Published online Apr 28, 2012. doi: 10.4329/wjr.v4.i4.159
Revised: March 31, 2012
Accepted: April 7, 2012
Published online: April 28, 2012
AIM: To validate a multimodal [structural and functional magnetic resonance (MR)] approach as coincidence brain clusters are hypothesized to correlate with clinical severity of auditory hallucinations.
METHODS: Twenty-two patients meeting Diagnostic and Statistical Manual of Mental Disorders (fourth edition, DSM-IV) criteria for schizophrenia and experiencing persistent hallucinations together with 28 healthy controls were evaluated with structural and functional MR imaging with an auditory paradigm designed to replicate those emotions related to the patients’ hallucinatory experiences. Coincidence maps were obtained by combining structural maps of gray matter reduction with emotional functional increased activation. Abnormal areas were correlated with the brief psychiatric rating scale (BPRS) and the psychotic symptom rating scale (PSYRATS) scales.
RESULTS: The coincidence analysis showed areas with coexistence gray matter reductions and emotional activation in bilateral middle temporal and superior temporal gyri. Significant negative correlations between BPRS and PSYRATS scales were observed. BPRS scores were negatively correlated in the middle temporal gyrus (right) (t = 6.86, P = 0.001), while negative PSYRATS correlation affected regions in both the superior temporal gyrus (left) (t = 7.85, P = 0.001) and middle temporal gyrus (left) (t = 4.97, P = 0.002).
CONCLUSION: Our data identify left superior and middle temporal gyri as relevant areas for the understanding of auditory hallucinations in schizophrenia. The use of multimodal approaches, sharing structural and functional information, may demonstrate areas specifically linked to the severity of auditory hallucinations.
- Citation: García-Martí G, Aguilar EJ, Martí-Bonmatí L, Escartí MJ, Sanjuán J. Multimodal morphometry and functional magnetic resonance imaging in schizophrenia and auditory hallucinations. World J Radiol 2012; 4(4): 159-166
- URL: https://www.wjgnet.com/1949-8470/full/v4/i4/159.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i4.159
Schizophrenia is a heterogeneous illness with neurobiological underpinnings that are not fully understood. Recent neuroimaging techniques have helped to disentangle brain mechanisms that may explain distinct aspects of this deteriorating condition. Several neuroimaging modalities may be combined to create multimodal approaches, which will undoubtedly help to enhance our knowledge clarifying the neurobiological basis of this illness.
Relevant data have been obtained from several magnetic resonance (MR) imaging unimodal studies using structural (morphometric MR imaging, mMRI), functional (functional MR imaging, fMRI), diffusion-tensor imaging and MR spectroscopy; mMRI and fMRI are currently the most extensively used techniques in the field. Structural abnormalities in patients with schizophrenia have repeatedly been demonstrated with the use of region of interest approaches and with non-hypothetically-driven techniques such as voxel-based morphometry (VBM)[1-7]. In 15 evaluated studies, Honea et al[1] found that gray matter reductions in the left medial and superior temporal areas were the most frequently reported. However, this has been confronted by a recent meta-analysis that failed to demonstrate a crucial role for the superior temporal gyrus in schizophrenia[8]. Several neuropathological processes may be merging in patients with schizophrenia and those related to the pathogenesis of auditory hallucinations may be overshadowed. In addition to technical difficulties inherent to meta-analytic techniques, disease heterogeneity may further obscure relevant findings for a particular manifestation such as auditory hallucinations, considering that some brain abnormalities may be present only in a particular subgroup of patients. This has been confirmed in a recent meta-analysis that has demonstrated a relevant role for the superior temporal gyrus and other fronto-temporal areas in the pathogenesis of auditory hallucinations[9]. However, although other brain areas may be involved, functional studies and sound theoretical models suggest that the superior temporal gyrus is a key area affecting auditory hallucinations[10].
Multimodal approaches based on a combination of techniques will help to improve our knowledge of this illness. Only a few multimodal studies are currently available for patients with schizophrenia. Calhoun et al[11] used a joint independent component analysis to analyze gray matter and fMRI activation images. They found group differences between schizophrenic patients and controls in the bilateral parietal, frontal and posterior temporal regions in gray matter associated with bilateral temporal regions activated by auditory oddball target stimuli. Bilateral anterior temporal lobe regions were found to have less gray matter in patients with schizophrenia and less hemodynamic activity for target detection. Resting state fMRI has several potential advantages over task-activation fMRI in terms of its clinical applicability[12]. Lui et al[13] used optimized VBM and resting state functional connectivity analysis in a sample of 68 antipsychotic-naïve first-episode schizophrenia patients and 68 matched healthy controls. They reported that clinical symptom severity assessed by Global Assessment of Function and Positive and Negative Syndrome Scale was associated with alterations in gray matter reductions in the right superior temporal gyrus, right middle temporal gyrus and functional temporal regions.
Functional studies designed to approach a specific symptom or dimension together with VBM studies on a highly homogeneous group of patients may be useful to clarify the biological basis of a disease. Horn et al[14] reported overlapping gray matter deficits in the left posterior superior temporal gyrus and left angular gyrus when using VBM and resting fMRI in 13 patients with schizophrenic formal thought disorder. They also observed positive correlations between the severity of formal thought disorder and both functional alterations and gray matter deficits in several brain regions.
One approach to validate a neuroimaging technique based on the MR analysis of coincidence between gray matter loss and emotional auditory functional activation[15] is to search for correlations between auditory hallucinations severity and multivariate coinciding clusters in specific brain areas.
The purpose of this study was to evaluate if MR functional abnormalities associated with auditory emotional stimuli coexist with focal brain reductions in schizophrenic patients with persistent auditory hallucinations. Our assumptions are focused on the functional segregation of the brain, looking for areas specifically linked to the pathogenesis of auditory hallucinations using a block design paradigm. We hypothesized that there will be a specific correlation with brief psychiatric rating scale (BPRS) and psychotic symptom rating scale (PSYRATS) scales between coinciding clusters in the superior temporal gyrus and the severity of auditory hallucinations.
The study sample comprised 50 subjects. Twenty-two patients exhibited DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) schizophrenia and persistent auditory hallucinations with a mean age of 29.8 ± 11.7 years (range 22-34 years) and a mean disease duration of 13.6 years. Twenty-eight control subjects were also evaluated using the same protocol; the mean age of the control group was 31.9 ± 11 years (range 20-70 years). All patients and subjects were right-handed and male to reduce confounding bias within the population. Handedness was assessed with the Edinburgh Questionnaire[16]. None of the patients had problems of hearing loss. Table 1 shows the most relevant clinical and demographic variables.
| Schizophrenia with auditory hallucinations (n = 22) | Control subjects (n = 28) | |
| Age (yr) | 29.78 ± 11.68 | 31.89 ± 11.01 |
| Age first hallucinations (yr) | 21.86 ± 12.21 | - |
| Illness duration (yr) | 13.55 ± 6.72 | - |
| GAF | 39.86 ± 10.34 | - |
| BPRS | 53.86 ± 8.51 | - |
| PSYRATS | 33.91 ± 15.21 | - |
| PANSS total | 73.36 ± 16.01 | - |
| PANSS positive | 19.86 ± 4.77 | - |
| PANSS negative | 18.23 ± 7.78 | - |
| PANSS general | 35.27 ± 8.39 | - |
All patients met the following selection criteria for persistent hallucinations: (1) persistence of hallucinations even after the use of two antipsychotic drugs at doses equivalent to at least 600 mg/d of chlorpromazine within the last year; (2) voices unmodified in any way by treatment; and (3) voices present at least once per day within the past year. Patients were assessed for general psychopathology using the BPRS[17] and for severity of auditory hallucinations using the PSYRATS[18]. At the end of the trial, every subject was asked if they have experienced hallucinations during the scanning.
The local ethics committee approved this study, and all the participants gave written informed consents.
MR images were acquired on a 1.5 T MR magnet (Intera, Philips Medical Systems, Best, Netherlands). fMRI images were obtained with a dynamic Echo Planar Imaging T2* weighted sequence (TR = 2000 ms, TE = 50 ms, 5 mm slice thickness with no inter-slice gap, 24 slices; acquisition matrix = 128 × 128, field of view = 220 mm and flip angle = 65°, voxel size = 1.72 mm × 1.72 mm × 5.0 mm). Additionally, a high spatial resolution 3D spoiled gradient echo T1-weighted MR sequence was employed to obtain an entire set of brain images with 96 axial slices covering the whole brain (TR = 7 ms, TE = 1.9 ms, 1.25 mm section thickness with no inter-slice gap; acquisition matrix = 256 × 256, field of view = 220 mm and flip angle = 8°, achieving a voxel size of 0.86 mm × 0.86 mm × 1.25 mm).
The auditory paradigm was previously defined by our group[19]. So that the selection of the words with emotional content was specific for psychosis, 82 patients with schizophrenia according to DSM-IV criteria and who had suffered auditory hallucinations according to their clinical record were selected. Patients were asked about the words of their hallucinations which were recorded on a tape recording machine. These recordings were transcribed and the most frequently appearing words were analyzed. A total of 13 emotional words were chosen and were distributed in four categories: 4 negative content imperative words, 3 insults, 2 with imperative tone, 2 exclamations related with emotions, and 2 having positive content. Additionally, 13 words with neutral content were selected to match the emotional ones according to their pleasantness and the number of syllables.
The fMRI design consists of 4 blocks with 20 s long stimuli mixed with 4 blocks with 20 s of rest. The order of both acquisitions (emotional and neutral) was random to avoid introducing bias (adaptation, tiredness, saturation surprise). Two sessions (neutral and emotional) were presented to each subject. Each session consisted of 80 dynamics (2 s long each one) covering the entire brain, assigning 10 dynamics to each block. The global duration of the sequence was 160 s. During the experiment, 4 blocks in rest and 4 during activation were alternately studied. Subjects wore earphones adjusted to their heads and connected by a pair of air tubes to an external audio compact disc player. Both sessions were separated by no less than 40 s.
SPM5 software (http://www.fil.ion.ucl.ac.uk/spm) was used to process structural and functional data. The tests were performed using MATLAB version 6.5 (The MathWorks, Natick, MA, USA) under LINUX platform.
Anatomical differences between patients and healthy subjects were measured using the optimized VBM protocol. Custom templates were created in order to minimize the bias induced by using standard anatomical templates in the normalization processes. This process involved the normalization of each raw image with the International Consortium for Brain Mapping 152 template as a reference, applying a 12-parameter affine transformation. The normalized images were segmented to obtain gray matter, white matter and cerebrospinal fluid maps, which were averaged and smoothed with an 8 mm × 8 mm × 8 mm full width at half maximum (FWHM) filter.
These templates were used for the normalization of each original T1 MR image. Normalized data was then segmented to obtain gray matter, white matter and cerebrospinal fluid tissue maps. At this stage, the segmentation process involved a cleaning process, removing non-useful tissue like scalp, skull and dural venous sinus. A non-linear spatial normalization process between segmented images and templates were estimated and applied to warp the original T1 images. Images were interpolated to 1 mm × 1 mm × 1 mm voxels. Finally, warped images were segmented and smoothed by a 12 mm × 12 mm × 12 mm FWHM Gaussian kernel.
To process fMRI data, spatial (realignment) and temporal (slice timing) corrections were applied to eliminate head motion effects and to correct for the delay between acquisition of the first and last slices. Images were then transformed to standard space (MNI350, Montreal Neurological Institute) and smoothed by a three-dimensional 6 mm × 6 mm × 6 mm FWHM kernel. In the individual analysis, a design matrix was defined for each subject. Both an ideal hemodynamic response function and the mean value of each fMRI session were included in the design matrix.
Contrast images of subtraction between emotional and non-emotional content words (both against the rest task) were then extracted for every schizophrenic and control subject. These images were considered to be maps of emotional activation associated with the auditory stimulus. A two sample t-test map was calculated testing the differences in activation between both groups of subjects and patients for the contrast of subtraction referred before (Random Effects Analyses were applied).
Statistical analyses were performed under the General Linear Model framework. Although the sample was quite homogeneous, two covariates of interest for each subject (age and total intracranial volume) were included in the designed model. Statistical parametric maps were obtained by performing Student-t tests voxel-by-voxel, using SPM one-tailed contrasts in order to measure interactions between groups (patients < controls and patients > controls).
To perform the correlation analysis between PSYRATS and BPRS scales with the affected areas, a new statistical model was built. Maps of coincidence, PSYRATS and BPRS scores, total intracranial volume and age factor were included in a General Linear Model design matrix.
Significance criteria were established by using a P < 0.005 and a correction for multiple comparisons following the false discovery rate (FDR) methodology. Only areas with a minimum expected number of voxels per cluster (k) of 45 were reported and labeled with the Automated Anatomical Labeling software. The coordinates were defined by the maximum Student-t value in the corresponding brain areas.
Both contrast images with gray matter reduction (gray matterpatients < gray mattercontrols) and maps of emotional hyperactivation in the schizophrenic patients (emotionalpatients > emotionalcontrols) were overlaid in order to depict the common abnormalities found out by both techniques. The coincidence map was then voxel-by-voxel generated multiplying the emotional functional images with the gray matter concentration differences maps, so that the higher the activation area and the higher the concentration differences, the more highlighted that area would appear. This procedure allowed determination of whether fMRI activation areas associated with hearing emotional words were coincident with focal brain gray matter reductions.
There were no significant differences in age between both groups (t = 0.81, P = 0.85). Additionally, total intracranial volume did not differ between schizophrenic patients (1266.8 ± 68.4 mL) and control subjects (1195.3 ± 64.2 mL) (t = 1.33, P = 0.72). As expected, schizophrenic patients with auditory hallucinations showed gray matter density reductions when compared with control subjects. The most relevant areas with significant gray matter reductions were the insula (bilateral), the superior temporal gyrus (bilateral), anterior cingulate gyrus (left), inferior parietal gyrus (right), middle temporal gyrus (bilateral) and amygdala (bilateral) (Table 2, P < 0.005 FDR corrected, k = 45).
| Coordinates (mm) | Label | Hemisphere | t value | Pcorrected | Brodmann area | ||
| X | Y | Z | |||||
| -42 | 10 | -11 | Insula | L | 7.41 | 0.001 | 48 |
| 59 | -33 | 50 | Insula | R | 7.39 | 0.001 | 48 |
| -54 | -19 | 4 | Superior temporal gyrus | L | 5.69 | 0.001 | 22 |
| -5 | 42 | 14 | Anterior cingulated gyrus | L | 5.36 | 0.001 | 32 |
| 66 | -16 | 3 | Superior temporal gyrus | R | 5.15 | 0.001 | 22 |
| 52 | -48 | 36 | Inferior parietal gyrus | R | 5.05 | 0.001 | 40 |
| -61 | -53 | 16 | Middle temporal gyrus | L | 4.85 | 0.002 | 21 |
| 19 | -3 | -13 | Amygdala | R | 4.75 | 0.002 | 34 |
| 60 | -42 | 4 | Middle temporal gyrus | R | 4.55 | 0.002 | 22 |
| -20 | 1 | -15 | Amygdala | L | 4.45 | 0.003 | 34 |
In fMRI experiments, significant areas of emotional auditory activation were primarily found in schizophrenic patients when comparing with control subjects in the middle temporal gyrus (bilateral), superior temporal gyrus (bilateral), amygdala (bilateral), hippocampus (left) and precuneus (right) (Table 3, P < 0.005 FDR corrected, k = 45).
| Coordinates (mm) | Label | Hemisphere | t value | Pcorrected | Brodmann area | ||
| X | Y | Z | |||||
| -53 | 4 | -17 | Middle temporal pole | L | 15.53 | 0.000 | 21 |
| 54 | -10 | -12 | Middle temporal gyrus | R | 14.68 | 0.000 | 22 |
| -56 | -23 | -3 | Superior temporal gyrus | L | 14.55 | 0.000 | 22 |
| 58 | -7 | -3 | Superior temporal gyrus | R | 14.69 | 0.000 | 22 |
| -22 | -1 | -17 | Amygdala | L | 11.89 | 0.000 | 34 |
| -21 | -29 | -3 | Hippocampus | L | 10.65 | 0.000 | 27 |
| 50 | -11 | -9 | Middle temporal gyrus | R | 9.88 | 0.001 | 22 |
| 1 | -52 | 15 | Precuneus | R | 9.71 | 0.001 | 30 |
| 21 | -5 | -17 | Amygdala | R | 8.05 | 0.001 | 34 |
The coincidence analysis showed areas with concomitant gray matter reductions and abnormal emotional activation in the middle temporal gyrus (bilateral) and superior temporal gyrus (bilateral) (Table 4) in schizophrenic patients.
| Coordinates (mm) | Label | Hemisphere | Brodmann area | ||
| X | Y | Z | |||
| -59 | -53 | 4 | Middle temporal gyrus | L | 21 |
| -53 | -17 | 4 | Superior temporal gyrus | L | 48 |
| 66 | -20 | 3 | Superior temporal gyrus | R | 22 |
| 62 | -47 | 10 | Middle temporal gyrus | R | 21 |
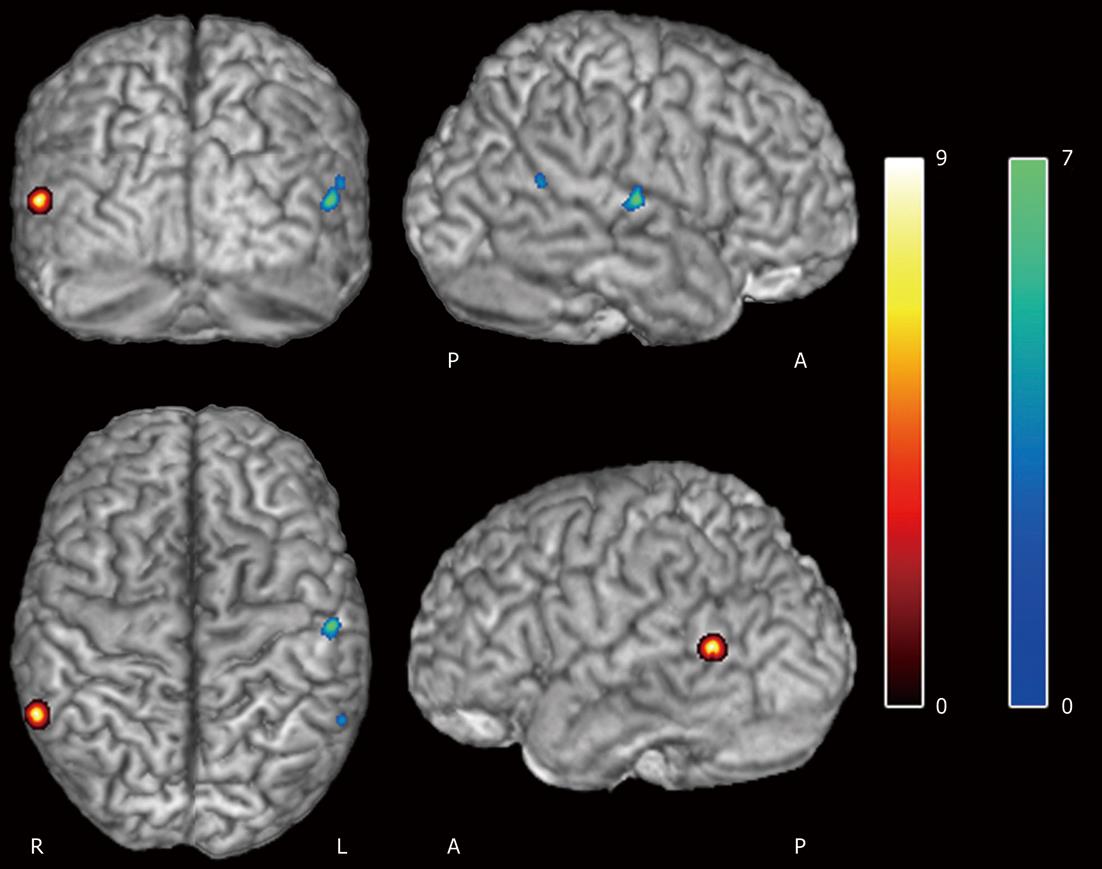
Values of the BPRS and PSYRATS scales evaluated in the schizophrenic patients group were 53.86 ± 8.51 and 33.91 ± 15.21, respectively. Significant negative correlations between these scales were observed with the coincidence areas (P < 0.005 FDR corrected, k = 45). The BPRS variable was negatively correlated in the middle temporal gyrus (right) (r2 = 0.62, P = 0.001), while negative PSYRATS correlation affected regions in both the superior temporal gyrus (left) (r2 = 0.68, P = 0.001) and middle temporal gyrus (left) (r2 = 0.51, P = 0.002) (Table 5, Figure 1). No linear correlation was found between BPRS and PSYRATS covariates (R = 0.08).
| Coordinates (mm) | Label | Hemisphere | t value | Pcorrected | Brodmann area | ||
| X | Y | Z | |||||
| BPRS | |||||||
| 61 | -50 | 5 | Middle temporal gyrus | R | 6.86 | 0.001 | 21 |
| PSYRATS | |||||||
| -56 | -15 | 7 | Superior temporal gyrus | L | 7.85 | 0.001 | 48 |
| -59 | -52 | 15 | Middle temporal gyrus | L | 4.97 | 0.002 | 21 |
By means of coincidence parametric maps applied on patients with schizophrenia, we have demonstrated the presence of neuronal clusters located in the left superior temporal and bilateral middle temporal gyri with both functional abnormal activation after an emotional auditory paradigm and gray matter reduction.
Auditory hallucinations bilaterally activate the superior and middle temporal gyri. Bilateral middle temporal gyri have been involved in emotion[20]. Structures activated during the perception of external voices are also activated during auditory hallucinations with the additional activation found in areas responsible for the processing of emotion[10]. Data are consistent on a negative correlation between the left superior temporal gyrus volumes and auditory hallucinations[2]. This finding was expected according to the emotional task that was used in the experiment which was designed to elicit the emotional response that patients with schizophrenia have when facing their auditory verbal hallucinations. It is a task aimed to draw out the key areas involved in the pathogenesis of auditory hallucinations and is therefore concordant with many studies of functional auditory stimulation.
It is not surprising that the superior temporal gyrus and middle temporal gyrus are key elements of all pathological models which have been proposed to explain auditory hallucinations. Most of the activity studies have shown that auditory hallucinations are also associated to regional blood flow increases in the left superior temporal gyrus and other fronto-temporal areas[9]. A neurophysiological interaction among psychopathology (auditory hallucinations), brain function (increased hemodynamic) and structure (gray matter deficits) has been previously hypothesized[15]. It is generally accepted that areas of decreased perfusion parallel decreases in gray matter concentration as occurs with age-related brain reduction, which is probably associated with a decrease in blood flow and metabolism in those areas. Nevertheless, our findings suggest the contrary effect, showing that in schizophrenic patients an abnormal hyperactivation is found in specific areas of maximal neural density decrement. These areas of coincidence where the same voxels have hemodynamic functional changes associated with the emotional auditory-triggered response and focal decreased density may express a compensation phenomenon in which regions with decreased volume need a larger hemodynamic response to a well-defined paradigm.
An influential model for the pathogenesis of auditory hallucinations in which the superior temporal and middle temporal gyri are key areas has been postulated[10]. A “top-down” network, presenting an altered activation in speech production areas (i.e., inferior frontal gyrus) and altered coupling with monitoring areas (anterior cingulate) and language reception areas (Wernicke’s area) was suggested. There would also be a “bottom-up” dysfunction through over-activation of secondary and occasionally primary sensory cortices that lead to the experience of vivid perceptions in the absence of sensory stimuli.
The neurobiological basis for temporal gyrus volume reduction is unknown. Abnormal brain maturation processes influenced by multiple genes interact with other potentially causative factors for psychoses such as substance abuse, stress and dysregulation of the hypothalamic-pituitary-adrenal axis function. Other factors may also interfere including poor diet and exercise, smoking, psychosocial and socioeconomic influences, and associated physical comorbidity as well as neuroleptics and other medications[21].
Structural and functional abnormalities in the superior temporal and middle temporal gyrus may also be relevant in the pathogenesis of schizophrenia. Several findings support the idea that decreasing superior temporal gyrus volume is a potential endophenotype for schizophrenia-spectrum disorders. Firstly, an MR imaging study in 29 young, non-psychotic subjects with a schizophrenic parent had reported bilateral superior temporal gyrus volume reduction compared to controls with no psychiatric family history[22]. Secondly, reduced superior temporal gyrus volume has been consistently reported in patients with schizotypal personality disorder[23]. The superior temporal gyrus is also particularly affected during the first years of illness through progressive gray matter reductions. This is the case even during the prodromal phase and probably has clinical implications[24].
The study presented here contains several limitations. Our relatively small sample size may have hampered the ability to detect significant correlations in other potentially relevant areas, although it has been reported that a sample of 20 or more subjects is sufficient to obtain reliable functional neuroimaging data[25]. The results obtained from a high homogeneous sample (only schizophrenic patients with persistent auditory hallucinations were included) cannot be generalized to all patients with schizophrenia. We assessed our multimodal approach to be a strategy for determining brain areas that should be further assessed to study the neurobiological basis of one particular symptom or aspect of a disorder. Additionally, hallucinatory experiences during fMRI scanning were answered subjectively according to the patients’ perception, with the subsequent lack of certainty derived from this qualitative approach. Finally, all our study patients were medicated with a wide range of first- and second-generation antipsychotics. Our findings can hardly be attributed to medication. In fact, a recent study in antipsychotic-naïve first-episode schizophrenia with [(18)F] fluoro-deoxyglucose (FDG) positron emission tomography has shown that patients experiencing auditory hallucinations during FDG uptake had significantly higher metabolic rates in the left superior and middle temporal cortices, and other brain areas[26]. Moreover, a neuroleptic-naïve sample would have been out of the scope of this study, as we have chosen to include a highly homogeneous sample of patients based on their refractory behavior to antipsychotic medications.
In conclusion, we identified the left superior and middle temporal gyri as relevant areas in patients with auditory hallucinations. Our findings provide support for the use of multimodal structural and functional MR approaches in the search for areas specifically linked to the pathogenesis of auditory hallucinations. This core approach appears to be a good strategy for studying the neurobiological basis of clinical dimensions which may be particularly indicated at this stage. Similar multimodal approaches could also be used for studying other features as long as specific paradigms for the functional study of such symptoms are provided.
Schizophrenia affects up to 1% of the population. Understanding the neural substrates of this heterogeneous disorder involves a precise study of the brain, in terms of anatomy and function.
To study the specific schizophrenia phenotypes is one of the most significant issues in psychiatry. The use of a multimodal (structural and functional) approach will help to develop new phenomenological models of anatomical and functional abnormalities in schizophrenic patients suffering from auditory hallucinations.
Several studies have reported both structural and functional alterations when evaluating patients with schizophrenia. Nevertheless, the clinical heterogeneity of the samples is associated with large variability in the results. This is the first study that uses a multimodal methodology to evaluate areas where functional and structural alterations coexist in a high specific sample of patients with schizophrenia and auditory hallucinations.
The use of the proposed methodology will be useful to obtain concordance maps showing areas with functional abnormalities and focal brain reductions in psychiatric and neurodegenerative diseases.
Semiautomatic morphometric methods are used to detect subtle differences in terms of gray and white matter between groups. The functional evaluation of a patient can detect activated areas that strongly correlate with the stimulation paradigm.
The authors present a validation study of a multimodal methodology (structural and functional MR) through the identification of key areas for the biological underpinnings of auditory hallucinations. The importance of this research is the potentiality of this approach to identify key areas for particular phenotypes. The authors combine this multimodal technique with an extreme phenotype approach, which is fairly innovative in psychiatry. The paper is well-written and readable with no ethical objections. The different sections are clearly differentiated.
Peer reviewer: Eduardo J Aguilar, MD, PhD, Coordinador de Salud Mental, Psiquiatría, Hospital de Sagunto, Avda. Ramón y Cajal s/n, 46520 Sagunto, Spain
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233-2245. [PubMed] |
| 2. | Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev. 2009;61:14-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | García-Martí G, Aguilar EJ, Lull JJ, Martí-Bonmatí L, Escartí MJ, Manjón JV, Moratal D, Robles M, Sanjuán J. Schizophrenia with auditory hallucinations: a voxel-based morphometry study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6199] [Cited by in RCA: 6216] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 5. | Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, Yamashita I, Chitnis XA, McGuire PK, Seto H. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci. 2004;254:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 279] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 9. | Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 11. | Calhoun VD, Adali T, Giuliani NR, Pekar JJ, Kiehl KA, Pearlson GD. Method for multimodal analysis of independent source differences in schizophrenia: combining gray matter structural and auditory oddball functional data. Hum Brain Mapp. 2006;27:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Tomasi D, Volkow ND. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Horn H, Federspiel A, Wirth M, Müller TJ, Wiest R, Wang JJ, Strik W. Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry. 2009;194:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Martí-Bonmatí L, Lull JJ, García-Martí G, Aguilar EJ, Moratal-Pérez D, Poyatos C, Robles M, Sanjuán J. Chronic auditory hallucinations in schizophrenic patients: MR analysis of the coincidence between functional and morphologic abnormalities. Radiology. 2007;244:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97-113. [PubMed] |
| 17. | Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799-812. |
| 18. | Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29:879-889. [PubMed] |
| 19. | Sanjuan J, Lull JJ, Aguilar EJ, Martí-Bonmatí L, Moratal D, Gonzalez JC, Robles M, Keshavan MS. Emotional words induce enhanced brain activity in schizophrenic patients with auditory hallucinations. Psychiatry Res. 2007;154:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, Simmons A, Andrew C, Brammer M, David AS. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res. 1998;83:127-138. [PubMed] |
| 21. | Pantelis C, Yücel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 389] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 22. | Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161:1121-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Goldstein KE, Hazlett EA, New AS, Haznedar MM, Newmark RE, Zelmanova Y, Passarelli V, Weinstein SR, Canfield EL, Meyerson DA. Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophr Res. 2009;112:14-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366-376. [PubMed] |
| 25. | Thirion B, Pinel P, Mériaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage. 2007;35:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 26. | Horga G, Parellada E, Lomeña F, Fernández-Egea E, Mané A, Font M, Falcón C, Konova AB, Pavia J, Ros D. Differential brain glucose metabolic patterns in antipsychotic-naïve first-episode schizophrenia with and without auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |