Published online Sep 28, 2011. doi: 10.4329/wjr.v3.i9.233
Revised: July 18, 2011
Accepted: July 25, 2011
Published online: September 28, 2011
AIM: To measure the dose distribution, related to the treatment planning calculations, in the contralateral mammary gland of breast cancer patients treated with accelerated hypofractionated 3-dimensional conformal radiotherapy.
METHODS: Thirty-four prospectively selected female patients with right breast cancer (pN0, negative surgical margins) were treated with breast-conserving surgery. A total dose of 42.5 Gy (2.66 Gy/fraction) was prescribed; it was requested that planning target volumes be covered by the 95% isodose line. The contralateral mammary gland was defined on CT simulation. The dose received was evaluated by dose volume histograms.
RESULTS: The measured contralateral breast doses were: (1) Dose maximum: 290-448 cGy [Equivalent (Eq) 337-522 cGy]; (2) Mean dose: 45-70 cGy (Eq 524-815 cGy); and (3) Median dose: 29-47 cGy (337-547 cGy) for total primary breast dose of 42.5 Gy in 16 equal fractions. The spearman rho correlation showed statistical significance between the contralateral breast volume and maximum dose (P = 0.0292), as well as mean dose (P = 0.0025) and median dose (P = 0.046) to the breast.
CONCLUSION: Minimizing the dose to the contralateral breast has to be one of the priorities of the radiation oncologist when using short schedules because of the radiosensitivity of this organ at risk. Further study is necessary to assess the long-term clinical impact of this schedule.
- Citation: Tolia M, Platoni K, Foteineas A, Kalogeridi MA, Zygogianni A, Tsoukalas N, Caimi M, Margari N, Dilvoi M, Pantelakos P, Kouvaris J, Kouloulias V. Assessment of contralateral mammary gland dose in the treatment of breast cancer using accelerated hypofractionated radiotherapy. World J Radiol 2011; 3(9): 233-240
- URL: https://www.wjgnet.com/1949-8470/full/v3/i9/233.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i9.233
Breast cancer is the most common (excluding skin) malignant neoplasm among women. In the United States, it was calculated to have an approximate lifetime risk of 13.4%; 184 450 cases are invasive and 67 770 cases are in situ carcinomas per year[1].
The purpose of radiation treatment following lumpectomy is to improve local control in the treated breast with as little toxicity as possible. Since radiation therapy efficacy has improved, the issues related to post-therapy complications have become very important. Contralateral breast dose from primary breast irradiation has been implicated in the risk of second breast malignancies.
Daily treatment over several weeks can be very inconvenient to many patients. A high number of studies[2-13] have shown that the goal of post-lumpectomy radiotherapy is also achieved with shorter than the conventional fractionation schedules. Whole breast radiotherapy for invasive breast cancer demonstrates equivalent efficacy and morbidity for conventional and hypofractionated treatment, as shown in a Canadian trial involving 1234 women with node-negative breast cancer and clear margins of excision after breast conserving surgery and axillary dissection. Women were randomly assigned to receive whole breast irradiation of 42.5 Gy in 16 fractions over 22 d (short arm) or 50 Gy in 25 fractions over 35 d (long arm).
Hypofractionation can increase the late normal tissue damage. The principal long-term effects that impair cosmesis are fibrosis and atrophy of the breast which are a result of the specific response of fibrocytes to irradiation.
The aim of the present study was to evaluate the delivery of accelerated hypofractionated 3-D conformal radiotherapy (3D-CRT) in the contralateral mammary gland in breast cancer patients.
Between October 2009 and September 2010, 34 women with a primary diagnosis of invasive carcinoma were enrolled in the treatment protocol. In the study were included patients > 50 years old, diagnosed with stage I-II, right-sided breast cancer. Large mammary glands with a distance from sternum to mid axillary line more than 25 cm were excluded from the study.
All patients underwent breast-conserving surgery (with axillary sampling or dissection). In particular, they had a lumpectomy before radiotherapy. They had no adjuvant chemotherapy. Exclusion criteria included previous treatment for a diagnosis of ductal carcinoma in situ or invasive breast carcinoma, omission of post-operative radiation, or surgical management with mastectomy.
Pathological results were abstracted from the original histopathology report. The specimens showed an invasive adenocarcinoma, non-high grade, negative margins (> 2 mm), no axillary lymph nodes involved.
Each patient underwent a virtual CT-simulation, in supine position, using dedicated devices. The patient’s arms were raised above the head using an arm support in carbon fiber (Sinmed©, Reeuwijk, The Netherlands).
For treatment planning, a CT scan covering a region from the 6th cervical vertebra to the middle part of the abdomen was obtained for each patient. The patients were scanned with 5 mm slice thickness in simulation CT scan and the CT datasets were transferred to the Prosoma® Treatment Planning System through the DICOM network.
All contouring of target volumes and normal structures [organs at risk (OARs)] were performed in the Prosoma Treatment Planning System. The following structures were delineated: clinical target volume (CTV), planning target volume (PTV), ipsilateral, contralateral lungs and contralateral breast. According to the ICRU[14,15], OAR is defined to be an uninvolved organ that, if given an excess radiation dose, might be damaged and would compromise the success of the course of radiation therapy.
The demonstrable tumor plus the microscopic disease constitute the CTV.
Margins are needed to surround the CTV to ensure that the CTV lies within the treatment field during the entire course of radiation therapy. These internal margins, in addition to the CTV, constitute the internal target volume (ITV).
In order to account for setup uncertainties, one adds a setup margin to the ITV to generate a PTV.
The CTV, PTV and OARs were outlined on all CT slices. The CTV was expanded to a PTV with 5 mm, with a constraint reverse expansion of 4 mm to the skin surface to avoid potential skin toxicity[16,17]. The PTV provided a margin around the CTV to compensate for the variability of treatment setup and motion of the breast or chest with breathing[17].
The patients were treated with adjuvant whole breast radiotherapy and they received no boost and no supraclavicular irradiation. Radiation therapy to the involved breast was planned to be administered within 12 wk of the most recent surgery. A dose of 42.5 Gy was delivered in 16 daily fractions over 3.5 wk (2.66 Gy/fraction, based on the Canadian randomized trial)[2,3]. Breast radiation was delivered using tangential fields to the entire breast and underlying chest wall, as previously described. The prescription dose of 42.5 Gy was defined for the 95% isodoses of the PTV. In particular, 95% of the PTV should have been covered within 95%-110% of the prescribed dose (39.9-46.2 Gy). Partial wedging or dynamic (Multi Leaf Collimator-MLC) was employed to improve dose homogeneity (7%). To evaluate the dose constraints for normal tissues we used the Toxicity criteria of the Radiation Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) NSABP B-39/RTOG 0413 protocol[18] corrected for hypofractionation, taking into account potential unfavorable anatomy[19]. The dose constraints for the OARs are described below: ipsilateral lung (without supraclavicular irradiation): V25 Gy < 5%, V17 Gy < 8%, V8 Gy < 10%, mean dose < 6.36 Gy; contralateral lung: V2.5 Gy < 15%; contralateral breast: dose max < 3% of the prescribed dose, mean dose as low as possible.
For the conventional technique, we used a virtual simulation. The entire breast was treated, using a parallel pair of two opposed tangential fields. Weighted beams and wedges were used as necessary. The fields were placed isocentrically, with matching posterior field borders. Dose calculation was performed and normalized to isocenter. The prescribed dose was 42.5 Gy delivered in 16 daily fractions, in whole-breast, given in 2.66 Gy fractions with accelerated hypofractionated 3D-CRT[20].
The treatment planning was performed in the Eclipse™
(Varian Medical Systems, United States) TPS. This treatment planning system includes the Pencil Beam algorithm for dose calculation. The beam arrangement consisted of 2 tangential beams, where the beam angles, apertures, weights and dynamic wedges were optimized by standard, forward planning. The photon beam energy was 6 MV, using the linear accelerator VARIAN 600C. To account for the tumor movement during treatment, 2 cm was extended beyond the skin surface in the anterior direction using the skin flash tool in the treatment planning system.
For the treatment technique, histograms of the contralateral breast were generated; a number of parameters, including mean, median and maximum dose to the breast, were evaluated.
During the radiation treatment the patients were monitored every week. Post treatment management included adjuvant endocrine therapy according to the National Comprehensive Cancer Network Guidelines. After the completion of the treatment, the patients were evaluated by a radiation oncologist every 3 mo. Acute skin and breast tissue reactions were also recorded. Toxicity was defined according to the RTOG/EORTC acute and late radiation morbidity scoring system[21].
Correlation of numerical variables was investigated by Pearson correlation coefficient. The whole analysis was performed by using the SPSS version 10 (Chicago, IL).
Thirty-four eligible women treated with adjuvant radiation following breast-conserving surgery were analyzed. The median age of the patients at the time of radiation was
65 years (range, 51-79 years). All patients underwent breast-conserving surgery with accompanying axillary sampling or dissection. All completed adjuvant whole breast radiotherapy with hypofractionated schedule (42.5 Gy in 16 fractions). Clinical and pathological characteristics were similar among the patients.
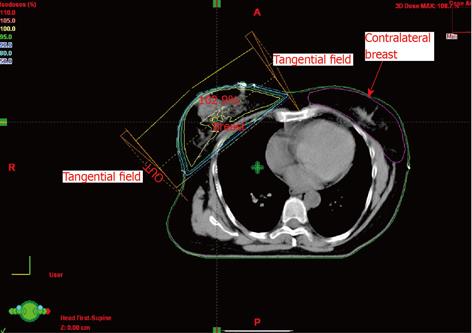
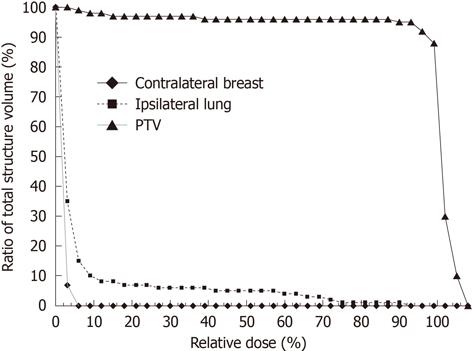
The doses to the opposite breast were generated from the dose volume histograms (DVHs) (Table 1). The doses represent the combined contribution from both the medial and lateral tangential beams. An isocentric technique was used for treatment. Scatter dose from the medial tangential field to the contralateral breast originates in the accelerator head and its accessories. The use of a medial wedge increased the contralateral breast dose due to an increase in scattered photons and in monitor units. The wedge angle used in our study ranged between 15° and 30°. For total primary dose of 4256 cGy, the measured dose maximum at the contralateral gland varies from 290-448 cGy. The mean dose varies from 45 to 70 cGy. The median dose was between 29-47 cGy. The average volume of the breast for the patients in question was 856 ± 327 cm3. The monitor units obtained from the pencil beam calculations and used for the treatment were in the range from 199 to 217. A representative dose distribution for the breast with the contralateral breast contouring is shown in Figure 1 with regard to axial and coronal planes. A representative cumulative dose volume histogram is shown in Figure 2. The spearman rho correlation showed statistical significance between the contralateral breast volume and maximum dose (P = 0.0292), as well as mean dose (P = 0.0025) and median dose (P = 0.046) to the breast (Table 2). Received doses in detail as extracted from DVHs are shown in Table 3.
| Treatment characteristics | Range |
| Dose max | 290-448 cGy |
| Mean dose | 45-70 cGy |
| Median dose | 29-47 cGy |
| Monitor units | 199-217 |
| Breast volume | 749-1474 cm3 |
| Correlation | Spearman rho | P value |
| Breast volume vs max dose to the breast | 0.0090 | 0.0292 |
| Breast volume vs mean dose to the breast | 0.0153 | 0.0025 |
| Breast volume vs median dose to the breast | 0.0028 | 0.046 |
| Breast volume vs gantry angle | 0.0042 | 0.2195 |
| No. | Age (yr) | Breast volume (cm3) | Breast dose (cGy) | |||
| Max | Mean | Median | MU | |||
| 1 | 79 | 1474 | 348 | 49 | 31 | 217 |
| 2 | 55 | 1090 | 380 | 45 | 41 | 209 |
| 3 | 63 | 803 | 367 | 54 | 47 | 203 |
| 4 | 61 | 749 | 290 | 45 | 35 | 199 |
| 5 | 71 | 789 | 448 | 63 | 46 | 203 |
| 6 | 56 | 857 | 345 | 56 | 36 | 204 |
| 7 | 67 | 942 | 401 | 47 | 32 | 210 |
| 8 | 72 | 801 | 412 | 52 | 39 | 200 |
| 9 | 61 | 998 | 434 | 51 | 40 | 202 |
| 10 | 56 | 796 | 399 | 52 | 45 | 210 |
| 11 | 58 | 893 | 387 | 45 | 33 | 199 |
| 12 | 72 | 956 | 402 | 70 | 33 | 204 |
| 13 | 71 | 1001 | 345 | 55 | 41 | 207 |
| 14 | 79 | 842 | 204 | 49 | 39 | 204 |
| 15 | 64 | 865 | 341 | 51 | 38 | 207 |
| 16 | 57 | 789 | 296 | 57 | 40 | 209 |
| 17 | 66 | 985 | 356 | 68 | 32 | 205 |
| 18 | 73 | 934 | 298 | 54 | 30 | 209 |
| 19 | 75 | 924 | 304 | 57 | 39 | 200 |
| 20 | 76 | 1023 | 326 | 61 | 45 | 210 |
| 21 | 59 | 1031 | 348 | 49 | 42 | 210 |
| 22 | 52 | 1320 | 401 | 51 | 40 | 211 |
| 23 | 57 | 980 | 295 | 59 | 32 | 205 |
| 24 | 58 | 1002 | 432 | 57 | 34 | 206 |
| 25 | 74 | 1007 | 422 | 49 | 37 | 209 |
| 26 | 70 | 983 | 427 | 61 | 40 | 200 |
| 27 | 60 | 879 | 346 | 58 | 40 | 204 |
| 28 | 74 | 765 | 307 | 49 | 38 | 214 |
| 29 | 68 | 795 | 401 | 60 | 39 | 208 |
| 30 | 72 | 1021 | 397 | 50 | 32 | 201 |
| 31 | 70 | 784 | 350 | 63 | 29 | 205 |
| 32 | 59 | 788 | 386 | 57 | 36 | 201 |
| 33 | 62 | 901 | 409 | 55 | 42 | 209 |
| 34 | 67 | 854 | 297 | 70 | 45 | 202 |
The choice of treatment for breast cancer is usually determined by tumor stage, patient age, co-morbidity, as well as by patient preferences. The long duration of treatment can adversely affect the quality of a patient’s life. The drawbacks of a prolonged schedule include inconvenience, loss of earnings and cost of traveling for 5 wk,
which can be significant for many women[22]. Shorter schedules, typically delivering a lower total dose in fewer, but larger than 2 Gy fractions, are more convenient for the patients by limiting the number of treatment attendances. Moreover, the reduced resource use in terms of personnel and machine time is advantageous for radiotherapy departments and translates into lower treatment costs. In order to formally validate this therapeutic approach from a societal perspective, however, cost-effectiveness evaluations weighing long-term outcome against the societal costs incurred for many years after treatment are needed[5,6,22]. The efficacy of this schedule has been analyzed by Whelan et al[2,3] and seems to be associated with no difference in 10-year LR (6.2% vs 6.7%, respectively), DFS, OS, or good/excellent cosmetic outcome (70% vs 71%).
In the linear quadratic model, fractionation sensitivity is expressed by the parameter α/β. If α/β is low (e.g., 1 Gy)
the tissue is much more sensitive to increasing dose per fraction than if α/β is high (e.g., 10 Gy), while cancerous tissues generally have rather high α/β ratios[20].
After the report of Yarnold et al[23] in patients irradiated following breast-conserving surgery with the standard 25 fractions or a 13-fraction radiotherapy scheme, it appears reasonable to use an α/β around 3 Gy in developing fractionation schedules for breast irradiation, which are iso-effective regarding overall late normal tissue effects. Although this study had insufficient statistical power to reliably determine the fractionation sensitivity of breast cancer, tentative results from the trial suggest that the α/β ratios are comparable for both breast fibrosis and local control endpoints. In the past, α/β values of 4-5 Gy have been derived for the radiation response of recurrent or inoperable breast cancer[24,25].
Moreover, an α/β ratio of 4 has been reported for human breast carcinoma cell lines[26-28].
3D-CRT and intensity modulated radiotherapy (IMRT) have allowed more conformal dose distributions to the breast, while selectively sparing surrounding normal tissues. During external beam radiotherapy, the contralateral breast receives radiation due to leakage from the collimator and scatter from primary irradiation[29]. Other factors that contribute to dose may include blocks, orientation of the fields, wedge size, wedge angle and the technique used for treatment[30]. Tangential fields and, if used, the anterior supraclavicular field contribute to contralateral scatter dose.
The dose to the contralateral breast can be reduced to some extent by reducing the medial wedge angle[30,31]. The closeness of the gantry angle to the contralateral breast is also associated with increase in dose. Contralateral scatter doses are highest for patients with large protruding breasts whose isocentric treatment plan needs the use of a large wedge and higher beam energy[30].
Radiation, especially at sub-therapeutic doses, has been proven to be carcinogenic[32-37]. According to the United Nations Scientific Committee on Effects of Atomic Radiation report[38], experimental exposure of animals to radiation and observations on exposed human populations have shown that ionizing radiations are general carcinogens capable of inducing tumors in almost all tissues of mammals irrespective of species. Dose to the contralateral breast as a result of radiotherapy of breast should not be ignored in radiotherapy, and more so in patients younger than 45 years. The breast tissue is highly sensitive and therefore the contralateral breast must be regarded as an organ at risk (sensitive organ) while planning for radiotherapy. As already reported[32-39], radiotherapy-associated risk of contralateral breast cancer (CBC) increases with decreasing age at first treatment [age < 35 years, hazard ratio (HR) = 1.78, 95% CI: 0.85 to 3.72; age > 45 years, HR = 1.09, 95% CI: 0.82 to 1.45]. This is very important, particularly in women irradiated at a younger age[32-34] and among women treated under the age of 45 years. Boice et al[32] have shown that the incidence of radiation-induced breast cancer is a linear function of dose received, with latent periods of over 10 years. Secondary tumors following radiotherapy may be observed around or well outside the margin of the PTV[35-37]. Other important considerations include dose to OARs, including the ipsilateral and contralateral lungs.
Women treated before age 45 years with post-lumpectomy radiotherapy experience 1.5-fold increased risk of CBC compared with those who had post-mastectomy radiotherapy. The joint effects of post-lumpectomy radiotherapy and strong family history for breast cancer on risk of CBC were found to be greater than expected when individual risks were summed (HR = 3.52, 95% CI: 2.07 to 6.02, P = 0.043).
Accelerated hypofractionated radiotherapy is currently used because of the similar local control and toxicity rates. To our knowledge, this is the first report on the estimation of the contralateral breast dose using the hypofractionated schedule[3].
Boice et al[32] have conducted a case control study in a cohort of 41 109 women diagnosed with breast cancer and analyzed the records. They found mean contralateral breast dose to be 282 cGy with a maximum of 710 cGy and relative overall increase in risk of contralateral breast malignancy due to treatment of primary by radiation to be 1.19. However, the risk of a second malignancy in the contralateral breast was 1.59, significantly high, in patients who underwent radiotherapy at a younger age than 45 years for primary breast malignancy. This indicates high risk for younger patients.
Bhatnagar et al[40] reported a comparison of contralateral breast dose during primary breast irradiation using IMRT and conventional tangential field technique. They observed the contralateral breast dose to be 7.74% ± 2.35% of the primary breast dose (5000 cGy) in IMRT treatment planning and 9.74% ± 2.04% of primary breast dose during conventional tangential field technique, i.e. about 20% reduction in contralateral breast dose with IMRT as compared to conventional tangential treatment with wedge. In our study, the measurements are in accordance with the conventional tangential field technique of Bhatnagar et al[40].
Tercilla et al[41] measured the contralateral breast dose during half beam block and isocentric treatment techniques for patients treated with primary breast irradiation with a Cobalt60 unit. They measured contralateral breast dose with thermoluminescent dosimeters (TLD) in 15 patients and the doses were 325-650 cGy during half beam block tangential field treatment and 200-450 cGy without half beam block tangential field treatment for a total primary breast dose of 5040 cGy in 28 equal fractions. They recommended non-use of half beam block techniques; however, this will increase the ipsilateral lung and rib dose[29,42]. Our doses are on the high side as compared to doses reported by Tercilla et al[41] because we treated the chest wall using slightly wider fields.
Bhatnagar et al[40,43] have studied the effect of breast size on scatter dose to the contralateral breast. They treated 65 patients with breast cancer using 6 MV photon with IMRT technique and measured contralateral breast dose using TLD[44]. The primary breast size volume was calculated by the planning system from CT slices. They found a mean contralateral dose of 7.2% of the primary breast dose (5000 cGy) and found that the contribution to contralateral breast dose is strongly dependent on primary breast size of the patient. Therefore, this has become of more concern in young breast cancer patients with bulky protuberant breasts.
According to Chougule[29], the dose at the contralateral breast nipple was 152.5 to 254.75 cGy for a total primary breast dose of 5000 cGy in 25 equal fractions (Co60 fields), which amounted to 3.05%-6.05% of total dose to the diseased breast. Furthermore, it was observed that the maximum contribution to the contralateral breast dose was due to the medical tangential half blocked field. Again in our case, although we used a strictly conformal technique with a full 3-D treatment planning, the measurements are of a higher order than those of Chougule[29], mainly because we did not take measurements only from the nipple where very much less scattered radiation dose is expected, but from the whole contralateral breast and especially from the neighboring breast tissues.
Muller-Runkel et al[42] have advocated covering of the contralateral breast with a thin lead sheet to reduce the scattered contribution to contralateral breast skin, though little can be done to reduce the dose from the lateral tangential field as the dose is caused by internal body scatter.
Using modern techniques of CRT and IMRT, the contralateral breast dose can be reduced by 10%-20% but it still is about 3.05%-6.05% (153-255 Gy) of a primary breast dose of 5000 cGy, which cannot be ignored. IMRT technique provides better dose uniformity as compared to other tangential field techniques, as well as significantly reducing the dose to the contralateral breast[40,43,44].
In a previous study, we have already reported the fine cosmesis in hypofractionated breast irradiation, as used in our institution[45]. In this report we are analyzing the dose at the contralateral breast. Further to ICRU reports[14,15], our results are in accordance with a previous study[30], showing that there are only two significant correlations concerning the contralateral breast volume and the dose. This was logical since the volume of the breast would be expected to be correlated with the incidence of direct or scattered field to be inserted. However, although in previous studies the gantry angle was correlated with higher doses to the contralateral breast[29,30,40,42-44], in our research we have not seen any significant correlation. The reason for this might be the fact that we used a smart immobilization device for the chest wall and the hands, which produces a detachment of the contralateral breast. Moreover, the majority of contralateral breast doses are from the scatter doses coming from the collimator. By using the asymmetric collimator technique, the unwanted scattering doses from the collimator can be minimized. In this current study, asymmetric collimators were used.
In terms of the dose uniformity all over the normal breast tissue (skin included), we did not used field in field techniques or a bolus in order to compensate low skin doses.
The main limitations of the present study are the small number of patients, the absence of in vivo dosimetry and the short follow up. Further dosimetric analysis and longer follow up are needed to evaluate the adverse late effects which could be increased because of the hypofractionation schedule used, such as for example, ischemic heart disease, symptomatic rib fracture, symptomatic lung fibrosis. In general, when hypofractionation is used, it is advisable that both possible dose inhomogeneity and normal tissue protection should be taken into account, while the use of three-dimensional conformal techniques should be mandatory[46]. In our clinical routine practice today, further to the use of three-dimensional conformal techniques, we are continuing the study by using in vivo dosimetry and further results will be reported after we have evaluated a sufficient number of patients. These results stress the necessity of meticulous patient observation and long follow up to the contralateral breast.
Lumpectomy followed by breast irradiation is an alternative to mastectomy in early-stage breast cancer. Adjuvant whole breast radiotherapy in patients diagnosed with invasive breast cancer improves local control. Delivering postoperative radiotherapy in a shorter period of time is as effective as longer treatment regimens. Hypofractionated adjuvant radiation schedules have been commonly used in Canada and the United Kingdoms based on data from early invasive breast cancer randomized studies, showing equivalent local control, survival and morbidity rates. Contralateral breast dose from primary breast irradiation has been implicated in the risk of second breast malignancies. The probability of developing contralateral breast cancer represents a serious concern. This study was conducted to measure the dose distribution, related to the treatment planning calculations, in the contralateral mammary gland, when the affected breast was treated with accelerated hypofractionated 3-dimensional conformal radiotherapy.
The hotspots or important areas in the research field related to the article are as following: (1) The use of dose volume histograms (DVH) for the assessment of the dose to the contralateral breast in a hypofractionated scheme; (2) The usefulness of the 3-dimensional conformal treatment planning technique for the accurate calculation of the dose in the organs at risk, as defined by the radiation oncologist (ipsilateral lung, contralateral breast, etc.).
To summarize, this is the first study dealing with the dose to the contralateral breast in a hypofractionated schedule, whereas all similar studies were concerned with the dose in the contralateral breast but under a conventional schedule (2 Gy per fraction instead of 2.66 Gy per fraction). Moreover, for the readers this article incorporates the importance of 3-dimensional treatment planning calculations.
Further application should be in-vivo dosimetry to the contralateral breast together with the modification of the fields (geometry and intensity modulation) for reducing the dose to the contralateral breast. Minimizing the dose to the contralateral breast has to be one of the priorities of the radiation oncologist in short schedules because of the radiosensitivity of this organ at risk. Further study is necessary to assess the long-term clinical impact of this schedule.
DVH is the histogram displaying the function between the delivered dose and the volume of a current target or organ. Three-dimensional conformal treatment planning technique concerns the calculation of the dose in each CT plane of the irradiated area. The ICRU defines an organ at risk to be an uninvolved organ that, if given an excess radiation dose, might be damaged and which would compromise the success of the course of radiation therapy. The demonstrable tumor plus the microscopic disease constitute the clinical target volume (CTV). Margins are needed to surround the CTV to ensure that the CTV lies within the treatment field during the entire course of radiation therapy. These internal margins, in addition to the CTV, constitute the internal target volume (ITV). In order to account for setup uncertainties, one adds a setup margin to the ITV to generate a planning target volume.
The study seems to be interesting, however there are some points to be reviewed.
Peer reviewer: Cem Onal, MD, Department of Radiation Oncology, Adana Research and Treatment Centre, Baskent University Medical Faculty, 01120 Yuregir, Adana, Turkey
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
| 1. | Jabbari S, Park C, Fowble B. Breast cancer. Handbook of evidence-based radiation oncology. 2nd ed. New York: Springer 2010; 263-305. |
| 2. | Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, Lada B, Lukka H, Perera F, Fyles A. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143-1150. [PubMed] |
| 3. | Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-520. [PubMed] [DOI] [Full Text] |
| 4. | Fujii O, Hiratsuka J, Nagase N, Tokiya R, Yoden E, Sonoo H, Murashima N, Iha S, Imajyo Y. Whole-breast radiotherapy with shorter fractionation schedules following breast-conserving surgery: short-term morbidity and preliminary outcomes. Breast Cancer. 2008;15:86-92. [PubMed] |
| 5. | Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for postlumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;47:1219-1228. [PubMed] [DOI] [Full Text] |
| 6. | Olivotto IA, Weir LM, Kim-Sing C, Bajdik CD, Trevisan CH, Doll CM, Lam WY, Basco VE, Jackson SM. Late cosmetic results of short fractionation for breast conservation. Radiother Oncol. 1996;41:7-13. [PubMed] |
| 7. | Yamada Y, Ackerman I, Franssen E, MacKenzie RG, Thomas G. Does the dose fractionation schedule influence local control of adjuvant radiotherapy for early stage breast cancer? Int J Radiat Oncol Biol Phys. 1999;44:99-104. [PubMed] [DOI] [Full Text] |
| 8. | Ash DV, Benson EA, Sainsbury JR, Round C, Head C. Seven-year follow-up on 334 patients treated by breast conserving surgery and short course radical postoperative radiotherapy: a report of the Yorkshire Breast Cancer Group. Clin Oncol (R Coll Radiol). 1995;7:93-96. [PubMed] [DOI] [Full Text] |
| 9. | Livi L, Stefanacci M, Scoccianti S, Dicosmo D, Borghesi S, Nosi F, Simontacchi G, Mangoni M, Paiar F, Ponticelli P. Adjuvant hypofractionated radiation therapy for breast cancer after conserving surgery. Clin Oncol (R Coll Radiol). 2007;19:120-124. [PubMed] [DOI] [Full Text] |
| 10. | Koukourakis MI, Tsoutsou PG, Abatzoglou IM, Sismanidou K, Giatromanolaki A, Sivridis E. Hypofractionated and accelerated radiotherapy with subcutaneous amifostine cytoprotection as short adjuvant regimen after breast-conserving surgery: interim report. Int J Radiat Oncol Biol Phys. 2009;74:1173-1180. [PubMed] [DOI] [Full Text] |
| 11. | Baillet F, Housset M, Maylin C, Boisserie G, Bettahar R, Delanian S, Habib F. The use of a specific hypofractionated radiation therapy regimen versus classical fractionation in the treatment of breast cancer: a randomized study of 230 patients. Int J Radiat Oncol Biol Phys. 1990;19:1131-1133. [PubMed] |
| 12. | Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331-341. [PubMed] [DOI] [Full Text] |
| 13. | Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss JM, Brown J, Dewar JA, Dobbs HJ. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098-1107. [PubMed] |
| 14. | International Commission on Radiation Units and Measurements (ICRU). Report 50. Prescribing, recording, and reporting photon beam therapy. Bethesda, MD: ICRU 1993; . |
| 15. | International Commission on Radiation Units and Measurements (ICRU). Report 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50). Bethesda, MD: ICRU 1999; . |
| 16. | Saibishkumar EP, MacKenzie MA, Severin D, Mihai A, Hanson J, Daly H, Fallone G, Parliament MB, Abdulkarim BS. Skin-sparing radiation using intensity-modulated radiotherapy after conservative surgery in early-stage breast cancer: a planning study. Int J Radiat Oncol Biol Phys. 2008;70:485-491. [PubMed] |
| 17. | Dijkema IM, Hofman P, Raaijmakers CP, Lagendijk JJ, Battermann JJ, Hillen B. Loco-regional conformal radiotherapy of the breast: delineation of the regional lymph node clinical target volumes in treatment position. Radiother Oncol. 2004;71:287-295. [PubMed] [DOI] [Full Text] |
| 18. | Hiatt JR, Evans SB, Price LL, Cardarelli GA, Dipetrillo TA, Wazer DE. Dose-modeling study to compare external beam techniques from protocol NSABP B-39/RTOG 0413 for patients with highly unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys. 2006;65:1368-1374. [PubMed] [DOI] [Full Text] |
| 19. | Williamson D, Dinniwell R, Fung S, Pintilie M, Done SJ, Fyles AW. Local control with conventional and hypofractionated adjuvant radiotherapy after breast-conserving surgery for ductal carcinoma in-situ. Radiother Oncol. 2010;95:317-320. [PubMed] [DOI] [Full Text] |
| 20. | Kurtz JM. The clinical radiobiology of breast cancer radiotherapy. Radiother Oncol. 2005;75:6-8. [PubMed] [DOI] [Full Text] |
| 21. | Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. [PubMed] [DOI] [Full Text] |
| 22. | Lievens Y. Hypofractionated breast radiotherapy: financial and economic consequences. Breast. 2010;19:192-197. [PubMed] [DOI] [Full Text] |
| 23. | Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9-17. [PubMed] [DOI] [Full Text] |
| 24. | COHEN L. Radiotherapy in breast cancer. I. The dose-time relationship theoretical considerations. Br J Radiol. 1952;25:636-642. [PubMed] [DOI] [Full Text] |
| 25. | Douglas BG, Castro JR. Novel fractionation schemes and high linear energy transfer. Prog Exp Tumor Res. 1984;28:152-165. [PubMed] |
| 26. | Williams MV, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys. 1985;11:87-96. [PubMed] [DOI] [Full Text] |
| 27. | Matthews JH, Meeker BE, Chapman JD. Response of human tumor cell lines in vitro to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1989;16:133-138. [PubMed] [DOI] [Full Text] |
| 28. | Joiner MC, Bentzen SM. Time-dose relationships: the linear-quadratic approach. Basic clinical radiobiology. London: Arnold 2002; 120-133. |
| 29. | Chougule A. Radiation dose to contralateral breast during treatment of breast malignancy by radiotherapy. J Cancer Res Ther. 2007;3:8-11. [PubMed] [DOI] [Full Text] |
| 30. | Fraass BA, Roberson PL, Lichter AS. Dose to the contralateral breast due to primary breast irradiation. Int J Radiat Oncol Biol Phys. 1985;11:485-497. [PubMed] [DOI] [Full Text] |
| 31. | Brooks PS. Dose to contralateral breast--a comparative study. Med Dosim. 1995;20:301-307. [PubMed] [DOI] [Full Text] |
| 32. | Boice JD, Harvey EB, Blettner M, Stovall M, Flannery JT. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781-785. [PubMed] [DOI] [Full Text] |
| 33. | Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038-1045. [PubMed] [DOI] [Full Text] |
| 34. | Unnithan J, Macklis RM. Contralateral breast cancer risk. Radiother Oncol. 2001;60:239-246. [PubMed] [DOI] [Full Text] |
| 35. | Dörr W, Herrmann T. Second primary tumors after radiotherapy for malignancies. Treatment-related parameters. Strahlenther Onkol. 2002;178:357-362. [PubMed] |
| 36. | Boice JD, Engholm G, Kleinerman RA, Blettner M, Stovall M, Lisco H, Moloney WC, Austin DF, Bosch A, Cookfair DL. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988;116:3-55. [PubMed] [DOI] [Full Text] |
| 37. | Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398-406. [PubMed] [DOI] [Full Text] |
| 38. | United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation. New York, NY: United Nations 1977; . |
| 39. | Hooning MJ, Aleman BM, Hauptmann M, Baaijens MH, Klijn JG, Noyon R, Stovall M, van Leeuwen FE. Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol. 2008;26:5561-5568. [PubMed] [DOI] [Full Text] |
| 40. | Bhatnagar AK, Heron DE, Deutsch M, Brandner E, Wu A, Kalnicki S. Does breast size affect the scatter dose to the ipsilateral lung, heart, or contralateral breast in primary breast irradiation using intensity-modulated radiation therapy (IMRT)? Am J Clin Oncol. 2006;29:80-84. [PubMed] [DOI] [Full Text] |
| 41. | Tercilla O, Krasin F, Lawn-Tsao L. Comparison of contralateral breast doses from 1/2 beam block and isocentric treatment techniques for patients treated with primary breast irradiation with 60CO. Int J Radiat Oncol Biol Phys. 1989;17:205-210. [PubMed] [DOI] [Full Text] |
| 42. | Muller-Runkel R, Kalokhe UP. Method for reducing scatter radiation dose to the contralateral breast during tangential breast irradiation therapy. Radiology. 1994;191:853-855. [PubMed] |
| 43. | Bhatnagar AK, Brandner E, Sonnik D, Wu A, Kalnicki S, Deutsch M, Heron DE. Intensity modulated radiation therapy (IMRT) reduces the dose to the contralateral breast when compared to conventional tangential fields for primary breast irradiation. Breast Cancer Res Treat. 2006;96:41-46. [PubMed] [DOI] [Full Text] |
| 44. | Bhatnagar AK, Brandner E, Sonnik D, Wu A, Kalnicki S, Deutsch M, Heron DE. Intensity-modulated radiation therapy (IMRT) reduces the dose to the contralateral breast when compared to conventional tangential fields for primary breast irradiation: initial report. Cancer J. 2004;10:381-385. [PubMed] [DOI] [Full Text] |
| 45. | Zygogianni AG, Kouvaris JR, Kouloulias V, Armpilia C, Antypas C, Vlachos L. Hypofractionated accelerated irradiation for stage I-II breast carcinoma: a phase II study. Breast J. 2010;16:337-338. [PubMed] [DOI] [Full Text] |
| 46. | Plataniotis G. Hypofractionated radiotherapy in the treatment of early breast cancer. World J Radiol. 2010;2:197-202. [PubMed] [DOI] [Full Text] |