Published online May 28, 2012. doi: 10.4329/wjr.v4.i5.224
Revised: April 5, 2012
Accepted: April 12, 2012
Published online: May 28, 2012
Portal vein thrombosis is an uncommonly reported complication of percutaneous transhepatic cholangiography (PTC). A thorough review of the available literature shows no reported cases. In this case, a 29 year old female presented on two separate occasions with portal vein thrombosis following PTC without drain placement. This unusual complication of image guided percutaneous biliary access is unreported in the literature and prompted evaluation of the patient’s coagulation parameters. A thrombophilia screen demonstrated a mutation in the Prothrombin (Factor II) gene. A thorough literature review shows no reported cases of portal vein thrombosis following percutaneous biliary access, is an unusual complication, and should raise suspicion of an underlying pro-coagulant state.
- Citation: Brennan IM, Ahmed M. Portal vein thrombosis following percutaneous transhepatic cholangiography-An unusual presentation of Prothrombin (Factor II) gene mutation. World J Radiol 2012; 4(5): 224-227
- URL: https://www.wjgnet.com/1949-8470/full/v4/i5/224.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i5.224
Genetic predisposition to hypercoagulability occurs with a number of inherited abnormalities including Factor V Leidin mutation, Factor II mutation and protein C and S deficiencies. Prevalence in the normal population is somewhat variable depending on the specific mutation with published rates in European populations of 8.8%-15% for Factor V Leidin mutation, 1.7%-3% for Factor II mutation, and 0.2%-0.4% and 0.03%-0.13% for Protein C and Protein S respectively[1]. Such risk factors not only predispose to more common thrombotic complications such as lower extremity deep venous thrombosis but also thrombosis in unusual anatomic sites or following low risk insults. Here we present a case of a young patient who developed thrombosis in an unusual location (segmental portal vein branches) following a procedure with no reported incidence of portal vein thrombosis which prompted screening for an underlying hypercoagulablestate.
A 29-year-old female was admitted electively to the interventional radiology service for percutaneous transhepatic cholangiography (PTC). The patient had a complex surgical history significant for a Roux en Y gastric bypass for morbid obesity 8 years prior. Three years following this she underwent laparoscopic cholecystectomy complicated by a bile leak from the gall bladder bed. In the subsequent three years she had ongoing upper abdominal pain and intermittently mildly elevated liver function tests. A contrast-enhanced computed tomography (CT) demonstrated mild intra- and extrahepatic biliary dilatation. Given her gastric bypass surgery, investigation with endoscopic retrograde cholangiopancreatography (ERCP) was not attempted and she underwent a diagnostic PTC at an outside hospital via a left lobe approach and left hepatic duct access. The cholangiogram revealed mildly dilated intrahepatic ducts but no flow of contrast into the duodenum. A 0.018 in wire was passed through the ampulla into the bowel resulting in rapid decompression of the biliary tree into the duodenum. At the time, the passage of the guide wire was thought to have displaced a small distal common bile duct stone. Free passage of contrast into the duodenum was seen at the end of the procedure. The catheter and wire were removed completely leaving no residual biliary access. The patient was discharged well the following day.
Two days following the PTC, the patient returned complaining of abdominal pain, nausea and vomiting. A contrast-enhanced CT scan performed at the time demonstrated new left portal vein thrombosis. The patient was started on a systemic heparin infusion per hospital protocol and transferred to our institution. Of note the patient had no history of oral contraceptive use. Liver function tests were checked and demonstrated an elevated total bilirubin (11.9 mg/dL), and magnetic resonance cholangiopancreatography (MRCP) was performed demonstrating persistent left portal vein occlusion with filling defects in a dilated left hepatic duct consistent with hemobilia. The heparin infusion was discontinued after 2 d (due to concern for ongoing hemobilia) and a repeat MRCP performed 5 d later showed resolution of the left lobe intrahepatic biliary dilatation.
Over the next 5 mo, repeat MRCP examinations revealed persistent left portal vein occlusion with progressive secondary left lobe atrophy. In this period the patient had two hospital admissions with upper abdominal pain, fever and elevated liver function tests. Given the patients on going symptoms and imaging findings of progressive left hepatic lobe atrophy a decision was made to perform a left hepatic lobectomy. Histopathology of the resected liver confirmed chronic portal vein occlusion with stage III fibrosis. Over the next 19 mo (and almost 8 years after the gastric bypass surgery) the patient continued to have upper and lower abdominal pain with multiple visits to the emergency department. Upper and lower GI camera endoscopy, capsule endoscopy and MR enterography were all within normal limits. Repeat MRCPs showed stable, mild, right lobe intrahepatic biliary dilatation.
A contrast-enhanced CT scan revealed that she had a re-accumulation of fluid above the gallbladder fossa in her surgical resection bed measuring approximately 3.2 cm × 2.6 cm. Her liver function tests in the emergency department included an alanine aminotransferase (ALT) of 105 IU/L, an aspartate aminotransferase (AST) of 89 IU/L, and alkaline phosphatase (AP) of 207 IU/L. Despite only mildly abnormal values, given the patient’s clinical history of recurrent episodes of upper abdominal pain combined with suggestion of mild biliary-ductal dilatation on cross-sectional imaging, the primary hepatobiliary surgeon requested that a PTC be performed of the remaining right hepatic biliary tree to evaluate the biliary anatomy, look for subtle strictures, and assess for possible flow stasis.
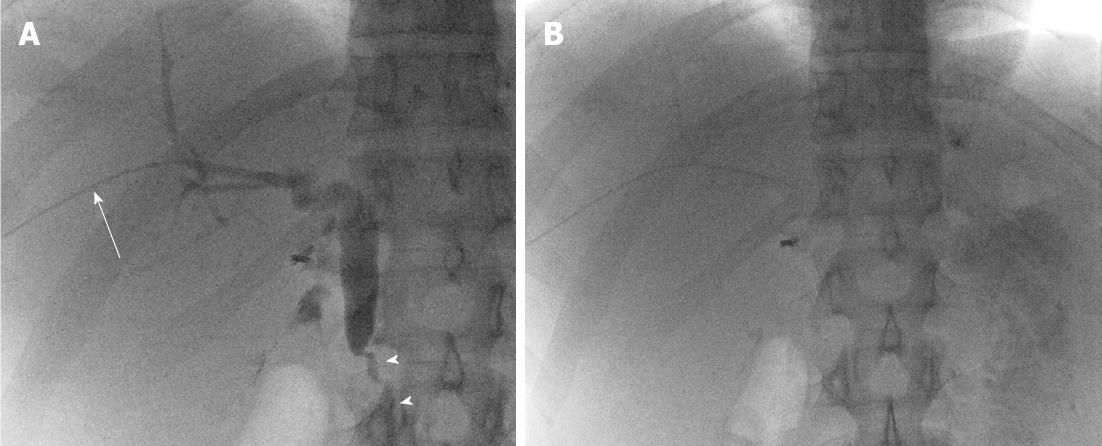
The patient underwent general anesthesia for the PTC procedure. Peri-procedural antibiotics were administered (Ceftriaxone 1 g IV) per our institutional protocol. Her liver function tests on the day of procedure were essentially normal (ALT 50 IU/L, AST 24 IU/L, AP 78 IU/L, total bilirubin 0.8 mg/dL). In addition the patient’s basic coagulation profile was within normal limits with a prothrombin time (PT) of 12.4 s, partial thromboplastin time (PTT) of 24.8 s and international normalized ratio (INR) of 1.0. A right lobe PTC was performed via a right intercostal approach using a 21 gauge, 15 cm long hollow-bore access needle (Percutaneous Entry Thinwall Needle, Cook Medical, Bloomington, IN). Using fluoroscopic guidance, the needle was advanced percutaneously into the right lobe hepatic parenchyma, and contrast was injected to opacify the biliary tree. A total of three passes were performed. Once biliary tree needle access was obtained through a peripheral biliary duct in Segment VIII, a 0.016 in guidewire (Headliner, Terumo, Tokyo, Japan) was advanced into the biliary tree. The needle was exchanged for the 3Fr inner dilator of an introducer sheath set (Accustick Sheath Set, Boston Scientific, Natick, MA). Cholangiography was then performed with contrast injection (75 cc, Optiray 320, Mallinckrodt Inc, Hazelwood, MO), and images were obtained in multiple projections. Cholangiography demonstrated a non-dilated right biliary system, without a visible stricture, and rapid passage of contrast through the common bile duct into the duodenum (Figure 1). Given the normal findings, no intervention was required (either biliary ductal dilatation or percutaneous drain placement). The 3Fr inner dilator was withdrawn from the biliary system, and the peripheral hepatic parenchymal tract was embolized with Gelfoam slurry (Surgifoam, Ferrosan, Soeborg, Denmark); cut into 2 mm cubes and mixed with 10 cc Optiray 320 contrast (using a three-way stopcock) under fluoroscopic guidance, as is standard practice in our department. No opacification of the biliary tree was identified on administration of the Gelfoam slurry.
The patient tolerated the procedure well, was admitted overnight for observation and discharged home well the next day. The patient represented to the emergency department 48 h post-procedure with worsening bloating, nausea, vomiting, mid abdominal pain and a fever to 1010F. Her liver function tests were ALT 148 IU/L, AST 74 IU/L, AP 174 IU/L, and a total bilirubin 1.2 mg/dL, increased since the PTC. Given this abnormal clinical presentation following PTC the patient was admitted for further evaluation.
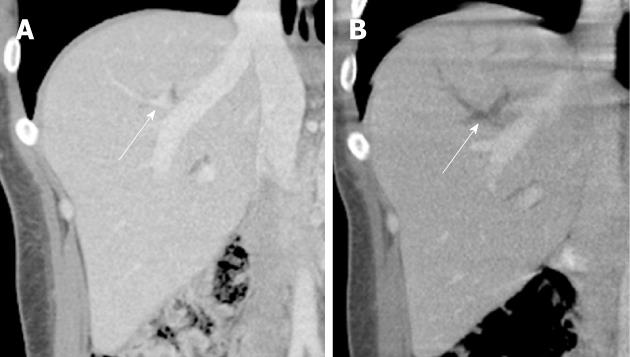
A contrast-enhanced CT demonstrated new right portal vein branch thrombosis in Segment VIII (corresponding to the segment and site in which biliary access had been obtained) (Figure 2). Of note, the main portal vein was patent. The patient was admitted and started on a systemic heparin infusion (per institutional protocol). Additionally, given the unusual nature of this complication, and the patient’s history of having a similar complication of portal vein branch thrombosis after a prior PTC, a hematology consult was recommended. A hypercoagulability work up was performed. The patient was found to be heterozygous for the prothrombin/Factor II (G20210A) mutation. Other assays including Factor V Leidin, anti-thrombin III, protein S and protein C were negative. Of note the patient was negative for lupus anticoagulant autoantibody.
The patient was subsequently transitioned to oral anticoagulation with warfarin for 6 mo.
A repeat contrast-enhanced CT 6 wk following PTC demonstrated persistent segmental portal vein thrombosis without progression, and with a wedge-shaped peripheral area of hyperarterialized perfusion involving Segment VIII.
Portal vein thrombosis (PVT) was first described in 1868 by Balfour and Stewart and is an uncommon but potentially life threatening condition[2]. In the absence of underlying liver disease/cirrhosis (where the reported prevalence of PVT is as high as 16%[3]), PVT in the general population is considered rare with a prevalence of 1% reported in a 24 000 patient autopsy study from Sweden[4]. Risk factors for the development of PVT can be divided into local and systemic causes. Systemic risk factors can be further subdivided into inherited prothrombotic risk factors such as Factor V Leidin mutation, Factor II mutation and Protein C and S deficiencies. Acquired systemic risk factors include myeloproliferative disorder, antiphospholipid syndrome and paroxysmal nocturnal hemoglobinuria. Administration of oral contraceptives also increases risk. Local causes include cirrhosis, pancreatitis, upper abdominal lymphadenopathy and abdominal sepsis such as diverticulitis or appendicitis. Reported iatrogenic causes include trauma during hepatobiliary surgery (especially liver transplantation), splenectomy, umbilical vein catheterization in the neonatal period and open and percutaneous portosystemic shunt procedures (TIPS)[5].
There are three isolated reports of PVT following percutaneous liver interventions. Habu et al[6] describe a case of PVT following percutaneous ethanol injection for treatment of HCC. Zheng et al[7] outline a case of portal vein thrombosis following radiofrequency ablation of a focal HCC. Finally, a recent case report from Kasahara et al[8] describes a bilioportal fistula and portal vein thrombosis in a pediatric liver transplant patient thought to be secondary to previous percutaneous biliary catheter placement. There are no reports in the literature describing intra or extra hepatic portal vein thrombosis following PTC alone.
The occurrence of PVT on two separate occasions following uncomplicated diagnostic PTC raised suspicion of an underlying hypercoagulablestate. The lack of data describing PVT following PTC is surprising given inadvertent portal vein cannulation frequently occurs when trying opacify the biliary tree, albeit with a 21G needle. Although this may simply be secondary to under reporting or under-diagnosis, it is likely that PVT likely occurs to a lesser degree in normal patients following PTC or PTBD, is either clinically occult, goes undetected, or resolves quickly. Furthermore, given the relative paucity of reports of clinically apparent post-PTC PVT in normal patients, this may require a two-hit process, in which patients already have an underlying susceptibility to hypercoagulability and are then exposed to iatrogenic injury to the portal vein branch wall during PTC. As such, we believe that the development of portal vein thrombosis following PTC in the absence of a known hypercoagulablestate merits investigation of the patient’s coagulation status for systemic risk factors.
A mutation in the Factor II gene was first described by Poort et al[9] in 1996. The mutation results in increased prothrombin synthesis with resulting elevated serum prothrombin concentration. The prothrombin/Factor II (G20210A) mutation is present in approximately 2% of the population and is estimated to incur a three fold increase in risk for thromboembolism[10]. A metaanalysis from Dentali et al[11] suggests a strong association between PVT and the prothrombin/Factor II (G20210A) mutation (4.5 fold increase in risk). A weaker association was noted between PVT and the Factor V Leidin mutation (2 fold increase in risk). They further suggest that all patients with PVT should undergo assessment for the prothrombin/Factor II (G20210A) mutation even in the absence of a precipitating event such as surgical intervention. A study from Chamouard et al[12] found a statistically significant increase in frequency of Factor II mutation in patients with previously “idiopathic” PVT when compared to controls or patients with lower extremity deep venous thrombosis Again the author suggests long term anticoagulation in Factor II mutation patients with a history of PVT.
Given the availability of rapid PCR assays for prothrombotic genetic mutations, consideration could be given to including this in the routine pre procedure work up of patients undergoing percutaneous biliary procedures. Whether the presence of a prothrombotic mutation would change procedural technique, periprocedural anticoagulation or imaging follow up is unclear however[13].
No firm guidelines exist on the management of patients with inherited risk factors for PVT. In our patient, the development of PVT on two occasions, at two institutions over a 5 year period following relatively minor trauma to the biliary tree prompted the hematology service to start warfarin therapy.
Symptomatic portal vein thrombosis following PTC is unreported in the literature. In our patient, the combination of a previously unreported iatrogenic risk factor (PTC) with an inherited risk factor (Factor IImutation) likely combined to result in symptomatic intrahepatic portal vein thrombosis. We believe portal vein thrombosis following PTC in the absence of a known hypercoagulablestate merits investigation of the patient’s coagulation status for inheritable risk factors. Patients with known Factor II mutation be at higher risk for PVT and may require periprocedural anticoagulation therapy if hepatobilary interventions are considered.
Peer reviewers: Hiroshi Yoshida,Professor, Department of Surgery, Nippon Medical School Tama Nagayama Hospital, 1-7-1, Nagayama, Tama-city, Tokyo 205-8512, Japan; Dr. Kazushi Kishi, Wakayama Medical University, Kimiidera 811-1, Wakayama 641-8510, Japan
S- Editor Cheng JX L- Editor A E- Editor Zheng XM
| 1. | Roberts LN, Patel RK, Arya R. Venous thromboembolism and ethnicity. Br J Haematol. 2009;146:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Wang JT, Zhao HY, Liu YL. Portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2005;4:515-518. [PubMed] |
| 3. | Chawla Y, Duseja A, Dhiman RK. Review article: the modern management of portal vein thrombosis. Aliment Pharmacol Ther. 2009;30:881-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Ogren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol. 2006;12:2115-2119. [PubMed] |
| 5. | Bayraktar Y, Harmanci O. Etiology and consequences of thrombosis in abdominal vessels. World J Gastroenterol. 2006;12:1165-1174. [PubMed] |
| 6. | Habu D, Nishiguchi S, Shiomi S, Tamori A, Sakaguchi H, Takeda T, Seki S, Ishibashi C, Asai H. Portal vein thrombosis following percutaneous ethanol injection therapy for hepatocellular carcinoma. Indian J Gastroenterol. 2002;21:162-163. [PubMed] |
| 7. | Zheng RQ, Kudo M, Inui K, Suetomi Y, Minami Y, Chung H, Kawasaki T. Transient portal vein thrombosis caused by radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol. 2003;38:101-103. [PubMed] |
| 8. | Kasahara M, Sakamoto S, Fukuda A, Shigeta T, Tanaka H, Mastuno N, Hashimoto M, Kondo Y, Nosaka S, Nakazawa A. Posttransplant bilioportal fistula with portal vein thrombosis: a case report. Transplant Proc. 2010;42:3862-3864. [PubMed] |
| 9. | Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698-3703. [PubMed] |
| 10. | Zöller B, García de Frutos P, Hillarp A, Dahlbäck B. Thrombophilia as a multigenic disease. Haematologica. 1999;84:59-70. [PubMed] |
| 11. | Dentali F, Galli M, Gianni M, Ageno W. Inherited thrombophilic abnormalities and risk of portal vein thrombosis. a meta-analysis. Thromb Haemost. 2008;99:675-682. [PubMed] |
| 12. | Chamouard P, Pencreach E, Maloisel F, Grunebaum L, Ardizzone JF, Meyer A, Gaub MP, Goetz J, Baumann R, Uring-Lambert B. Frequent factor II G20210A mutation in idiopathic portal vein thrombosis. Gastroenterology. 1999;116:144-148. [PubMed] |