Published online Aug 28, 2012. doi: 10.4329/wjr.v4.i8.379
Revised: August 20, 2012
Accepted: August 27, 2012
Published online: August 28, 2012
AIM: To evaluate the response of hepatocellular carcinoma (HCC) to transarterial chemoembolization (TACE) using a simplified protocol of parametric contrast-enhanced ultrasound (pCEUS).
METHODS: Eighteen patients with HCC (18 target tumors, diameter: 2.8-12 cm) were evaluated before, and 20 d after TACE. The distribution and morphology of TACE-induced necrosis in these tumors precluded accurate evaluation by visual assessment or by simple measurements. For pCEUS, a 4.8 mL bolus of SonoVue (Bracco, Milan, Italy) was intravenously administered and analysis of tumor perfusion during the initial phase of enhancement (0-30 s post injection) was performed with dedicated software (Qontrast, Bracco, Milan, Italy). Time-intensity curves were plotted and three parameters were calculated: peak intensity (PI, in percentage %), time to peak (TTP in seconds, s) and area under the curve during wash-in (AUC-WI, in arbitrary units, a.u). Magnetic resonance imaging was the standard imaging modality for post-treatment evaluation. Changes in tumor size were recorded and response was assessed according to response evaluation criteria in solid tumors criteria.
RESULTS: A statistically significant decrease in PI and AUC-WI was observed in the treated tumors post TACE; PIpre: 21.5% ± 8.7% (mean ± SD), PIpost: 12.7% ± 6.7%, P < 0.001, AUC-WI pre: 17493 ± 9563 a.u, AUC-WI post: 9585 ± 5494 a.u, P < 0.001. A slight increase in TTP was noted post TACE, but this was not statistically significant; TTP pre: 13.1 ± 4.3 s, TTP post: 13.6 ± 4.2 s , P = 0.058). The changes in the aforementioned parameters were not accompanied by significant tumor shrinkage.
CONCLUSION: pCEUS, even when limited to the study of the arterial phase of tumoral enhancement, can detect and quantify early perfusional changes in HCC post TACE.
- Citation: Moschouris H, Malagari K, Marinis A, Kornezos I, Stamatiou K, Nikas G, Papadaki MG, Gkoutzios P. Hepatocellular carcinoma treated with transarterial chemoembolization: Evaluation with parametric contrast-enhanced ultrasonography. World J Radiol 2012; 4(8): 379-386
- URL: https://www.wjgnet.com/1949-8470/full/v4/i8/379.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i8.379
One of the many evolving roles of contrast-enhanced ultrasonography (CEUS) in oncologic imaging is in the evaluation of the efficacy of transarterial chemoembolization (TACE) of liver tumors[1-3]. CEUS has been proved to be efficient in differentiating residual (enhancing) from necrotic (non-enhancing) tumor after TACE, and the therapeutic effect can be assessed by subjective, visual evaluation or by simple calculations (for example, by uni- or bi-dimensional measurements of the residual enhancing tumor at a representative section).
In order to achieve a more accurate and quantitative assessment of the enhancement of liver tumors using CEUS, dedicated software has been developed, which allows for pixel-by-pixel analysis of the changes in signal intensity (SI) of CEUS images acquired at a defined plane over a selected period of time and for calculation of several perfusion parameters[4]. Quantitative (parametric) CEUS has already been used to assess the effect of antiangiogenetic agents on liver tumors; however, this technique could also be applied for the study of liver tumors post-TACE, particularly in cases in which the chemoembolic effect cannot be easily estimated with the aforementioned simple methods.
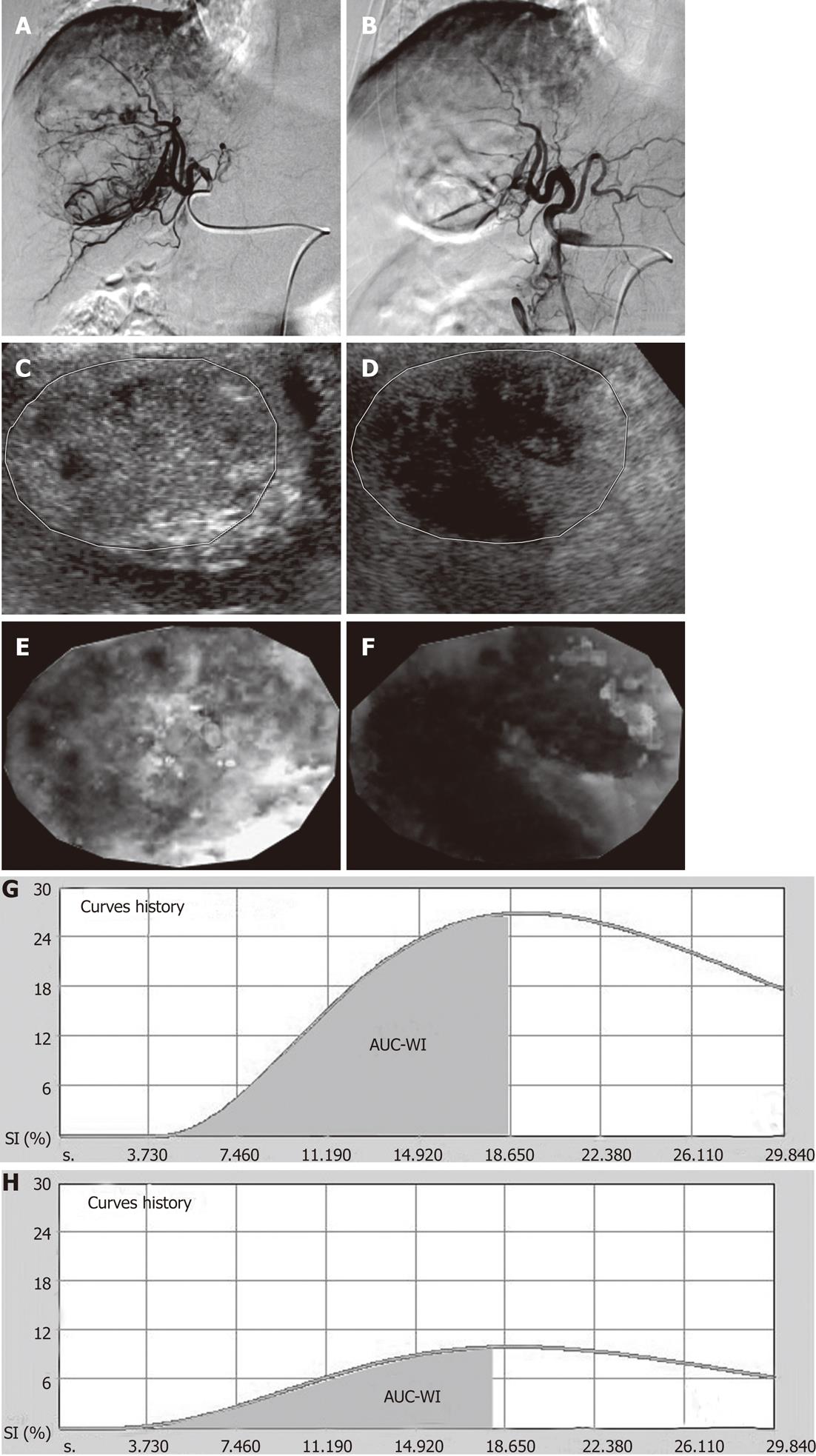
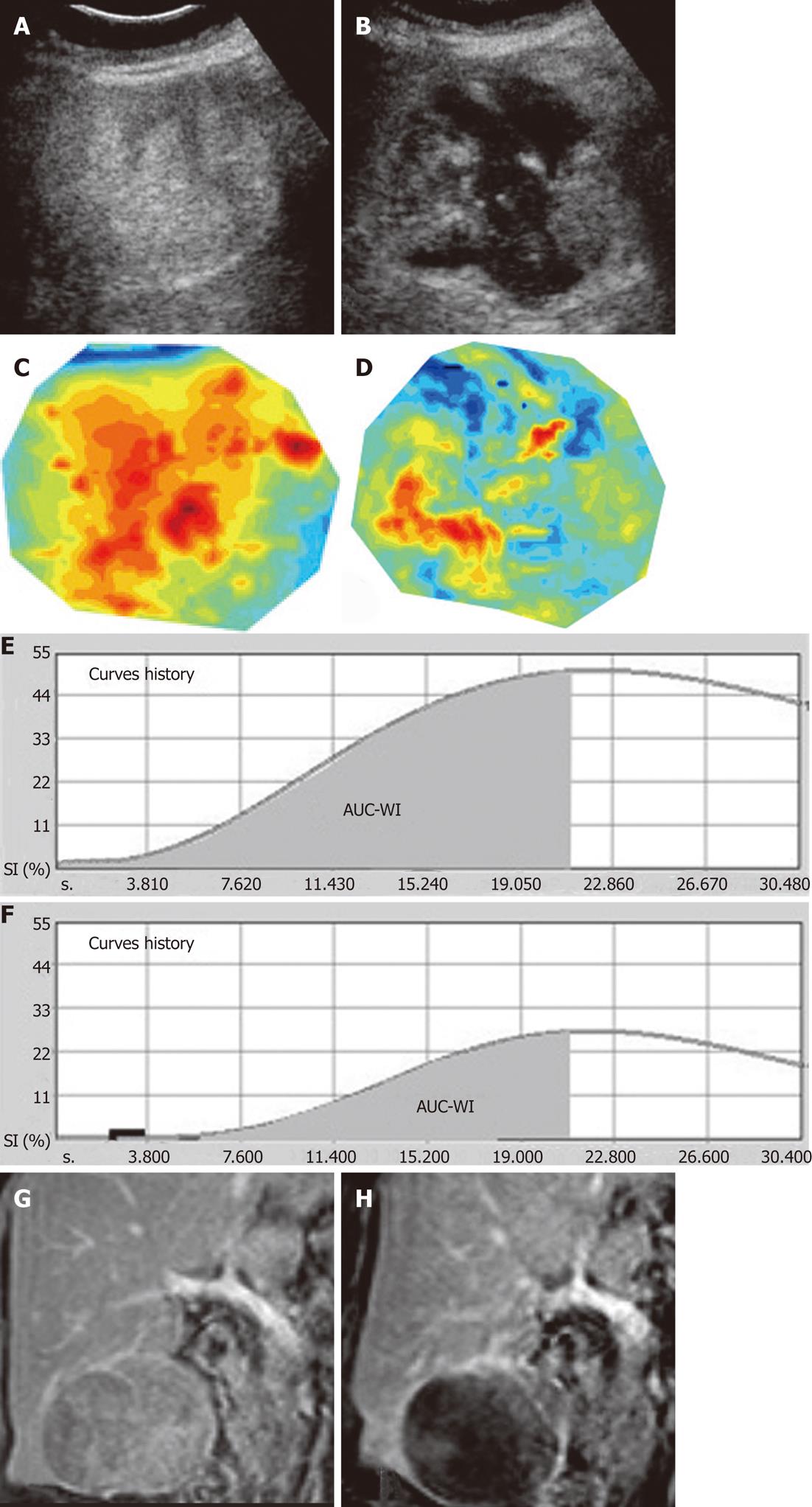
Eighteen non-consecutive patients (14 men and 4 women; mean age: 70.3 years; range: 54-84 years) with hepatocellular carcinoma (HCC), were included in this study. 10 of the studied patients had solitary lesions and 8 had multifocal involvement. In the latter, one tumor (superficial and lacking significant necrosis at baseline) was selected as the target lesion for the subsequent CEUS study. The longest diameter (sonographic measurement) of the target tumors ranged from 2.8-12 cm (mean: 7.5 ± 2.6 cm). All target tumors were suitable for a detailed and reproducible sonographic study. Moreover, after TACE, all target tumors exhibited multiple, ill-defined necrotic (non-enhancing) areas as well as islets of viable tissue with varying degrees of enhancement; this complex appearance precluded accurate evaluation of the therapeutic effect by simple (uni- or bi-dimensional) measurements of the residual enhancing tumor tissue.
The 18 patients in this study were a subgroup of a total of 63 patients who were eligible for, and underwent TACE in our institution during a period of 21 mo. Forty-five out of sixty-three patients were not included in the study for the following reasons: (1) Twenty-one patients (33.3%) were unsuitable for an optimal and reproducible CEUS study. Thirteen of those patients had deep-seated lesions (> 10 cm from the skin surface). The remaining 8 patients showed poor co-operation, regarding breathholding; (2) in 20 patients, TACE had caused a well-defined, easily measurable enhancement defect, and parametric analysis was deemed unnecessary; and (3) four patients showed complete disappearance of tumoral enhancement indicative of complete necrosis.
TACE was performed using a technique similar to that described in previous studies[1,2]. All patients were treated with subsegmental and/or segmental TACE. TACE was performed with drug-eluting microspheres (DC-Beads Biocompatibles Ltd, Surrey, United Kingdom). Each patient received 2-4 mL of DC beads (diameters: 100-300 μm and 300-500 μm) preloaded with doxorubicin (Adriblastina, Pfizer Italia S.r.L., Nerviano, Milano, Italy, dose: 25-37.5 mg drug/mL of hydrated beads). Additional bland embolization was performed in 7 cases (Embozene microspheres, CeloNova BioSciences Inc., Newnan, GA, United States). Post-embolization angiogram showed partial devascularization in all 18 target tumors.
CEUS examination consisted of two phases, the second phase being performed 15 min after the first. Each phase required the intravenous administration of a 4.8 mL bolus of second generation echo-enhancer based on sulfur hexafluoride (SonoVue®, Bracco S.p.A., Milan, Italy), followed by 10 mL of sterile solution. A Philips HD11 XE (Philips Ultrasound, Andover, MA, United States) ultrasononographic unit equipped with dedicated, contrast specific, low mechanical index software and a convex probe (frequency: 2.5-5 MHz) were utilized.
The first phase was a non-parametric study (npCEUS), during which the entire target tumor and the surrounding liver parenchyma were scanned for approximately 120 s (i.e. during the arterial, portal and parenchymal phases of enhancement). During this phase, an overall impression of the tumor enhancement was provided and the imaging plane, which depicted the target tumor at its largest diameter, was defined. This was the reference imaging plane for the subsequent, parametric study. During npCEUS, scan parameters (depth, focus, pulse repetition frequency, mechanical index, gain, depth-gain compensation) were optimized for a clear, artifact-free depiction of tumoral enhancement at the reference plane.
The second phase was exclusively focused on the study of tumoral enhancement at the reference plane for the first 30 s after injection of the echo enhancer. The probe was manually stabilized at the reference plane and the patient was instructed to hold his breath for 30 s, or to take very shallow breaths for the same period. Care was taken to avoid any change in imaging plane and in scan parameters that were defined from the first phase. The second phase of CEUS examination resulted in a cineloop of 399 frames, which depicted the initial phase of the enhancement of each studied tumor at the plane of its largest diameter. This cineloop was stored as a digital archive (Audio Video Interleave) in the hard disc of the sonographic unit and was transferred to a personal computer for off-line parametric analysis. The second phase of CEUS along with the subsequent analysis was referred to as “parametric CEUS” (pCEUS).
Each of the 18 patients underwent the above-described biphasic CEUS examination 1-2 d prior to TACE, as well as 18-22 d post TACE, using the same imaging plane and the same scan parameters. We did not evaluate the patients early (i.e. 1-2 d) post TACE; it has been observed, that increased tumor echogenicity shortly after TACE may interfere with detection of tumoral enhancement on CEUS[3].
One session of TACE was studied per patient. All npCEUS and pCEUS studies were performed by the same consultant radiologist with 7 years experience in CEUS and 20 mo experience in utilization of pCEUS software. The presented study was approved by the institutional review board of our hospital.
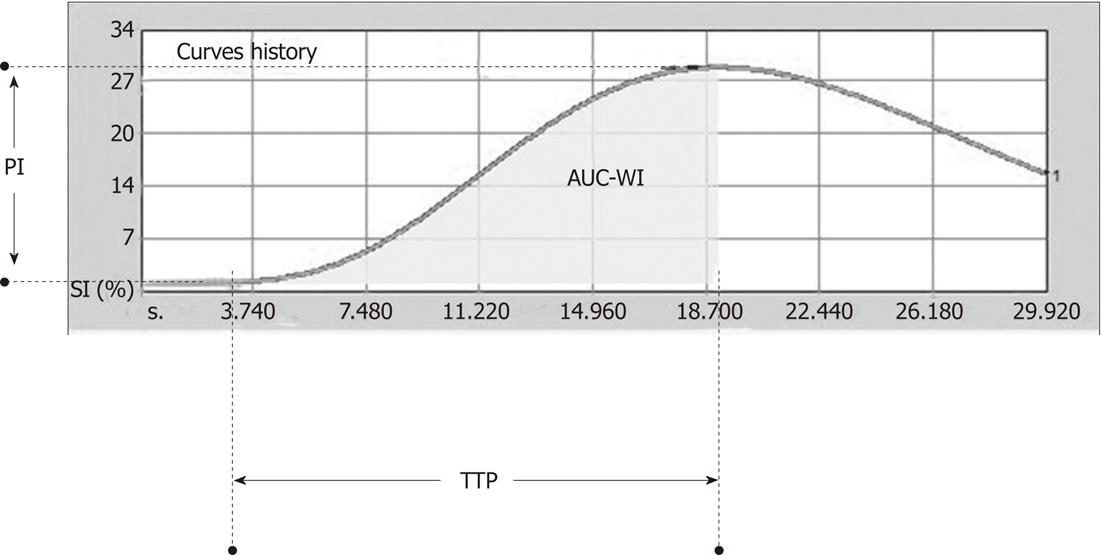
pCEUS digital archives were processed with dedicated software (Qontrast 4.00, Bracco, Milan, Italy) that evaluated tumoral perfusion using the video intensity data (i.e. non-linearized, compressed data)[4]. A region of interest (ROI) that encompassed the whole area of the tumor at the reference plane was manually drawn, and the software processed, pixel-by-pixel, the changes in SI, which occurred within the ROI over the selected 30 s period of initial enhancement. A time-intensity curve (TIC) and parametric graphs were produced. Since perfusion analysis was limited to the initial 30 s of tumoral enhancement, only the following semi-quantitative parameters were calculated (Figure 1): (1) peak intensity (PI, in percentage %): defined as the increase in SI, from baseline SI to maximal SI measured in the selected ROI during the selected period of enhancement; (2) time to peak (TTP, in seconds): defined as the time period from the onset of tumoral enhancement until the moment maximal SI was reached; and (3) area under the curve during wash-in (AUC-WI, in arbitrary units, a.u): defined as the area under TIC from the onset of tumoral enhancement until the moment maximal SI was reached.
Of note, Qontrast software can compensate for minor changes in imaging plane (caused, for example, by very shallow breathing). In case of more pronounced changes in imaging plane, frame-by-frame editing can be performed, and the respective frames can be manually selected and characterized as “wrong”. These are ignored during parametric analysis[4]. If wrong frames exceeded 10% of the total number of frames of the video clip, we rejected the respective pCEUS study as technically inadequate.
Contrast-enhanced magnetic resonance (MR) performed 2-5 d prior to intervention and approximately 2 mo (55-68 d) post intervention was used as the standard imaging modality for the evaluation of TACE in all patients. The longest diameter of the target tumor and the sum of the longest diameters of the tumors were measured on axial MR sections prior to, and post TACE; changes were recorded and tumor response was estimated according to response evaluation criteria in solid tumors (RECIST). Moreover, similar to CEUS, a decrease in tumoral enhancement was visually appreciated in all target tumors on dynamic MR images post TACE; however, the distribution, borders’ definition and varying intensity of enhancement reduction precluded accurate measurement of the TACE-induced necrosis.
Descriptive statistics were produced for continuous variables. The normality of distribution of the studied parameters (PI, AUC-WI, TTP) was evaluated with the Kolmogorov-Smirnov test. The statistical significance of the changes in PI, AUC-WI and TTP was evaluated with the paired t-test. The Pearson’s correlation coefficient between changes in longest tumor diameter and changes in perfusion parameters was calculated. A P value of < 0.05 was considered significant. Statistical calculations were performed with SPSS 19.0 (233 South Walker Drive Chicago, IL 60606-6412, United States).
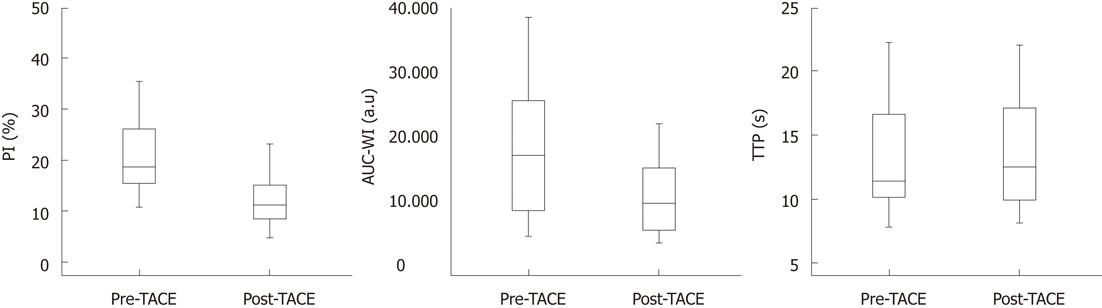
Prior to TACE, PI ranged from 10.8%-43.8% (mean: 21.5% ± 8.7%). Post TACE, PI ranged from 4.8-31.3% (mean: 12.7% ± 6.7%). The decrease in PI after TACE was statistically significant (P < 0.001). A decrease in PI was observed in all patients after TACE. The ratio of the decrease in PI [(PIpre-PIpost/PIpre) × 100%] ranged from 12.3%-64.4% (mean: 40.9% ± 16.1%). All tumors reached their PI within the studied period of 30 s after injection of the echo-enhancer.
Prior to TACE, TTP ranged from 7.8-22.4 s (mean: 13.1 ± 4.3 s). Post TACE, TTP ranged from 8.1-22.1 s (mean: 13.6 ± 4.2 s). The increase in TTP caused by TACE failed to reach statistical significance (P = 0.058). Post TACE, TTP was reduced (0.2-0.7 s shorter) in 6 patients, was prolonged (0.1-3.5 s) in 11 patients and remained unchanged in one patient.
Prior to TACE, AUC-WI ranged from 4152-38489 a.u (mean: 17493 ± 9563 a.u). Post TACE, AUC-WI ranged from 2635-21701 a.u (mean: 9585 ± 5494 a.u). The decrease in AUC-WI after TACE was statistically significant (P < 0.001). A decrease in AUC-WI was observed in all patients after TACE, the ratio of the decrease [(AUC-WI pre- AUC-WI post/AUC-WI pre) × 100%] ranged from 12.5%-70.3% (mean: 42.5% ± 17.4%).
TACE-induced changes in the aforementioned parameters are schematically shown in Figure 2. Representative cases of pCEUS assessment of TACE are shown in Figures 3 and 4.
Based on axial MR images, the change in the longest tumor diameters caused by TACE was not statistically significant (longest diameter pre-TACE: 77 ± 27 mm, longest diameter 2 mo post-TACE: 75 ± 26 mm, P = 0.09). Moreover, this change had no significant correlation with the aforementioned changes in PI (r = -0.27, P = 0.28), AUC-WI (r = -0.14, P = 0.56) and TTP (r = -0.18, P = 0.47). All patients were classified as “stable disease” according to RECIST criteria.
The time required to perform a pCEUS study (including parametric analysis) did not exceed 20 min.
This work was based on a simplified protocol of pCEUS, in which only the initial period (practically, the arterial phase) of tumoral enhancement was studied. Arterial-phase pCEUS was originally proposed by Yoshida et al[5], who used this technique to study the effect of the antiangiogenetic agent, sorafenib, on liver tumors implanted in rabbits. Compared to a complete perfusional study (lasting at least 120 s), arterial-phase pCEUS seems to be simpler and less time-consuming. Moreover, it is much easier to maintain constant and favorable imaging conditions for 30 s than for 120 s. On the other hand, significantly more parameters can be derived from a complete perfusional study. Additionally, arterial-phase pCEUS might not be suitable for the study of tumors with a slower “wash-in” phase, which require more than 30 s to reach their peak enhancement.
In our study, pCEUS revealed a statistically significant decrease in PI and in AUC-WI of the target tumors after TACE, indicating a potential value of these parameters for the quantification of the therapeutic effect of TACE. Parametric imaging is probably not necessary in tumors with total necrosis, or when TACE has caused a well-defined, easily measurable, enhancement defect. In contrast, pCEUS could be applied when simple measurements of the residual enhancing tissue cannot be performed (i.e. when TACE has caused multiple, ill-defined enhancement defects, or hypoperfused areas, instead of clear-cut necrosis).
Our results showed a large variability in the studied parameters and of their changes after TACE. This could be attributed to the small number of patients studied. It could also reflect the varying efficacy of TACE and the varying degrees of vascularity of the studied tumors; HCC is typically considered a hypervascular, arterially enhancing tumor, however, the degree of arterial hyperenhancement is not expected to be constant, depending, for example, on the degree of tumor differentiation and on the extent of spontaneous (pretreatment) necrosis.
No significant correlation was found between post-therapeutic changes in PI and AUC-WI and changes in longest tumor diameter. We do not consider that this reduces the value of our observations; it rather reflects the insensitivity of size-based criteria in evaluating the therapeutic effect, at least for the first weeks after TACE. On the contrary, we recognize as a major limitation of our work, the fact that we did not correlate our results with overall survival or with another valid response criterion; all of our patients were alive upon completion of this study and all had received additional TACE sessions. In this first attempt to assess TACE using pCEUS, we focused on the description of the technique and on the evaluation of the feasibility of pCEUS; it is clear that the prognostic value of CEUS parameters require further research.
Experience regarding post-therapeutic parametric evaluation with CEUS is primarily based on the assessment of antiangiogenetic agents[5-8], and a significant proportion of the relevant studies have been performed on animals. It is not clear which parameter better reflects the therapeutic effect. In the aforementioned study by Yoshida et al[5], the treated tumors exhibited a delay in TTP, but no significant change in PI. However, these authors included not the entire tumoral area, but only viable tumor components in their ROIs. A significant increase in TTP (and not in other parameters) was also associated with good response in the study by Schirin-Sokhan et al[6], who evaluated liver metastases treated with chemotherapeutic and antiangiogenic agents. In another study[7], a delay in TTP as well as a decrease in PI was observed post treatment with a vascular disruptive agent (AVE8062). In a third animal study[8], reduction in PI correlated well with immunohistochemically proven necrosis after administration of pazopanib. Another study[9] showed that perfusion indices, which were equivalent to our PI and AUC-WI, correlated positively with segmental blood flow and microcirculatory perfusion. Based on the last 2 observations, we can hypothesize that the reduction in PI and AUC-WI in our study reflects the TACE-induced necrosis and reduction in tumoral blood flow.
Although we found a slight increase in TTP post TACE, we could not establish a statistical significance for this observation. Methodological (i.e. small patient number) and technical factors could account for this result. We also hypothesize that TACE may not necessarily have the same effect on TTP as antiangiogenic treatment; TACE causes tumor ischemia and devascularization with different mechanisms to those of antiangiogenetic agents. Several factors (site and degree of vessel occlusion, diameter of target vessel, additional administration of bland embolic agents) could be responsible for the variability in our results; however, the analysis of these factors was beyond the scope of this preliminary work.
The clinical application of pCEUS seems to be more challenging and more demanding compared to pCEUS of experimental tumors; a significant disadvantage of this method is that it can be applied only in selected patients, who are fit for a detailed, high-quality CEUS study and who can undergo follow-up under fully reproducible conditions. In another work[10], motion artifacts caused technical failure of pCEUS in 30% of the studied patients. An additional limitation of pCEUS is that it provides perfusional information only for the selected section of the tumor and not for the entire lesion.
We conclude that a simplified protocol of pCEUS could be a useful adjunct to standard pre- and post-interventional imaging in selected cases of liver tumors, in which the efficacy of TACE cannot be adequately evaluated by visual assessment and by simple measurements. Additional studies are required, in order to define which parameters optimally estimate the therapeutic effect and to correlate these parameters with established response criteria.
One of the many evolving roles of contrast-enhanced ultrasonography (CEUS) in oncologic imaging is in the evaluation of the efficacy of transarterial chemoembolization (TACE) of liver tumors. CEUS has been proved to be efficient in differentiating residual (enhancing) from necrotic (non-enhancing) tumor after TACE, and therapeutic effect can be assessed by subjective, visual evaluation or by simple calculations.
Quantitative (parametric) CEUS has already been used to assess the effect of antiangiogenetic agents on liver tumors. In this study, the authors used this technique for the study of liver tumors post-TACE.
In this study, pCEUS revealed a statistically significant decrease in higher peak intensity and in area under the curve during wash-in of the target tumors after TACE, indicating the potential value of these parameters for the quantification of the therapeutic effect of TACE.
A simplified protocol of pCEUS could be a useful adjunct to standard pre- and post-interventional imaging in selected cases of liver tumors, in which the efficacy of TACE cannot be adequately evaluated by visual assessment and by simple measurements.
It is a well written paper with important results. Some clarifications on the methodology are needed.
Peer reviewers: Rajasvaran Logeswaran, PhD, Associate Professor, Faculty of Engineering, Multimedia University, 63100 Cyberjaya, Malaysia; Dr. Charikleia Triantopoulou, Konstantopouleion Hospital, 3-5, Agias Olgas street, 14233 Athens, Greece
S- Editor Cheng JX L- Editor Webster JR E- Editor Xiong L
| 1. | Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269-280. [PubMed] [DOI] [Full Text] |
| 2. | Moschouris H, Malagari K, Papadaki MG, Kornezos I, Matsaidonis D. Contrast-enhanced ultrasonography of hepatocellular carcinoma after chemoembolisation using drug-eluting beads: a pilot study focused on sustained tumor necrosis. Cardiovasc Intervent Radiol. 2010;33:1022-1027. [PubMed] [DOI] [Full Text] |
| 3. | Kono Y, Lucidarme O, Choi SH, Rose SC, Hassanein TI, Alpert E, Mattrey RF. Contrast-enhanced ultrasound as a predictor of treatment efficacy within 2 weeks after transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Operator’s Manual of the parametric software (Qontrast). Greece. : Esaote SpA. 2006;8-17. |
| 5. | Yoshida K, Hirokawa T, Moriyasu F, Liu L, Liu GJ, Yamada M, Imai Y. Arterial-phase contrast-enhanced ultrasonography for evaluating anti-angiogenesis treatment: a pilot study. World J Gastroenterol. 2011;17:1045-1050. [PubMed] [DOI] [Full Text] |
| 6. | Schirin-Sokhan R, Winograd R, Roderburg C, Bubenzer J, do Ó NC, Guggenberger D, Hecker H, Trautwein C, Tischendorf JJ. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol. 2012;18:541-545. [PubMed] [DOI] [Full Text] |
| 7. | Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, Vrignaud P, Bissery MC, Brulé A, Koscielny S. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100-111. [PubMed] |
| 8. | Zhu XD, Zhang JB, Fan PL, Xiong YQ, Zhuang PY, Zhang W, Xu HX, Gao DM, Kong LQ, Wang L. Antiangiogenic effects of pazopanib in xenograft hepatocellular carcinoma models: evaluation by quantitative contrast-enhanced ultrasonography. BMC Cancer. 2011;11:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Eichhorn ME, Klotz LV, Luedemann S, Strieth S, Kleespies A, Preissler G, Lindner M, Jauch KW, Reiser MF, Clevert DA. Vascular targeting tumor therapy: non-invasive contrast enhanced ultrasound for quantitative assessment of tumor microcirculation. Cancer Biol Ther. 2010;9:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Goetti R, Reiner CS, Knuth A, Klotz E, Stenner F, Samaras P, Alkadhi H. Quantitative perfusion analysis of malignant liver tumors: dynamic computed tomography and contrast-enhanced ultrasound. Invest Radiol. 2012;47:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |