Published online Jun 28, 2012. doi: 10.4329/wjr.v4.i6.278
Revised: April 16, 2012
Accepted: April 23, 2012
Published online: June 28, 2012
AIM: To present our initial experience with computed tomography guided radiofrequency ablation (RFA) of osteoid osteoma (OO) in our institution.
METHODS: RFA was performed on eight patients (5 males and 3 females) with clinically and radiologically diagnosed OO (femoral neck, n = 4; femoral diaphysis, n = 2; tibial diaphysis, n = 1; fibular diaphysis, n = 1). Ablation was performed using an electrode with a 10-mm exposed tip for a total of 4-6 min at a targeted temperature of 90 degrees Celsius. No cooling system was used. The intervention was accepted as technically successful if the tip of the electrode could be placed within the center of the nidus. We defined clinical success as a disappearance within 2 wk after treatment of symptoms that had manifested at presentation.
RESULTS: All procedures were technically successful. No major or immediate complications were observed. Clinical success was achieved in six of eight patients in the first procedure. A second procedure was performed for two patients who had recurrent or continued pain, and one of these cases was successfully treated. The overall rate of success was 87.5% (7/8). No complication was observed.
CONCLUSION: Our preliminary results indicate a favorable success rate and no complications and are compatible with the previous reports of RFA of OO.
- Citation: Asayama Y, Nishie A, Ishigami K, Kakihara D, Ushijima Y, Takayama Y, Fujita N, Tajima T, Yoshimitsu K, Matsuda S, Iwamoto Y, Honda H. CT-guided radiofrequency ablation of osteoid osteoma in the long bones of the lower extremity. World J Radiol 2012; 4(6): 278-282
- URL: https://www.wjgnet.com/1949-8470/full/v4/i6/278.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i6.278
Osteoid osteoma (OO) is a benign but painful bone lesion that primarily occurs in children and young adults[1]. The most common symptom is bone pain, which often worsens at night and is typically relieved by aspirin or other nonsteroidal anti-inflammatory drugs[2]. Other less common, but possible symptoms include growth disturbances, bone deformity, and painful scoliosis. Physical examination may disclose focal tenderness; however, signs of inflammatory disease are almost always absent. Pain may be mediated by a proliferation of nerve fibers in the lesion[3] and possibly by the release of prostaglandin. On plain-film studies, OO is seen as dense reactive bone with a radiolucent nidus at the core, which may be difficult to visualize. Computed tomography (CT) is the most effective approach to the diagnosis and localization of OO, and therefore is the imaging modality of choice[4].
As regards treatment, there are currently three options: conservative medical treatment, surgical treatment, and percutaneous intervention. Pain may disappear after several years of conservative treatment, with an average time to pain resolution of 5-6 years[5]; however, long-term medical therapy may be unacceptable due to refractory pain and complications with the chronic use of anti-inflammatory agents[6]. Surgery has been considered as a curative treatment. Because intraoperative localization of the lesion, which is usually smaller than 10 mm in maximum diameter, can be very difficult, surgical removal of the tumor often necessitates significant bone resection. Since the first report in the literature by Rosenthal and colleagues in 1992[6], CT-guided radiofrequency ablation (RFA) has been accepted as a demonstrably safe, minimally invasive, and cost-effective treatment for OO. In this report, the authors present their initial experience with CT-guided RFA of OO in eight patients.
From December 2005 to April 2010, eight patients were referred for percutaneous management of OO (5 males, 3 females; 4-18 years old; mean, 12.9 years). Lesions were located in the femoral neck (n = 4), femoral diaphysis (n = 2), tibial diaphysis (n = 1), and fibular diaphysis (n = 1). The mean maximum size of the nidi was 8.8 mm. Lesions have typical radiological findings; all patients underwent X-ray and CT examination, and a nidus was visible in each case. Diagnosis was made by the consensus of four radiologists (Asayama Y, Kakihara D, Ushijima Y, and Tajima T). The required clinical criteria for RFA included the presence of pain that was worse at night and was relieved by the administration of oral anti-inflammatory medications. Before the procedure, blood cell count and blood clotting analyses were performed (platelet count > 50 000/mL; international normalized ratio < 1.3). Patients undergoing treatment also required specific imaging criteria, including the documentation of bony sclerosis, cortical thickening, and the presence of a radiolucent nidus on plain film, CT imaging, or both. Our institutional review board approved this study. Written consent was requested in all cases from the patients themselves or their parents or legal guardians. All families decided to proceed with RFA after 5 to 15 mo (mean, 9.1 mo) of treatment with nonsteroidal anti-inflammatory drugs.
Procedures were performed on an inpatients basis. All patients were placed under general endotracheal anesthesia. Contiguous CT (Aquilion 16, Toshiba Medical systems, Tokyo, Japan) scans with a section thickness of 1-5 mm were obtained to localize the OO. Using the images, we adjusted the position of the patient’s limb and marked the skin at the planned access point. The skin was prepared and draped. Osseous access was established with an 11-gauge bony biopsy needle. Kirschner wire (Mizuho Ikakougyo, Tokyo, Japan) was advanced to the nidus via a coaxial system. Biopsies were performed using 19- to 21-gauge biopsy needles. Via a track created by the drill hole, an RF electrode with a 10-mm unprotected tip (Cool-tip; Covidien, Mansfield, Ma.) was introduced into the nidus. After the RF electrode was connected to the RF generator, RFA was performed without a cooling system. The temperature at the tip of the electrode was monitored during the procedure. Ablation was performed for a total of 4-8 min at a targeted temperature of 90 degrees Celsius, with as-needed manual adjustment of the output controls during the procedure in order to maintain stable lesion temperature. Postprocedural CT was performed to confirm the lack of soft tissue swelling and hematoma.
Follow-up occurred in the form of a clinical visit to our hospital 1 wk after treatment. After the initial follow-up examination, clinical assessment was performed every 3-6 mo, either by a visit to the outpatient clinic or via telephone.
Procedures were regarded as technically successful if the tip of the RF electrode could be placed within the center of the nidus and could be heated to the desired temperature[1]. We defined a good response (clinical success) as the disappearance within 2 wk after treatment of the symptoms that had manifested upon presentation. We also defined a recurrence as the residual occurrence or recurrence of symptoms that resembled the symptoms that had manifested at presentation and had reappeared or persisted for more than 2 wk after treatment was performed[7]. Visual analogue scale was evaluated before and after treatment. The use of non-steroidal anti-inflammatory drugs was also asked to the patients.
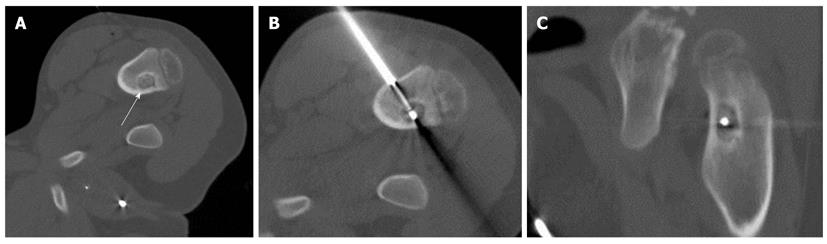
The clinical characteristics are presented in Table 1. The RFA was technically successful in all cases, and there were no procedural or postprocedural fractures, hematomas or significant muscle injuries by CT images obtained immediately after RFA. The duration of follow-up ranged from 7 to 58 mo (median, 648 d). There was a relief of pain in six of eight patients within 2 d after the procedure. All medication for pain was discontinued after the procedure in these six patients (primary success rate, 75%). One patient (case 5) (Figure 1) reported that he felt distinctly better within the first 24 h after the procedure, and did not require any medication. However, he returned to our hospital due to recurrent pain 4 mo after the procedure. He was treated successfully with a second RFA procedure and continues to be pain-free. In another patient (case 3), a tumorous lesion in the right femur was found on MRI by the patient’s local physician; that patient underwent open biopsy, but no histological confirmation was obtained. Afterward, careful reexamination at our hospital revealed a nidus near the surgical defect in the patient’s right femoral neck. Although an initial RFA procedure was performed upon localization of the nidus, this initial intervention did not result in complete relief. After 2 mo of continued pain, the patient elected to undergo a repeat procedure, ut the pain continued. This patient received follow-up treatment with medication. The secondary clinical success rate was 87.5% (7/8).
| No. | Age (yr) | Sex | Location | Size (mm) | Duration of pain (mo) | No. of tip position in the first ablation | First clinical success | Pre RFA | Post RFA | Recurrence | Second clinical success | ||
| VAS | NSAIDs | VAS | NSAIDs | ||||||||||
| 1 | 18 | M | Femur | 4 | 9 | 1 | Yes | 2 | Yes | 0 | No | No | N/A |
| 2 | 17 | M | Fibula | 7 | 14 | 1 | Yes | 1 | Yes | 0 | No | No | N/A |
| 3 | 17 | F | Femur | 11 | 6 | 2 | No | 10 | Yes | 0 | Yes | 2 mo later | No |
| 4 | 17 | M | Femur | 11 | 15 | 3 | Yes | 5 | Yes | 1 | No | No | N/A |
| 5 | 8 | M | Femur | 14 | 11 | 1 | No | 8 | Yes | 2 | No | 4 mo later | Yes |
| 6 | 7 | F | Femur | 6 | 7 | 1 | Yes | 9 | Yes | 0 | No | No | N/A |
| 7 | 4 | M | Femur | 9 | 6 | 1 | Yes | N/A | Yes | N/A | No | No | N/A |
| 8 | 15 | F | Tibia | 8 | 5 | 1 | Yes | 6 | Yes | 2 | No | No | N/A |
Biopsy specimens used for histological diagnosis were obtained in six of eight patients at the time of the procedure. Histological confirmation of OO was not obtained in any of these cases.
Since percutaneous RFA for OO was first reported by Rosenthal and co-workers in 1992[6], a number of researchers have demonstrated that RFA is a safe and effective treatment for OO. In the present study, clinically successful treatment was performed in 87.5 % of cases. This result is comparable to those reported by many other groups, who also have achieved a high efficacy rate ranging from 74%-100%[1,8-11]. These rates of success compare favorably to those of surgical treatment (88%-97%)[2] and other less-invasive therapies such as CT-guided percutaneous resection[12] and laser ablation[13]. Fluoroscopic RFA was performed previously, however cross-sectional imaging have made accurate lesion location possible[4].
It remains controversial whether or not a cooling system should be used[14]. In the early stages of the development of the RFA technique, some authors reported performing RFA with non-cooled tip probes using a 5-mm exposed tip[9,14]. When the nidus of an OO exceeds 1 cm at its greatest dimension, the use of two or more electrode positions is often necessary to successfully ablate the lesion[2]. To resolve this issue, various new types of probe have been developed to be equipped with a cooling system (and a longer probe) or a higher output generator, and to be implemented for longer treatment times. Originally, Tillotson and colleagues evaluated the ablation effect in the normal femur of a living dog model. In their report, osteonecrosis of 0.9-1.3 cm developed with the use of a 5-mm exposed tip and no cooling system. After the publication of that report, several authors reported the advantages of RF ablation with a water-cooled probe compared to the benefits of RF ablation with non-cooled electrodes, as a larger amount of tissue could be successfully ablated using the former system[15,16]. Cantwell et al[17] reported that RF ablation with a high-energy delivery technique using a 1- or 2-cm exposed, water perfusion-cooled probe is safe, and this approach has a high success rate. On the contrary, unlike in cases involving the ablation of soft tissues such as liver tumors, no statistically significant difference was noted in the dog model reported by Martel and co-workers in terms of the size of the area of osteonecrosis generated by internally cooled electrodes and that generated by non-cooled electrodes[18]. Furthermore, since the probe-cooling approach limits an operator’s ability to monitor the therapy, unexpected complications such as wound infections and skin burns can occur[2,15,16]. Cantwall et al[17] also demonstrated that RFA at higher energy and when using a cooled tip increased postprocedural pain and prolonged the interval to symptom resolution, as compared to results reported in a published series using a traditional 5-mm non-cooled probe and a low-output generator. To avoid complications and postprocedural pain, we elected to use a non-cooled system. In addition, because 5-mm electrodes were not commercially available in our country, we used a 10-mm exposed tip, which appeared to be adequate for ablating lesions measuring 8.8 mm on average in this study.
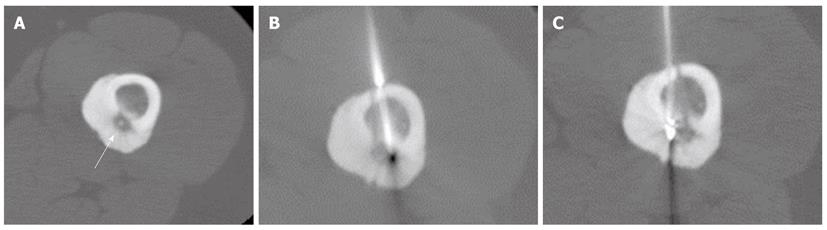
In cases of OO measuring over 1 cm in diameter, the use of two or more electrode positions is often necessary[2,9]. In case 5 of the present study, the nidus measured 14 mm in diameter; we placed the tip of the electrode only in the center of nidus, and performed RFA only once. Four months after the initial ablation, the patient returned to our clinic complaining of recurrent pain and elected to undergo a repeat procedure, which was successful. In one patient (case 4) with a nidus measuring 11 mm, we selected three different sites for placement of the electrodes at the initial treatment, which was successful (Figure 2). An unsuccessful procedure was conducted in one patient (case 3) with a large nidus exceeding 1 cm; in this patient, a repeat procedure was also ineffective at ameliorating the pain. The reason for the failure of treatment in this case remains unclear. Our primary recurrence rate of 25% (2 of 8 patients) was comparable with that of previous study [23 (24%) of 97 patients][7] but higher than those of previous reports of RFA[10,19]. It is also higher than that reported after percutaneous extraction [six (16%) of 38 patients][12], and that reported after surgical resection [0 (0%) of 97%)][20]. A possible explanation for the difference is immature technique. Multiple ablations at the same RFA session are necessary to reduce the risk of recurrence[8,9].
No complications were encountered in the present series. These results are comparable to those of previous reports of RFA of OO[2,8,10]. In contrast, lesion resection leaves a bone defect that may be vulnerable to fracture and, in some cases, may necessitate internal fixation and bone grafting[2]. Few complications of RFA for OO have been described in the literature to date. Skin burns may occur with superficial procedures. Extreme precautions should be taken with RFA for spinal and hand OO to avoid neural injuries. A classical restriction for percutaneous treatment is the presence of a lesion near neurological structure (distance < 5 mm), due to the risk of spinal cord damage by hyperthermia-induced cytotoxicity[21-23]. In addition, RFA may cause chondral damage via thermal necrosis, when one is dealing with juxta-articular lesions[24]. In our series, all lesions were located in the long bone of the lower extremities, which facilitated the avoidance of both major nerves and major complications. Postprocedural imaging of the lesion site may be beneficial for the evaluation of immediate complication however, it is not necessary that CT is routinely performed after procedure[2].
This study has several limitations. One major disadvantage was that histological confirmation was not obtained in any of the cases. The specimens were only fragments of bony material and exudate. In bone biopsies performed before RFA, OO was confirmed in 36 to 100%[25]. Histological confirmation based on the smaller fragments collected from the drill is similarly frequent as previously reported for standard bone biopsy[26]. Thus, even though we used relatively a small biopsy needle (19-21 gauge), this low rate of confirmation should not be due to the small size of the needle, but rather due to technical error. However, histological verification was unnecessary for the diagnosis of OO in all the present cases, since these patients experienced typical clinical symptoms of OO, and diagnosis was confirmed in each case by typical imaging findings. Second, the number of patients in this study was very small, as the Japanese insurance benefit does not include reimbursement for RFA of OO, as noted above. A phase I-II multi-institutional prospective study of RFA for OO is now being undertaken by the Japan Interventional Radiology in Oncology Study Group.
In conclusion, our preliminary results indicate a favorable success rate and no complication.
Osteoid osteoma (OO) is a painful benign bone tumor seen in young people. Although surgical removal is the definitive treatment, the need for extensive dissection has a problem. Since 1992, computed tomography (CT)-guided radiofrequency ablation (RFA) has been accepted as a demonstrably safe, minimally invasive, and cost-effective treatment for OO. In this report, the authors present their initial experience with CT-guided RFA of OO in eight patients.
In this study, the authors reconfirm safety and effectiveness of RFA for OO in Japan. Further, the authors also perform a systematic review of most of the study describing this treatment.
In cases of OO measuring over 1 cm in diameter, the use of two or more electrode positions is often necessary.
Percutaneous RFA should be the method of choice for treating OOs.
Major revision is needed. Conclusions performed at the manuscript are only valuable if more literature is described and discussed. Ethics of the clinical research in regard to anesthesia and a postprocedural CT scan has to be proved. Language needs corrections.
Peer reviewers: Mohamed Ragab Nouh, MD, Radiodiagnosis Department, Faculty of medicine, Alexandria University, 1 Kolya El-Teb Streeet, Mohata El-Raml, Alexandria 21521, Egypt; Lars Victor Baron von Engelhardt, MD, Department of Orthopedic and Trauma Surgery, St. Josef Hospital - Ruhr-University Bochum, Gudrunstrasse 56, 44791 Bochum, Germany
S- Editor Cheng JX L- Editor A E- Editor Zheng XM
| 1. | Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001;12:717-722. [PubMed] |
| 2. | Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, Menendez L. Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics. 2009;29:2127-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Schulman L, Dorfman HD. Nerve fibers in osteoid osteoma. J Bone Joint Surg Am. 1970;52:1351-1356. [PubMed] |
| 4. | Venbrux AC, Montague BJ, Murphy KP, Bobonis LA, Washington SB, Soltes AP, Frassica FJ. Image-guided percutaneous radiofrequency ablation for osteoid osteomas. J Vasc Interv Radiol. 2003;14:375-380. [PubMed] |
| 5. | Parlier-Cuau C, Champsaur P, Nizard R, Hamze B, Laredo JD. Percutaneous removal of osteoid osteoma. Radiol Clin North Am. 1998;36:559-566. [PubMed] |
| 6. | Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183:29-33. [PubMed] |
| 7. | Vanderschueren GM, Taminiau AH, Obermann WR, Bloem JL. Osteoid osteoma: clinical results with thermocoagulation. Radiology. 2002;224:82-86. [PubMed] |
| 8. | Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 364] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Vanderschueren GM, Taminiau AH, Obermann WR, van den Berg-Huysmans AA, Bloem JL. Osteoid osteoma: factors for increased risk of unsuccessful thermal coagulation. Radiology. 2004;233:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Rimondi E, Mavrogenis AF, Rossi G, Ciminari R, Malaguti C, Tranfaglia C, Vanel D, Ruggieri P. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: percutaneous radio-frequency ablation. Radiology. 1995;197:451-454. [PubMed] |
| 12. | Sans N, Galy-Fourcade D, Assoun J, Jarlaud T, Chiavassa H, Bonnevialle P, Railhac N, Giron J, Morera-Maupomé H, Railhac JJ. Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology. 1999;212:687-692. [PubMed] |
| 13. | Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Miyazaki M, Aoki J, Miyazaki A, Nakajima T, Koyama Y, Shinozaki T, Endo K. Percutaneous radiofrequency ablation of osteoid osteoma using cool-tip electrodes without the cooling system. Jpn J Radiol. 2011;29:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Peyser A, Applbaum Y, Khoury A, Liebergall M, Atesok K. Osteoid osteoma: CT-guided radiofrequency ablation using a water-cooled probe. Ann Surg Oncol. 2007;14:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol. 2005;56:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Cantwell CP, O'Byrne J, Eustace S. Radiofrequency ablation of osteoid osteoma with cooled probes and impedance-control energy delivery. AJR Am J Roentgenol. 2006;186:S244-S248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Martel J, Bueno A, Domínguez MP, Llorens P, Quirós J, Delgado C. Percutaneous radiofrequency ablation: relationship between different probe types and procedure time on length and extent of osteonecrosis in dog long bones. Skeletal Radiol. 2008;37:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815-821. [PubMed] |
| 20. | Campanacci M, Ruggieri P, Gasbarrini A, Ferraro A, Campanacci L. Osteoid osteoma. Direct visual identification and intralesional excision of the nidus with minimal removal of bone. J Bone Joint Surg Br. 1999;81:814-820. [PubMed] |
| 21. | Mylona S, Patsoura S, Galani P, Karapostolakis G, Pomoni A, Thanos L. Osteoid osteomas in common and in technically challenging locations treated with computed tomography-guided percutaneous radiofrequency ablation. Skeletal Radiol. 2010;39:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Martel J, Bueno A, Nieto-Morales ML, Ortiz EJ. Osteoid osteoma of the spine: CT-guided monopolar radiofrequency ablation. Eur J Radiol. 2009;71:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Akhlaghpoor S, Aziz Ahari A, Arjmand Shabestari A, Alinaghizadeh MR. Radiofrequency ablation of osteoid osteoma in atypical locations: a case series. Clin Orthop Relat Res. 2010;468:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Migues A, Velan O, Solari G, Pace G, Slullitel G, Araujo ES. Osteoid osteoma of the calcaneus: percutaneous radiofrequency ablation. J Foot Ankle Surg. 2005;44:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol. 2004;14:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Akhlaghpoor S, Aziz Ahari A, Ahmadi SA, Arjmand Shabestari A, Gohari Moghaddam K, Alinaghizadeh MR. Histological evaluation of drill fragments obtained during osteoid osteoma radiofrequency ablation. Skeletal Radiol. 2010;39:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |