Published online Jun 28, 2012. doi: 10.4329/wjr.v4.i6.265
Revised: May 22, 2012
Accepted: May 29, 2012
Published online: June 28, 2012
AIM: To assess the attenuation of non-calcified atherosclerotic coronary artery plaques with computed tomography coronary angiography (CTCA).
METHODS: Four hundred consecutive patients underwent CTCA (Group 1: 200 patients, Sensation 64 Cardiac, Siemens; Group 2: 200 patients, VCT GE Healthcare, with either Iomeprol 400 or Iodixanol 320, respectively) for suspected coronary artery disease (CAD). CTCA was performed using standard protocols. Image quality (score 0-3), plaque (within the accessible non-calcified component of each non-calcified/mixed plaque) and coronary lumen attenuation were measured. Data were compared on a per-segment/per-plaque basis. Plaques were classified as fibrous vs lipid rich based on different attenuation thresholds. A P < 0.05 was considered significant.
RESULTS: In 468 atherosclerotic plaques in Group 1 and 644 in Group 2, average image quality was 2.96 ± 0.19 in Group 1 and 2.93 ± 0.25 in Group 2 (P≥ 0.05). Coronary lumen attenuation was 367 ± 85 Hounsfield units (HU) in Group 1 and 327 ± 73 HU in Group 2 (P < 0.05); non-calcified plaque attenuation was 48 ± 23 HU in Group 1 and 39 ± 21 HU in Group 2 (P < 0.05). Overall signal to noise ratio was 15.6 ± 4.7 in Group 1 and 21.2 ± 7.7 in Group 2 (P < 0.01).
CONCLUSION: Higher intra-vascular attenuation modifies significantly the attenuation of non-calcified coronary plaques. This results in a more difficult characterization between lipid rich vs fibrous type.
- Citation: Maffei E, Martini C, Arcadi T, Clemente A, Seitun S, Zuccarelli A, Torri T, Mollet NR, Rossi A, Catalano O, Messalli G, Cademartiri F. Plaque imaging with CT coronary angiography: Effect of intra-vascular attenuation on plaque type classification. World J Radiol 2012; 4(6): 265-272
- URL: https://www.wjgnet.com/1949-8470/full/v4/i6/265.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i6.265
Computed tomography coronary angiography (CTCA) is a clinical imaging modality for the detection and exclusion of significant coronary artery disease (CAD). Most of the clinical value is related to the high negative predictive value and the most suitable population who could undergo this investigation consists of symptomatic patients with intermediate cardiovascular risk, with or without preliminary stress tests[1-3].
The incremental value of CTCA is the capability of detecting atherosclerotic plaques[3]. There are several reports dealing with the presumed capability of CTCA for characterizing those plaques[4-13]. In particular, some authors have reported CTCA as capable of distinguishing between fibrous and lipid rich plaques based on attenuation values [Hounsfield units (HU)][4,6-13]. Other experimental reports on small numbers of patients have reported a possible artifact created by lumen attenuation which may significantly affect non-calcified plaque attenuation[14-16]. This finding is likely to affect reproducibility and reliability of non-calcified plaque attenuation measurements with a subsequent inaccurate characterization.
The aim of our study is to measure the impact of intra-vascular attenuation on non-calcified coronary plaques components, with particular focus on distribution and classification, using CTCA.
We retrospectively enrolled 400 consecutive patients (249 men, 151 women; mean age 65.1 ± 11.1 years, range 31-88 years) who underwent CTCA for suspected CAD. Patients were retrospectively collected from the hospital database and the procedures (i.e., CTCA) were performed by the same experienced team. For an overview of the patient population see Table 1.
| Population | Total (n = 400) | Group 1 (n = 200) | Group 2 (n = 200) |
| Age (yr), mean ± SD (range) | 65.1 ± 11.1 (31-88) | 63.6 ± 11.2 (38-87) | 66.5 ± 10 (31-88) |
| Gender (M/F) | 249/151 | 126/74 | 123/77 |
| Symptoms | |||
| Typical angina | 123 (30.8) | 60 (30) | 63 (31.5) |
| Atypical chest pain | 204 (51) | 101 (50.5) | 105 (52.5) |
| Unstable angina | 34 (8.5) | 14 (7) | 20 (10) |
| AMI | 15 (3.8) | 15 (7.5) | 0 (0) |
| High risk asymptomatic | 22 (5.5) | 10 (5) | 12 (8.3) |
| Cardiovascular risk factors | |||
| Hypertension | 250 (62.5) | 118 (59) | 132 (66) |
| Dyslipidemia | 202 (50.5) | 92 (46) | 110 (55) |
| Diabetes | 58 (14.5) | 30 (15) | 28 (14) |
| Smoking | 98 (24.5) | 47 (23.5) | 51 (25.5) |
| Family history | 171 (42.8) | 90 (45) | 81 (40.5) |
| BMI (kg/m2), mean ± SD (range) | 27.1 ± 3.9 (20-47) | 26.7 ± 4.1 (20-47) | 27.4 ± 3.8 (20-45) |
| RF/patient (mean ± SD) | 2.1 ± 1.3 | 2.0 ± 1.2 | 2.2 ± 1.4 |
| Calcium score (agatston score, mean ± SD) | 336.7 ± 627.4 | 303.3 ± 628.8 | 370.2 ± 627.4 |
| HR during CTCA (bpm), mean ± SD (range) | 59.5 ± 7.3 (42-75) | 59.5 ± 8.0 (43-75) | 59.5 ± 6.5 (42-75) |
Patients were included when they had sinus rhythm, no history of CAD, had never undergone percutaneous angioplasty or coronary artery bypass graft surgery, and were able to maintain breath-hold for at least 12 s. Exclusion criteria for CTCA were: absolute contraindication to the administration of intravenous contrast material (e.g., known allergy, renal insufficiency or thyroid disorders) and possible pregnancy. All patients gave their informed consent for the procedure. Due to the retrospective nature of the study the ethics committee approval was waived.
Examinations were performed with a 64-slice CT scanner (Group 1: 200 patients: Sensation 64, Siemens, Forchheim, Germany; Group 2: 200 patients: VCT, General Electric, Milwaukee, WI, USA) according to standard protocols elsewhere described[17-19]. All patients underwent an unenhanced scan for the quantification of coronary calcium followed by an angiographic scan. Scan parameters were: the lowest collimation available; the pitch appropriately reduced for oversampling of the information; 120 kV; 600-900 mAs. We administered 80-100 mL of contrast material [Group 1: Iomeprol, Iomeron 400 mgI/mL, Bracco (Iodine burden = 32-40 grI); Group 2: Iodixanol, Visipaque 320 mgI/mL, GE Healthcare (Iodine burden = 25.6-32 grI)] at an injection rate of 5-6 mL/s [depending on the quality of the venous access; Iodine Delivery Rate (IDR): Group 1 = 2.0-2.4 mgI/s and Group 2 = 1.6-1.92 mgI/s] with an automatic injector (Stellant, MedRad, Pittsburgh, PA, USA) attached to an 18- to 20-gauge needle cannula inserted in an antecubital vein. With the aim of optimizing enhancement of the coronary arteries, the bolus-tracking technique was used to synchronize arrival of the contrast material in the coronary arteries with the beginning of the scan, and a bolus of saline solution was administered following the bolus of contrast material[20,21].
Images were reconstructed with the minimum slice thickness available or, alternatively, with an appropriate slice thickness for maintaining an adequate signal-to-noise ratio (0.625-0.75 mm). Reconstruction increment was 0.4 mm. Field of view used for evaluation of the coronary arteries was the minimum allowed by the individual anatomy of the patient (120-160 mm). Retrospective reconstructions based on the ECG signal were performed to obtain an image quality with the least motion artifacts. The time windows used were the mid-diastolic to end-diastolic phase (from -300 to -450 ms prior to the subsequent R wave and/or 60%-80% of the R-R interval). When deemed necessary (e.g., in the event of persistent residual cardiac motion that reduced image quality), additional reconstructions in the end-systolic phase were analyzed (+225 ms to +325 ms after the previous R wave and/or 25%-40% of the R-R interval). Radiation dose delivered was estimated for both Groups (Group 1 = 17 mSv; Group 2 = 22 mSv).
All scans were analyzed by two observers with at least 5 years experience in cardiovascular imaging in consensus reading (Cademartiri F and Maffei E). All coronary artery segments visualized were considered assessable for the presence of significant stenosis according to the modified American Heart Association 16-segment classification (Figure 1)[22]. Curved multiplanar reconstructions (MPR), maximum intensity projections and volume rendering images were used to identify coronary artery lesions. Longitudinal and axial MPRs were then performed on the individual atherosclerotic plaques and stenosis. Plaques were defined as structures located at the coronary artery wall clearly distinguishable from the surrounding sub-epicardial fat and from the enhanced coronary lumen[8].
Evaluation of coronary stenosis was performed by means of visual semi-quantitative score. Segments were classified as normal/not significantly diseased (non diseased or with wall irregularities or lumen reduction < 50%) or significantly diseased (≥ 50% lumen reduction).
Overall quality of each dataset was assessed on a 4-point Likert scale considering residual motion, attenuation, sharpness: 0 = low/not assessable; 1 = adequate; 2 = good; 3 = optimal.
Vascular attenuation was assessed with regions of interest (ROI), as large as allowed by vessel size and avoiding calcifications, performed in the ascending aorta, at the origin of the left main coronary artery (LM) and the origin of the right coronary artery (RCA).
Coronary plaques were assessed for quality and for attenuation of the non-calcified component. Plaque quality was assessed on a 4-point Likert scale considering residual motion, attenuation, sharpness: 0 = low/not assessable; 1 = adequate; 2 = good; 3 = optimal. Attenuation of the non-calcified component of coronary plaques was measured in each non-calcified and mixed plaque with ROI as large as possible but avoiding pseudo-enhancement from neighboring structures (i.e., enhanced lumen and calcifications).
Noise was defined as the standard deviation of HU in air. Signal to noise ratio (S/N) was calculated using vascular attenuation as the signal and the SD of HU in air as the noise (S/N = Arterial HU/SD HU in Air).
Categorical variables are described as frequencies (percentage), continuous variables as mean ± SD. Groups were compared using the Student’s t and χ2 or Fisher exact tests, as appropriate. Because coronary artery calcium score had highly skewed, non-normal distributions, the Mann-Whitney U test was used.
Each measured plaque (both for mixed and non-calcified plaques) was classified as fibrous when attenuation was ≥ 30 HU and lipid rich when the attenuation was < 29 HU (threshold = 50 HU). The same re-classification was performed using 50 HU and 70 HU thresholds. Statistical analysis was performed with a dedicated software package (Statistical Package for the Social Sciences, version 11.5, SPSS Inc., Chicago, IL, USA). For all tested comparisons a P < 0.05 was considered significant.
The two populations did not differ concerning demographics, symptoms, risk factors, calcium score (Agatston 337 ± 627) and heart rate (60 ± 7 bpm; range 42-75 bpm) during CTCA (P > 0.05, Table 1).
Of the 400 patients evaluated, 242 (60.5%) were found not to have significant disease. Conversely, at least one significant/obstructive stenosis was detected in 158 (39.5%) patients. Overall, 5678 coronary segments were evaluated and 722 significant lesions were detected resulting in an overall prevalence of 12.7%. There was no significant difference in the prevalence of obstructive disease between the Groups 1 and 2 (37.5% vs 41.5%, P > 0.05). Concerning severity of obstructive disease, we observed a significantly higher number of patients with multi-vessel disease in Group 2 (P < 0.05).
Global quality was not significantly different between the Groups 1 and 2 (2.8 ± 0.4 vs 2.9 ± 0.3, P > 0.05; Table 2).
| Total | Group 1 | Group 2 | P-value | |
| Global quality assessment | 2.85 ± 0.34 | 2.83 ± 0.37 | 2.86 ± 0.31 | 0.425 |
| Global vascular attenuation (HU) | 370.6 ± 82.5 | 380.0 ± 65.2 | 353.5 ± 69.0 | < 0.0001 |
| Plaque attenuation (HU) | 42.7 ± 23.5 | 48.3 ± 22.5 | 38.7 ± 21.4 | < 0.0001 |
| Lumen attenuation (HU) | 344.1 ± 87.1 | 366.9 ± 85.1 | 327.4 ± 72.8 | < 0.0001 |
| Difference lumen vs plaque (HU) | 301.4 ± 62.6 | 318.7 ± 88.9 | 289.2 ± 74.0 | < 0.0001 |
| Aortic attenuation (HU) | 374.7 ± 67.4 | 380.1 ± 64.2 | 335.2 ± 70.6 | < 0.0001 |
| Aortic noise (HU) | 26.4 ± 8.6 | 31.6 ± 8.3 | 21.2 ± 4.9 | < 0.0001 |
| RCA attenuation (HU) | 372.3 ± 173.8 | 384.5 ± 76.5 | 336.7 ± 72.3 | < 0.0001 |
| LM attenuation (HU) | 364.8 ± 70.6 | 375.3 ± 66.6 | 354.4 ± 73.1 | 0.003 |
| Noise (HU) | 23.3 ± 7.1 | 25.8 ± 6.5 | 18.0 ± 5.0 | < 0.0001 |
| S/N | 18.7 ± 8.4 | 15.6 ± 4.7 | 21.2 ± 7.7 | < 0.0001 |
Per-segment quality (on 5678 segments) was not significantly different between the Groups 1 and 2 except for segment 16 (3.0 ± 0 vs 2.9 ± 0.3, P < 0.05).
Per-plaque quality was not significantly different between the two Groups except for segments 8 and 10 in which this was significantly higher in Group 1 (P < 0.05).
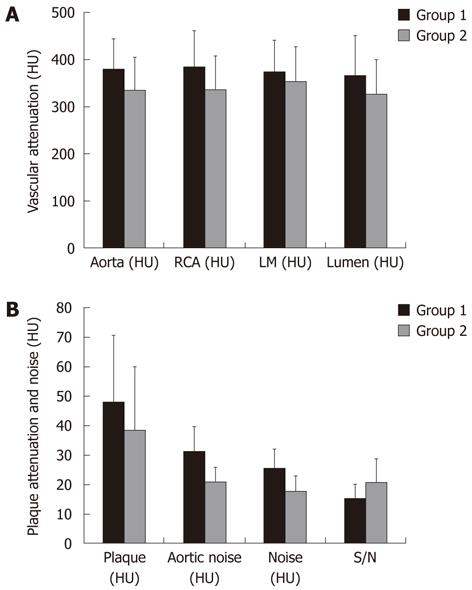
Global vascular attenuation as well as Aortic (Group 1 = 380 ± 64 HU vs Group 2 = 335 ± 71 HU), RCA (Group 1 = 385 ± 77 HU vs Group 2 = 337 ± 72 HU), and LM (Group 1 = 375 ± 67 HU vs Group 2 = 354 ± 73 HU) attenuations were significantly higher in Group 1 as compared to Group 2 (P < 0.01). We detected 766 atherosclerotic plaques in Group 1 (280 calcified; 486 mixed or non-calcified) and 907 in Group 2 (253 calcified; 654 mixed or non-calcified). Of these, 468 atherosclerotic plaques in Group 1 and 644 in Group 2 were suitable for assessment of the attenuation of the non-calcified component (i.e., size and location).
Intra-coronary lumen attenuation adjacent to measurable plaques was significantly higher for Group 1 (Group 1 = 367 ± 85 HU vs Group 2 = 327 ± 73 HU, P < 0.01; Table 2, Figure 2).
Global non-calcified plaque attenuation was significantly higher for Group 1 (Group 1 = 48 ± 23 HU vs Group 2 = 39 ± 21 HU, P < 0.01; Table 2, Figure 2).
The standard deviation of HU (i.e., noise) was significantly higher for Group 1 both on Aorta (Group 1 = 32 ± 8 HU vs Group 2 = 21 ± 5 HU, P < 0.01) and in Air (Group 1 = 26 ± 7 HU vs Group 2 = 18 ± 5 HU, P < 0.01).
S/N was significantly higher for Group 2 (Group 1 = 16 ± 5 HU vs Group 2 = 21 ± 8 HU, P < 0.01; Table 2, Figure 2).
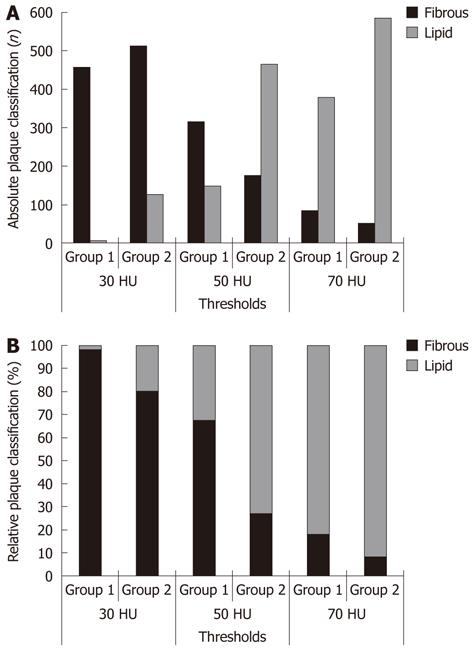
Using a threshold of 50 HU to differentiate lipid rich and fibrous plaques, 151 (32.3%) plaques and 467 (72.5%) plaques were classified as lipid rich in Group 1 and Group 2, respectively (P < 0.05) (Table 3, Figure 3). The ratio between lipid rich and fibrous plaques was 0.48 in Group 1 and 2.6 in Group 2.
| Threshold | Type | Group 1 | Group 2 | P-value | ||
| n (%) | Ratio (lipid/fibrous) | n (%) | Ratio (lipid/fibrous) | |||
| 30 HU | Lipid rich | 9 (1.9) | 0.020 | 129 (20.0) | 0.250 | < 0.01 |
| Fibrous | 459 (98.1) | 515 (80.0) | ||||
| 50 HU | Lipid rich | 151 (32.3) | 0.476 | 467 (72.5) | 2.638 | < 0.01 |
| Fibrous | 317 (67.7) | 177 (27.5) | ||||
| 70 HU | Lipid rich | 381 (81.4) | 4.379 | 588 (91.3) | 10.500 | < 0.01 |
| Fibrous | 87 (18.6) | 56 (8.7) | ||||
| Total | 468 | 644 | ||||
Using a threshold of 30 HU to differentiate lipid rich and fibrous plaque, 9 (1.9%) plaques and 129 (20.0%) plaques were classified as lipid rich in Group 1 and Group 2, respectively (P < 0.05). The ratio between lipid rich and fibrous plaques was 0.020 in Group 1 and 0.25 in Group 2.
Using a threshold of 70 HU to differentiate lipid rich and fibrous plaques, 381 (81.4%) plaques and 588 (91.3%) plaques were classified as lipid rich in Group 1 and Group 2, respectively (P < 0.05). The ratio between lipid rich and fibrous plaques was 4.48 in Group 1 and 10.5 in Group 2.
Lipid rich/fibrous ratio represents the relative proportion of lipid rich vs fibrous plaques. As expected, there was a significant trend towards a shift from fibrous to lipid rich plaques when thresholds were progressively increased in both groups (P < 0.05). However, the proportion of lipid rich plaques was consistently higher in Group 2 regardless of the threshold applied. The effect was more evident with a threshold of 50 HU and was reduced with 30 HU and 70 HU thresholds.
CTCA can detect and potentially characterize atherosclerotic coronary plaques[4-9]. According to a recent meta-analysis of 14 studies and 340 patients, the overall sensitivity for the detection of coronary plaque was 81%-86%, with a better sensitivity for calcified compared to non-calcified plaques[9].
In-vitro and ex-vivo CTCA studies have demonstrated that the measured attenuation values of the vascular wall are affected by high intravascular attenuation, as a result of high iodine concentrations[10,14,23]. This phenomenon has been previously described as pseudo-enhancement[24-26] and it is caused by insufficient spatial resolution resulting in partial volume and beam hardening artifacts[27]. Preliminary observations suggested an association between the coronary lumen enhancement and measured plaque attenuation in clinical cardiac CT scans[16].
In our study, the same team performed all the examinations and all the analyses using the same tools. The patient populations were similar, while the CT scanner and the contrast medium were different. The protocol used was state of the art for each individual solution. The aim of our study was to demonstrate in-vivo the high variability of plaque attenuation due to intra-vascular attenuation and how it affects the classification of coronary plaques.
Overall image quality was not significantly different between the two groups. Global intra-vascular attenuation and non-calcified plaque attenuation were significantly higher for Group 1, as expected from a higher iodine concentration. Per-segment image quality and non-calcified plaque quality were not significantly different between the two Groups. There was also significantly higher non-calcified plaque attenuation in Group 1. Conversely, S/N was significantly higher for Group 2. This finding is difficult to explain if we only consider a single parameter (e.g., intra-vascular attenuation). In fact, it is most likely the result of multiple parameters, including convolution kernel filtering.
When we applied thresholds for the definition and classification of lipid rich and fibrous plaques we observed a significantly larger number of lipid rich plaques in Group 2 (P < 0.05). This is a finding that could be expected from the lower average attenuation of non-calcified plaques in Group 2. In clinical practice, lower plaque attenuation means a better capability for characterizing coronary atherosclerosis.
Several studies have examined the potential of CTCA to visualize coronary atherosclerotic plaques with particular focus on those that are non-obstructive[4,6-13]. The reported sensitivity of CTCA for the detection of non-calcified components of coronary artery plaques was lower (53%-78%) as compared to mixed (78%) or calcified (94%-95%) plaques, using IVUS as the reference standard[7,8].
Schroeder et al[4] studied the composition of 34 coronary plaques on CTCA using ICUS as a reference standard. The mean attenuation values for hypo-echoic, hyper-echoic, and calcified plaques on ICUS were 14 HU, 91 HU, and 419 HU, respectively, on CTCA. Leber et al[8] studied 68 coronary vessels with CTCA and ICUS and found that the mean attenuation values for hypo-echoic, hyper-echoic, and calcified plaques on ICUS were 49 HU, 91 HU, and 391 HU, respectively on CTCA.
Plaque echogenicity as determined by ICUS corresponded well to plaque attenuation as measured by CTCA[4,8]. Thus, the CTCA attenuation values are presumed to reflect the dominant plaque composition (e.g., plaques with a large lipid core with a low echogenicity on ICUS might be identified on the basis of low attenuation value).
While the data from small series suggest that CTCA may distinguish between lipid, fibrous and calcified tissue, there appears to be significant overlap between the density values for the different tissues. In a coronary artery phantom study, Schroeder et al[10] measured the attenuation of two plaques of rubber material with three decreasing concentrations of intra-luminal contrast material. The density of the contrast material in the lumen significantly affected density measurements within the plaque. The higher the concentration of contrast medium in the lumen, the higher were the density measurements within the plaque[10].
The incremental prognostic potential of CTCA has been recently demonstrated[28]. The classification in different types of coronary plaque further helps in stratifying prognosis[28]. Studies on the predictive value of hypo-attenuating (i.e., lipid rich) plaques suggest that CTCA may identify patients with higher risk of developing an acute myocardial infarction in the future[29,30]. Based on our data, it seems that a reliable classification of non-calcified plaques as vulnerable or stable (i.e., predominantly lipid rich or fibrous) based on CT attenuation measurements may be difficult. In addition, the possibility of monitoring non-calcified plaques over time, for example to evaluate the effects of medical treatment on plaque burden and characteristics, may be similarly difficult. However, in terms of size evaluation, CT may play a role. It appears from our data that a lower attenuation is more suitable for adequate attenuation measurements of non-calcified component of atherosclerotic plaques.
The study was performed with a retrospective design, collecting data at two sites and the sub-groups (i.e., Group 1 and Group 2) are slightly different. However, the aim of the study was to demonstrate the influence of intra-vascular attenuation on plaque characterization.
The CT equipment and the contrast medium used were different for the two populations. However, considering the aim of the study it would have been impossible to scan twice the same patient with a different contrast medium, and the large number of patients offers a good benchmark for our evaluation.
We observed a higher number of plaques in Group 2 as reflected by the higher prevalence of multi-vessel disease and by the higher average calcium score. This may be explained by the fact that although the two populations were similar they were not the same.
We did not perform intra-vascular ultrasound for the classification of plaques. However, the aim of the study was not to validate CTCA for the classification (i.e., fibrous vs lipid rich) of coronary plaques. This has been performed previously in other studies. Instead, the aim of the study was to assess the impact of intra-vascular attenuation on the classification of plaques. In addition, it would have been impossible to perform IVUS in all our patients for ethical reasons.
Radiation dose remains a consistent issue in CTCA. We performed the study in a period in which “step and shoot”/prospective ECG triggering was not available in our institution on the scanners described. Since the study was retrospective, no additional radiation dose was delivered to the patients. The use of prospective ECG triggering would have significantly reduced the flexibility of reconstruction window optimization for the purpose of plaque imaging. In addition, several studies are reporting data on the reliability of low-dose CTCA performed with prospective ECG triggering with a dose of 1-4 mSv[31-37].
High intra-vascular attenuation modified significantly the attenuation of non-calcified coronary plaques. As a result, the detection of fibrous vs lipid rich plaques was significantly affected. Image quality was not significantly affected by different settings (i.e., CT scanner and contrast medium used). S/N was significantly better in the Group using lower iodine concentration and with lower intra-vascular attenuation.
The best strategy to characterize coronary artery plaques might be the one aiming at intermediate intra-vascular attenuation as determined by a lower Iodine Delivery Rate.
Plaque imaging is an extremely difficult task for almost any imaging modality, especially when dealing with coronary arteries. Computed tomography coronary angiography (CTCA) is the only non invasive imaging modality able to visualize coronary arteries and atherosclerotic plaques. Controversies regarding the optimization of CTCA contrast material administration are present in the literature. In particular, there is discussion on whether a higher or lower concentration best fits the purpose of plaque imaging.
The use of high vs low iodine concentration may determine significant differences in detection and distribution of coronary plaque types. This is specifically the conclusion of the study and leads to caution in attributing more detailed features to coronary plaques when assessed by means of CTCA.
The innovation in this case is related to the observation that different contrast materials, in terms of compounds and iodine concentrations, determine a different prevalence of lipid-rich plaques as detected by CTCA.
Different contrast materials can be used but the interpretation of coronary plaque type by means of CTCA data should be driven by caution and several considerations regarding iodine load affect the actual plaque classification. A lower iodine concentration is likely to provide a more realistic quantitative assessment and therefore a more accurate one.
Plaque imaging is the new frontier of non invasive cardiovascular prevention. The so-called vulnerable plaque is still difficult to identify by means of non invasive tools, especially at the level of coronary arteries. Even though CTCA is a viable tool for coronary plaque imaging, operators should use caution in attributing vulnerable features regardless of contrast material protocols.
In this study, authors demonstrated their experience of correlating the intravascular attenuation and characterization of coronary plaques, and concluded that high intravascular attenuation significantly interferes with the detection of non-calcified plaques. The paper is well written, and results have potential clinical applications.
Peer reviewers: Masahiro Jinzaki, MD, Associate Professor, Department of Diagnostic Radiology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; Zhonghua Sun, PhD, Discipline of Medical Imaging, Department of Imaging and Applied Physics, Curtin University of Technology, GPO Box U 1987, Perth, Western Australia 6845, Australia
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
| 1. | Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497. [PubMed] |
| 2. | Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, Hundley WG, Manning WJ, Printz BF, Stuber M. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation. 2008;118:586-606. [PubMed] |
| 3. | Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29:531-556. [PubMed] |
| 4. | Schroeder S, Kopp AF, Baumbach A, Meisner C, Kuettner A, Georg C, Ohnesorge B, Herdeg C, Claussen CD, Karsch KR. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37:1430-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 507] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | Becker CR, Nikolaou K, Muders M, Babaryka G, Crispin A, Schoepf UJ, Loehrs U, Reiser MF. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol. 2003;13:2094-2098. [PubMed] |
| 6. | Nikolaou K, Sagmeister S, Knez A, Klotz E, Wintersperger BJ, Becker CR, Reiser MF. Multidetector-row computed tomography of the coronary arteries: predictive value and quantitative assessment of non-calcified vessel-wall changes. Eur Radiol. 2003;13:2505-2512. [PubMed] |
| 7. | Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, Pohle K, Baum U, Anders K, Jang IK. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14-17. [PubMed] |
| 8. | Leber AW, Knez A, Becker A, Becker C, von Ziegler F, Nikolaou K, Rist C, Reiser M, White C, Steinbeck G. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241-1247. [PubMed] |
| 9. | Springer I, Dewey M. Comparison of multislice computed tomography with intravascular ultrasound for detection and characterization of coronary artery plaques: a systematic review. Eur J Radiol. 2009;71:275-282. [PubMed] |
| 10. | Schroeder S, Flohr T, Kopp AF, Meisner C, Kuettner A, Herdeg C, Baumbach A, Ohnesorge B. Accuracy of density measurements within plaques located in artificial coronary arteries by X-ray multislice CT: results of a phantom study. J Comput Assist Tomogr. 2001;25:900-906. [PubMed] |
| 11. | Leber AW, Knez A, White CW, Becker A, von Ziegler F, Muehling O, Becker C, Reiser M, Steinbeck G, Boekstegers P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol. 2003;91:714-718. [PubMed] |
| 12. | Schoenhagen P, Tuzcu EM, Stillman AE, Moliterno DJ, Halliburton SS, Kuzmiak SA, Kasper JM, Magyar WA, Lieber ML, Nissen SE. Non-invasive assessment of plaque morphology and remodeling in mildly stenotic coronary segments: comparison of 16-slice computed tomography and intravascular ultrasound. Coron Artery Dis. 2003;14:459-462. [PubMed] |
| 13. | Nikolaou K, Becker CR, Muders M, Babaryka G, Scheidler J, Flohr T, Loehrs U, Reiser MF, Fayad ZA. Multidetector-row computed tomography and magnetic resonance imaging of atherosclerotic lesions in human ex vivo coronary arteries. Atherosclerosis. 2004;174:243-252. [PubMed] |
| 14. | Cademartiri F, Mollet NR, Runza G, Bruining N, Hamers R, Somers P, Knaapen M, Verheye S, Midiri M, Krestin GP. Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol. 2005;15:1426-1431. [PubMed] |
| 15. | Cademartiri F, Runza G, Mollet NR, Luccichenti G, Belgrano M, Somers P, Knaapen M, Verheye S, Bruining N, Hamers R. Influence of increasing convolution kernel filtering on plaque imaging with multislice CT using an ex-vivo model of coronary angiography. Radiol Med. 2005;110:234-240. [PubMed] |
| 16. | Cademartiri F, Runza G, Palumbo A, Maffei E, Martini C, McFadden E, Somers P, Knaapen M, Verheye S, Weustink AC. Lumen enhancement influences absolute noncalcific plaque density on multislice computed tomography coronary angiography: ex-vivo validation and in-vivo demonstration. J Cardiovasc Med (Hagerstown). 2010;11:337-344. [PubMed] |
| 17. | Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724-1732. [PubMed] |
| 18. | Mollet NR, Cademartiri F, van Mieghem CA, Runza G, McFadden EP, Baks T, Serruys PW, Krestin GP, de Feyter PJ. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318-2323. [PubMed] |
| 19. | Cademartiri F, Runza G, Belgrano M, Luccichenti G, Mollet NR, Malagutti P, Silvestrini M, Midiri M, Cova M, Pozzi Mucelli R. Introduction to coronary imaging with 64-slice computed tomography. Radiol Med. 2005;110:16-41. [PubMed] |
| 20. | Cademartiri F, Mollet N, van der Lugt A, Nieman K, Pattynama PM, de Feyter PJ, Krestin GP. Non-invasive 16-row multislice CT coronary angiography: usefulness of saline chaser. Eur Radiol. 2004;14:178-183. [PubMed] |
| 21. | Cademartiri F, Nieman K, van der Lugt A, Raaijmakers RH, Mollet N, Pattynama PM, de Feyter PJ, Krestin GP. Intravenous contrast material administration at 16-detector row helical CT coronary angiography: test bolus versus bolus-tracking technique. Radiology. 2004;233:817-823. [PubMed] |
| 22. | Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5-40. [PubMed] |
| 23. | Cademartiri F, Runza G, Mollet NR, Luccichenti G, Belgrano M, Bartolotta TV, Galia M, Midiri M, Pozzi Mucelli R, Krestin GP. Impact of intravascular enhancement, heart rate, and calcium score on diagnostic accuracy in multislice computed tomography coronary angiography. Radiol Med. 2005;110:42-51. [PubMed] |
| 24. | Wang ZJ, Coakley FV, Fu Y, Joe BN, Prevrhal S, Landeras LA, Webb EM, Yeh BM. Renal cyst pseudoenhancement at multidetector CT: what are the effects of number of detectors and peak tube voltage? Radiology. 2008;248:910-916. [PubMed] |
| 25. | Birnbaum BA, Hindman N, Lee J, Babb JS. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology. 2007;244:767-775. [PubMed] |
| 26. | Abdulla C, Kalra MK, Saini S, Maher MM, Ahmad A, Halpern E, Silverman SG. Pseudoenhancement of simulated renal cysts in a phantom using different multidetector CT scanners. AJR Am J Roentgenol. 2002;179:1473-1476. [PubMed] |
| 27. | Luccichenti G, Cademartiri F, Pezzella FR, Runza G, Belgrano M, Midiri M, Sabatini U, Bastianello S, Krestin GP. 3D reconstruction techniques made easy: know-how and pictures. Eur Radiol. 2005;15:2146-2156. [PubMed] |
| 28. | van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Kroft LJ, Boersma E, Pazhenkottil A, Valenta I, Pundziute G, de Roos A. Incremental prognostic value of multi-slice computed tomography coronary angiography over coronary artery calcium scoring in patients with suspected coronary artery disease. Eur Heart J. 2009;30:2622-2629. [PubMed] |
| 29. | Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49-57. [PubMed] |
| 30. | Matsumoto N, Sato Y, Yoda S, Nakano Y, Kunimasa T, Matsuo S, Komatsu S, Saito S, Hirayama A. Prognostic value of non-obstructive CT low-dense coronary artery plaques detected by multislice computed tomography. Circ J. 2007;71:1898-1903. [PubMed] |
| 31. | Hirai N, Horiguchi J, Fujioka C, Kiguchi M, Yamamoto H, Matsuura N, Kitagawa T, Teragawa H, Kohno N, Ito K. Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology. 2008;248:424-430. [PubMed] |
| 32. | Shuman WP, Branch KR, May JM, Mitsumori LM, Lockhart DW, Dubinsky TJ, Warren BH, Caldwell JH. Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology. 2008;248:431-437. [PubMed] |
| 33. | Stolzmann P, Leschka S, Scheffel H, Krauss T, Desbiolles L, Plass A, Genoni M, Flohr TG, Wildermuth S, Marincek B. Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology. 2008;249:71-80. [PubMed] |
| 34. | Scheffel H, Alkadhi H, Leschka S, Plass A, Desbiolles L, Guber I, Krauss T, Gruenenfelder J, Genoni M, Luescher TF. Low-dose CT coronary angiography in the step-and-shoot mode: diagnostic performance. Heart. 2008;94:1132-1137. [PubMed] |
| 35. | Lell M, Marwan M, Schepis T, Pflederer T, Anders K, Flohr T, Allmendinger T, Kalender W, Ertel D, Thierfelder C. Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol. 2009;19:2576-2583. [PubMed] |
| 36. | Achenbach S, Marwan M, Schepis T, Pflederer T, Bruder H, Allmendinger T, Petersilka M, Anders K, Lell M, Kuettner A. High-pitch spiral acquisition: a new scan mode for coronary CT angiography. J Cardiovasc Comput Tomogr. 2009;3:117-121. [PubMed] |
| 37. | Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, Kuettner A, Daniel WG, Uder M, Lell MM. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340-346. [PubMed] |