various interpretative algorithms have been used to characterize benign adrenal lesions. Benign lesions have < 10 non-contrast HU, an adrenal standardized uptake maximum value (SUVmax) of < 3.1, and a SUVmax/liver SUVavg ratio of < 2.5[51]. In general, when unilateral or bilateral adrenal nodules have more activity than the liver in cancer patients, malignancy should be suspected because malignant adrenal lesions are more likely to be metabolically active than benign adenomas[1]. Figure 8 shows an adrenal metastasis on PET-CT. Multiple studies have revealed that PET can differentiate benign from malignant masses in cancer patients, with a sensitivity of 74% to 100% and specificity of 66% to 100%[5,47]. In non-cancer patients, the sensitivity is 73%-100% and the specificity is 70%-100%[5,52]. Note that the most common primary malignancies to metastasize to the adrenal gland are lung cancer, breast cancer, melanoma, kidney cancer, colon cancer and thyroid cancer[8]. Although PET can identify malignant lesions, it cannot differentiate the particular type of malignancy, such as metastases, adrenal cortical carcinoma, malignant pheochromocytomas or lymphoma[8]. PET-CT is useful for differentiating lymphomatous from non-lymphomatous involvement (e.g., hyperplasia or nonfunctioning adenoma), in which the degree of adrenal lymphoma equals that of other extra-adrenal areas of lymphomatous involvement; thus, it can be used to assess treatment response[8,53].
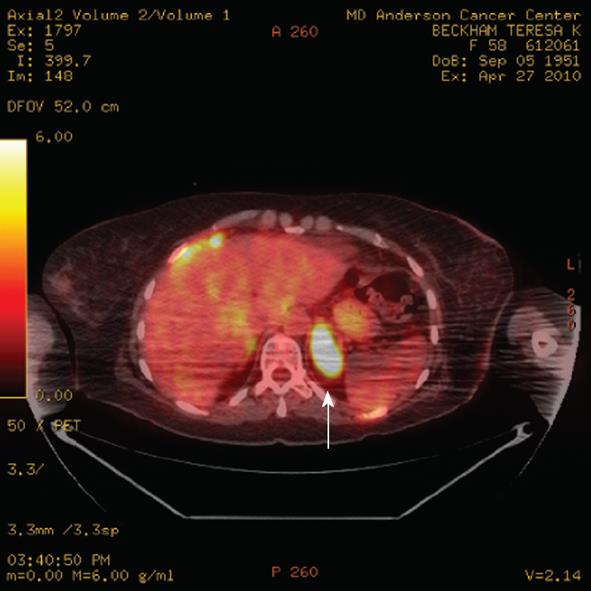
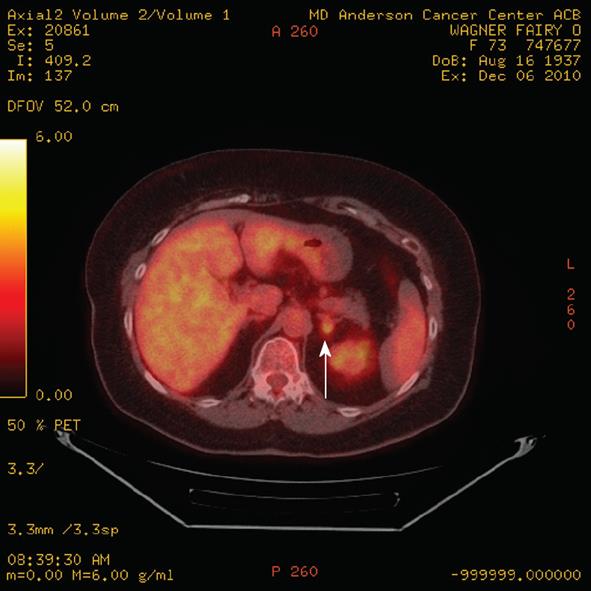
Figure 8 Axial positron emission tomography/computed tomography demonstrates a left adrenal 4.
5 cm metastasis (arrow) from breast cancer. The standardized uptake values of the metastasis was 11.9. Note the incidental streaking artifact.
PET-CT has been used to differentiate adenomas from non-adenomas by combining the SUV ratios with unenhanced CT measurements[49]. The combination of PET and unenhanced CT is more accurate than either modality alone[51,53]. PET-CT had a superior performance to unenhanced CT in 2 studies because of the limited sensitivity (57% and 66%) of unenhanced CT for lipid-poor adenomas and other benign lesions that mimic malignancy[47,54]. On PET, adenomas typically do not demonstrate increased activity, although false-positive results do occur[55]. Generally, adenomas that demonstrate FDG activity demonstrate mild activity, whereas malignant lesions exhibit marked FDG activity[45]. In cases of mild FDG activity, delayed-contrast CT scans with wash-out measurements may be useful for further characterization[56], although one study demonstrated that delayed enhanced CT provides better characterization than PET-CT[57].
Most PET scanners have a spatial resolution of approximately 5 mm, which can miss a lesion. In addition, there are false negatives and false positives. False negative results can occur when an adrenal gland is too small to detect (5-10 mm) or when hemorrhage, tumor necrosis, post-treatment changes or underlying low-grade tumors (e.g., neuroendocrine, renal cell carcinomas and bronchoalveolar type) are present[5,58]. False positive results occur approximately 3%-13% of the time, typically as the result of an infectious or inflammatory process. In addition, some benign cortical adenomas, adrenal cortical hyperplasia, adrenal endothelial cysts and benign pheochromocytomas may demonstrate more activity than the liver, causing false positive results[2,5,8,59]. Figure 9 shows a false positive adrenal lesion on PET-CT.
Figure 9 A positron emission tomography/computed tomography fused axial image demonstrates a left adrenal mass (arrow) with increased fluorodeoxyglucose activity slightly greater than the liver in a patient with non small cell lung cancer.
Biopsy revealed an adenoma, rendering this a false positive finding.
In summary, PET-CT is useful for identifying masses that are indeterminate on CT and MRI. PET-CT can help distinguish between benign and malignant origin and help in therapeutic management. No FDG uptake in an adrenal nodule in an FDG-avid cancer patient suggests a benign process; thus, unnecessary surgery could be avoided. Avid adrenal uptake is a strong indicator of malignant or secreting lesions and further intervention should be considered, such as adrenal biopsy or surgery[60]. Another drawback is that PET-CT cannot differentiate among different malignancy types or characterize tissue, as described above.
BIOPSY
Given the advancements in imaging techniques in CT, PET and MRI, only a small number of adrenal masses remains indeterminate. In patients with known malignancies, these masses are candidates for percutaneous biopsy. First, pheochromocytomas should be excluded with laboratory studies and imaging because percutaneous biopsies can elicit adrenal hypertensive crises. Percutaneous CT-guided adrenal biopsies are relatively safe in patients with underlying non-adrenal primary malignancies. A single percutaneous negative biopsy is regarded as sufficient on the basis of a study in which 225 CT-guided, single-pass biopsy samples with benign findings yielded a 98%-100% negative predictive value[61]. There is a risk of minor complications that require no treatment. Major complications, such as seeding, pneumothoraces and infection, occur approximately 3% of the time and require treatment interventions[2,62].
SUMMARY
The non-invasive characterization of adrenal lesions continues to improve with current cross-section imaging modalities. CT is useful, particularly because of its non-contrast and wash-out features. MRI is especially useful with CSI and for MR spectroscopy. PET-CT can differentiate benign from malignant masses through qualitative analysis and detect the presence of extra-adrenal metastases in cancer patients.
The preferred work-up scheme for the indeterminate adrenal mass includes evaluation with a non-contrast CT. If a lesion is less than 10 HU < 10 on a non-contrast CT, it is a benign lipid-rich adenoma and no further work-up is needed. A lipid-poor adenoma can be differentiated from a metastasis utilizing CT wash-out patterns or MRI. If a lesion remains indeterminate, PET imaging may be of use in the appropriate clinical setting, in which lesions with low or no FDG activity are mostly benign. In the few cases in which adrenal lesions remain indeterminate, surgical sampling, such as percutaneous biopsy, can be performed.