Published online Apr 28, 2011. doi: 10.4329/wjr.v3.i4.114
Revised: January 25, 2011
Accepted: February 1, 2011
Published online: April 28, 2011
Spermatic cord leiomyosarcomas (LMSs) are rare tumors which may cause significant morbidity and mortality if inadequately diagnosed or treated. We report a case of a paratesticular LMS in a 60-year-old man who presented with a right scrotal mass. The patient was evaluated by scrotal ultrasound and computed tomography of the abdomen and pelvis (including scans of the scrotum), which revealed a large extratesticular mass. The lesion proved to be malignant and the patient underwent radical orchiectomy with high cord ligation. To improve the assignment of this lesion, we further analyze the imaging features of LMS and correlate them with pathologic findings.
- Citation: Kyratzi I, Lolis E, Antypa E, Lianou MA, Exarhos D. Imaging features of a huge spermatic cord leiomyosarcoma: Review of the literature. World J Radiol 2011; 3(4): 114-119
- URL: https://www.wjgnet.com/1949-8470/full/v3/i4/114.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i4.114
Leiomyosarcoma (LMS) accounts for 5%-10% of soft tissue sarcomas. However, LMS of the spermatic cord is rare and only approximately 110 cases have been reported in the literature so far[1]. The spermatic cord is the most common site of extratesticular neoplasia but only 30% of them are malignant, 90% of which are sarcomas. Approximately 10% of paratesticular sarcomas are LMSs[2]. This type of lesion is reported in all age groups but is mostly diagnosed in the 6th decade[3]. A case of LMS is presented with description of its imaging features [ultrasound (U/S), computed tomography (CT)].
A 60 year-old male presented at the outpatient clinic with a right hydrocele and a small right scrotal lump, 4 mo after having had a mesh repair of bilateral inguinal hernias and mesh repair of an incisional midline hernia. Further work up was recommended during the consultation but the patient did not comply. Eighteen months later he presented once more at the outpatient clinic with a slightly painful, firm and obviously larger than previously right scrotal mass. The patient denied any lower urinary tract symptoms. On physical examination a firm, lobulated mass was palpated in the right hemiscrotum extending proximal up to a few centimeters from the right external inguinal ring. The right spermatic cord was thick and hard on palpation. Recurrence of the right inguinal hernia with mass migration into the scrotum was excluded clinically. Right inguinal lymph nodes were palpated, but they were soft, painless and mobile.
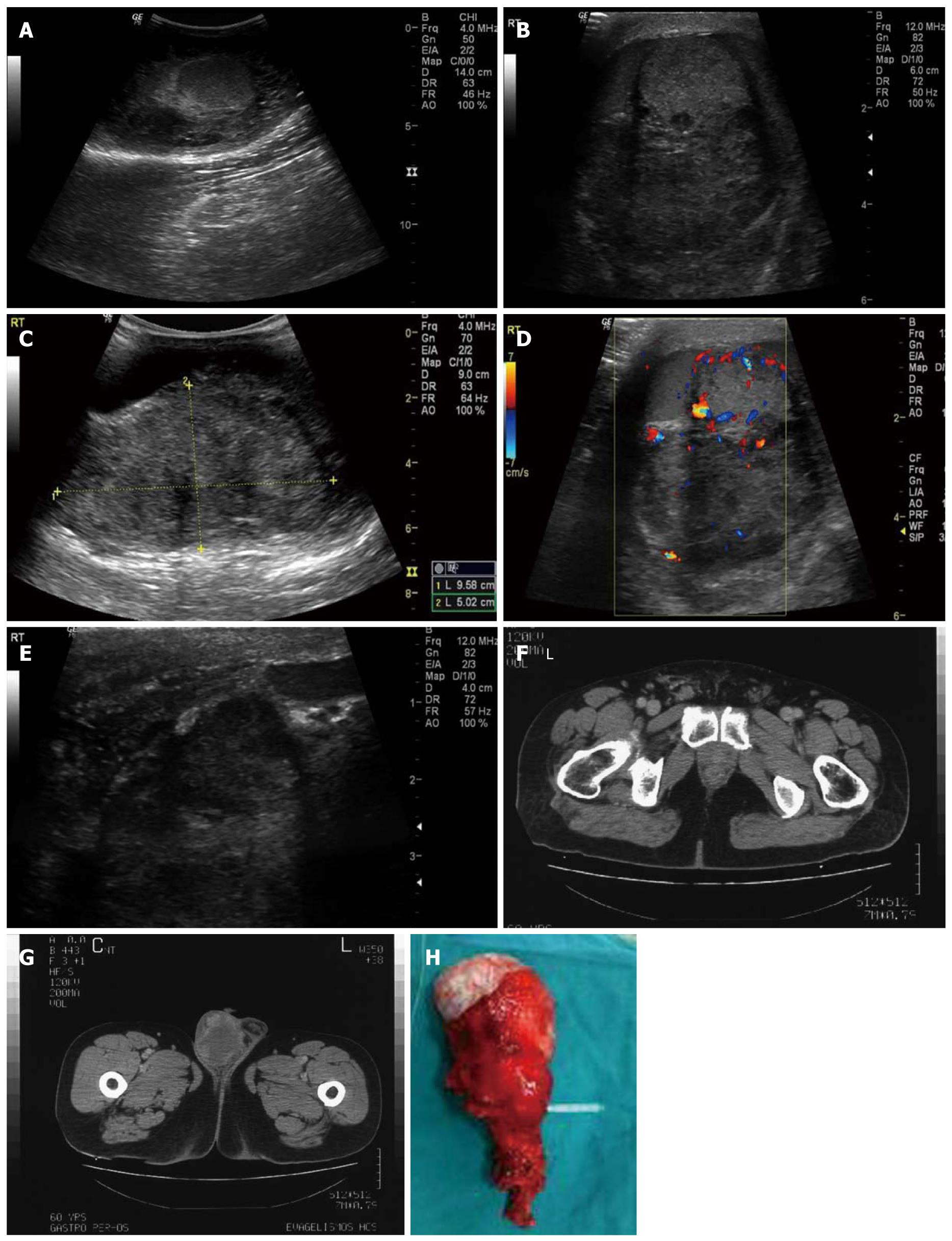
Scrotal ultrasound was performed using a 10 MHz linear transducer and a 4 MHz convex transducer, revealing a large mass of mixed echogenicity with calcifications, measuring approximately 10 cm × 5 cm × 8 cm, located posterior-superiorly to the right testis (Figure 1A). The mass was depicted in close proximity to the testis, without obvious signs of infiltration (Figure 1B and C). Colour Doppler revealed increased, mostly peripheral, irregular vascularity (Figure 1D), as well as dilatation and congestion of the vessels within the spermatic cord. The right testis had normal dimensions, echotexture and vascularity. It was impossible to visualize the right epididymis. A significant complex hydrocele was also present. The examination was further expanded into the inguinal canal, to exclude a possible groin hernia. There were no signs of hernia; the walls of the ductus deferens, however, were thick in comparison to the left side (Figure 1E). The wall of the right hemiscrotum was also thick. A few inguinal lymph nodes were detected, without signs of inflammation or infiltration. The findings of the left hemiscrotum were unremarkable. Even though the extratesticular location of the mass was indicative of a benign etiology, the irregularity of the vascularity along with the inability to depict the epididymis led us to the admission of the patient for further investigation.
All laboratory examinations were within normal limits, including α-fetoprotein and β-human chorionic gonadotropin (β-HCG). Plain chest X-ray was normal. A CT scan of the abdomen and the pelvis with intravenous contrast material was performed and the scrotum was also included. The right spermatic cord was edematous with dilated vessels along its course (Figure 1F). A mass with a greater diameter of approximately 10 cm was located in the right hemiscrotum, with peripheral enhancement after intra venous injection of contrast material (Figure 1G). Soft tissue densities (HU 20-65), which did not imply the presence of fat, were detected throughout the mass. A few inguinal lymph nodes were depicted but without suspicious characteristics of infiltration. No para-aortic or pelvic lymph nodes were detected. There was no evidence of other pathologic conditions. Malignancy was confirmed by a core biopsy.
A transinguinal right radical orchiectomy was carried out with high cord ligation. Two right inguinal lymph nodes were sampled. The patient’s post-operative course was uneventful.
Macroscopically, a firm, solid, grey-white tumor measuring 12 cm × 9.5 cm × 6 cm involved the right spermatic cord, displacing the testicle inferiorly, without invading it (Figure 1H). The right epididymis was not recognized. Seven neoplastic lesions along the spermatic cord with undefined borders were noted with diameters up to 2.5 cm.
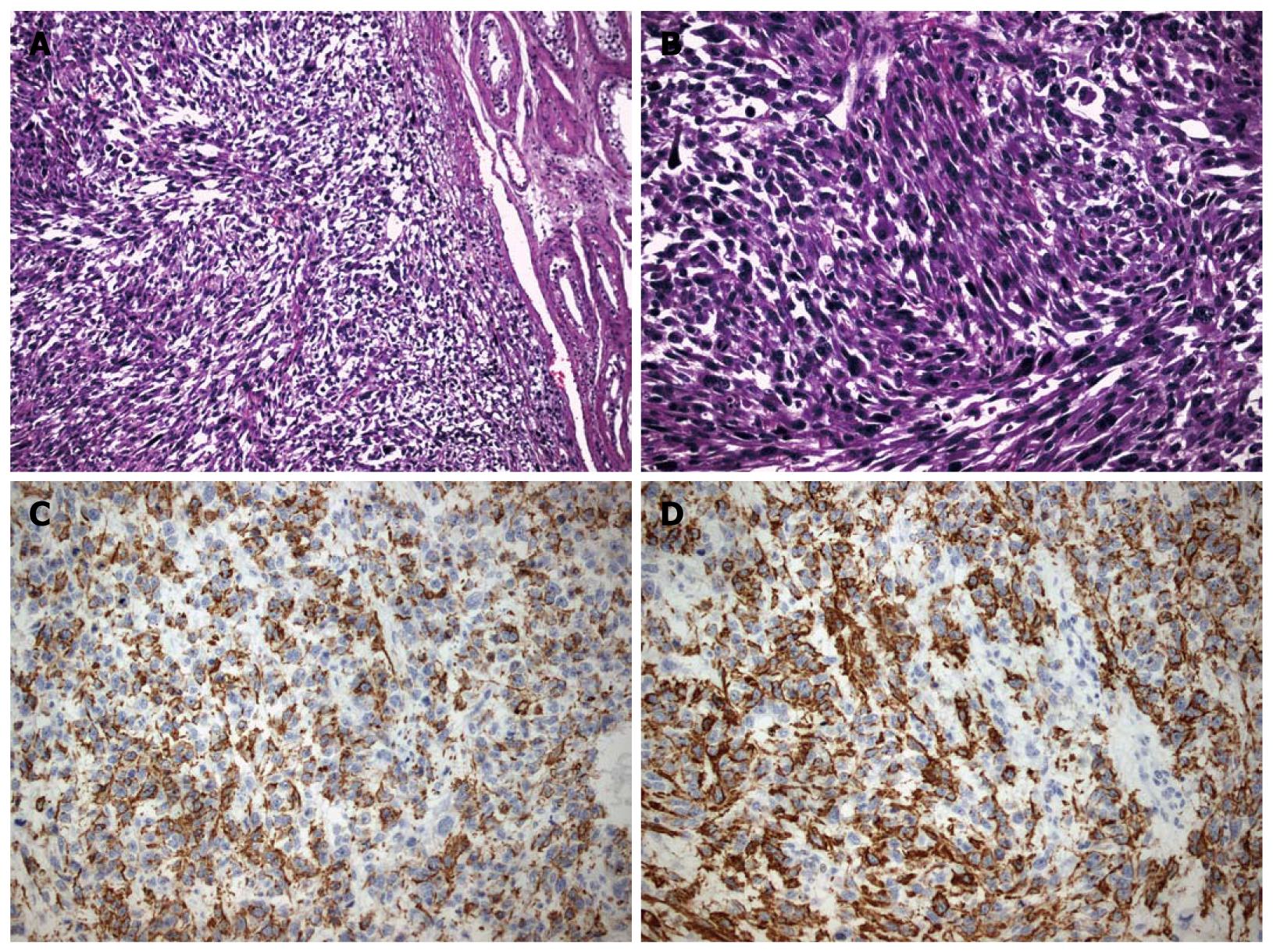
Microscopically, an adequate degree of cellular and nuclear atypia as well as pleomorphism were revealed. In addition to that, multiple mitoses and widespread, coagulant necrotic areas were seen. Immunohistochemical stain results were: desmin (+), specific muscle actin (a-SMA) (+), CD68 (+), myogenic differentiation 1 (MyoD1) (+), Caldesmon (-) (Figure 2A-D). The findings were compatible with the diagnosis of a highly malignant, grade III leiomyosarcoma of low differentiation. Other pathological findings concerning extension of the mass included multiple neoplastic emboli in the tumor periphery, infiltration of the vessel walls and absence of testicular or tunica infiltration. The sampled lymph nodes were not infiltrated.
Chest CT was performed after the pathological diagnosis was established and revealed multiple nodules throughout the parenchyma of the lungs, compatible with secondary deposits. It was not carried out immediately after malignancy was diagnosed by core biopsy, because it would not have changed the surgical plan. The operation could not have been avoided because the patient was symptomatic and the mass was locally advanced. Moreover, the operation aimed at controlling local disease, regardless secondary lung depositions. Nevertheless, due to the rarity of the disease, there were not sufficient data and clear guidelines regarding the surgical management of these tumors in relation to the stage.
Two courses of adjuvant chemotherapy were administered (doxorubicin hydrochloride, trabectedin), after which progressive disease was still detected on chest CT. A second line of chemotherapy (dacarbazine, cyclophosphamide, vincristine sulfate) was administered and the metastatic disease showed evidence of remission in follow up CT after the third course (approximately 6 mo after the operation). By the end of the sixth course of the second line of chemotherapy, however, progressive disease was detected (increase of the size and number of secondary lung depositions, as well as a block of external iliac lymph nodes). Chemotherapy was switched to paclitaxel and gemcitabine, which is currently administered to the patient (9 mo after the operation).
Malignant neoplasms of non testicular origin located in the scrotum are uncommon and are usually sarcomas. In a series of 1583 adult soft tissue sarcomas at the Memorial Sloan-Kettering Cancer Center, 43 were urological and 14 (0.8%) were paratesticular (5 rhabdomyosarcoma, 4 leiomyosarcoma, 3 liposarcoma, 1 malignant fibrous histiocytoma and 1 undifferentiated sarcoma)[4]. One of the largest series of solid extratesticular masses in literature with 91 patients included, all of whom underwent surgical resection, reports an overall malignancy rate of 3%[5]. However, another series of 19 patients with extratesticular masses evaluated with scrotal U/S, reports a malignancy rate of 16%, with a limitation of selection bias[6]. Even though a few reviews of a small number of series are available, LMS seems to be the second most common histological variety following liposarcoma, with a peak incidence in the sixth decade[7]. Most patients present with a painless or slightly painful mass in the scrotum as our patient did. Only one case was reported where the mass was extremely painful and this was related to overproduction of β-HCG[8].
LMS is the result of neoplastic transformation of smooth muscle cells or multi-potential mesenchymal cells in various sites of the body. Its behavior is related to the site, histological grade of the lesion and the presence of nodal or distant metastases. It is subdivided topographically into 3 groups: LMS of the deep soft tissue, LMS of the cutaneous and subcutaneous tissue and LMS of vascular origin. According to the American Joint Committee on Cancer Staging System, paratesticular sarcomas should belong to the deep subtype[9].
Paratesticular LMS originates from the spermatic cord, the scrotum (testicular tunica, dartos muscle and scrotal subcutis) or the epididymis. The most common type arises from undifferentiated mesenchymal cells of the cremasteric muscle and vas deferens. The epididymal form is less frequent and arises from the smooth muscle surrounding the basement membrane of the epididymis canal. The dartous layer is the origin of the scrotal types. The first two aforementioned types drain into the retroperitoneal lymph nodes in contrast with the last type, which drains into the inguinal, external and internal iliac nodes[10,11].
Grading of paratesticular LMS is based on the evaluation of the number of mitoses (the mean number of mitoses in 5 HPF [high power field] in a part of tumor with the highest mitosis rate and cellularity), the percentage of necrosis and the severity of nuclear pleomorphism[3]. This LMS was classified grade III due to its multiple necrosis, widespread necrotic areas nuclear atypia and nuclear pleomorphism.
Radical orchiectomy is the cornerstone of treatment in the management of this neoplasm, but the reported survival rates indicate the need for additional treatment[3,9]. It is important to note that negative histological margins are particularly hard to achieve during primary surgery[10]. Comprehension of the pattern of spread is essential, but this task is difficult by the rare occurrence of this disease. The most common means of dissemination are by regional lymph nodes spread (external, common iliac, hypogastric and retroperitoneal lymph nodes), haematogenous metastases (most commonly to the lungs) and by local extension (local infiltration of the scrotum, inguinal canal or pelvis, along the pathway of vas deferens)[11]. Involvement of the anterior abdominal wall is also possible[12]. In 1966, Kyle stated that the ratio of haematogenous to lymphatic spread is 3:1[13]. Further series suggested that lymph node dissection (especially retroperitoneal) should not be performed unless enlarged lymph nodes are encountered on CT scans or palpated during surgery[13,14].
Even though the study of a rare disease treated over several decades contains inherent biases that makes firm conclusions difficult to draw, the results of several studies suggest that adjuvant radiation, following radical orchiectomy, may control local microscopic disease and reduce the risk of locoregional relapse[10]. At present the role of chemotherapy remains controversial and restricted to the presence of metastatic disease[7].
In this case report, ultrasound examination of the patient revealed a heterogeneous mass, with calcifications and hypoechoic to anechoic areas, with irregular, mostly peripheral vascularity overtaking the right hemiscrotum, pressing the testicle inferiorly, without obviously obscuring its borders. It was impossible to depict the right epididymis. The majority of the LMSs described in the literature are heterogeneous lesions like the aforementioned[6,11,15,16] although some LMSs appear to be hypoechoic[8,14]. Calcifications are not mentioned in the majority of the cases described[1,2,6,8,11,14-16]. Colour Doppler ultrasonography shows either minimal[8], or increased vascularity[11,16]. The appearance is mostly related to the size of the lesion and the differentiation of the mesenchymal components[16]. This mass appears to be the largest LMS ever to have been reported in literature until now, with a maximum diameter of 12 cm. LMSs described until now ranged in size from 2-9 cm with a mean of 5 cm[8,17]. CT scan was not performed for the evaluation of the lesion itself, but in order to estimate the extent of disease, since the mass was suspected to be malignant. The only relevant bibliographic references besides staging concern the exclusion of the extension of retroperitoneal sarcoma into the scrotum[12]. A non-homogeneous mass with irregular, peripheral contrast enhancement and HU between 20 and 65, indicative of cystic, solid and calcified areas were found. A thickened and edematous spermatic cord with distended vessels was also depicted. The above CT findings parallel the sonographic ones. In addition to that, absence of areas with negative HU excluded the presence of fat within the lesion.
The afore-mentioned are pathologically correlated to necrotic areas within the tumor (cystic areas), infiltration of the epididymis (epididymis not visualized), origin from the spermatic cord (high position of the lesion within the scrotum), absence of testicular infiltration (definite testicular borders), neoplastic lesions within the spermatic cord (thick and edematous appearance) and infiltration of the vessels (vessel distention within the cord) (Table 1).
| U/S findings | CT findings | Pathologic findings |
| Hypoechoic to anechoic areas | Heterogeneous lesion with cystic areas | Necrotic areas |
| Disability to visualize the epididymis | Epididymal infiltration | |
| Location in the root of the hemiscrotum, superiorly to the testis | Location in the root of the hemiscrotum, superiorly to the testis | Spermatic cord origin |
| Definite testicular borders | No testicular infiltration | |
| Thick and edematous appearance of spermatic cord | Thick and edematous appearance of spermatic cord | Neoplastic lesions within the cord |
| Distended vessels within the cord | Distended vessels within the cord | Spermatic cord vessels’ infiltration |
| HU 20-65 | Cystic, solid and calcified areas - absence of fat |
Even though there are circumstances where MR imaging is very helpful in the assessment of the scrotum, since it is far more specific than U/S (depiction of lipomas, fibrous pseudotumors, polyorchidism), it was considered that it would not limit the aforementioned differential diagnosis[18] or change the surgical procedure.
Although the ultrasound findings alone should have raised the probability of malignancy, the differential diagnosis of the extratesticular lesions in general is not so limited. Apart from purely cystic extratesticular lesions (epididymal cyst, scrotal tunica cyst) most of the solid lesions, either benign (adenoid tumor, papillary epididymal cystadenoma, fibrous pseudotumor, inguinoscrotal hernia, lipoma, leiomyoma) or malignant (rhabdomyosarcoma, liposarcoma, leiomyosarcoma, mesothelioma), frequently have overlapping characteristics, making it extremely difficult to exclude malignancy[18,19]. Considering the above imaging features the mass was more compatible with a leiomyosarcoma (exclusion of rhabdomyosarcoma due to the age of the patient), even though the diagnosis of a benign leiomyoma or a fibrous pseudotumor could not be completely excluded[18-20].
In conclusion, dealing with an extratesticular lesion can be confusing and troublesome, especially when a young patient is involved. Malignant extratesticular tumors are rare, but even if the malignancy rate of these lesions is much lower than that of the intratesticular masses, it is high enough to be of concern. Sonography should be the initial imaging modality since it can determine the origin of the lesion and even though the imaging characteristics are not adequate to reach a single diagnosis, the heterogeneous appearance along with the irregular, often increased vascularity of the tumor may allow the diagnosis of a sarcoma. Correlation with case history of the patients and CT/MR findings can further limit the differential diagnosis and lead to a better management of the patient.
Peer reviewer: Ahmed A Shokeir, MD, PhD, FEBU, Professor, Urology Department, Urology and Nephrology Center, Mansoura University, Mansoura 35516, Egypt
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Vogelaar FJ, Schuttevaer HM, Willems JM. A patient with an inguinal mass: a groin hernia? Neth J Med. 2009;67:399-400. |
| 2. | Lopes RI, Leite KR, Lopes RN. Paratesticular leiomyosarcoma treated by enucleation. Int Braz J Urol. 2006;32:66-67. |
| 3. | Mohammadi Torbati P, Zham H. Epithelioid type of paratesticular leiomyosarcoma: a case report and literature review. Urol J. 2004;1:215-217. |
| 4. | Russo P. Urologic sarcoma in adults. Memorial Sloan-Kettering Cancer Center experience based on a prospective database between 1982 and 1989. Urol Clin North Am. 1991;18:581-588. |
| 5. | Beccia DJ, Krane RJ, Olsson CA. Clinical management of non-testicular intrascrotal tumors. J Urol. 1976;116:476-479. |
| 6. | Frates MC, Benson CB, DiSalvo DN, Brown DL, Laing FC, Doubilet PM. Solid extratesticular masses evaluated with sonography: pathologic correlation. Radiology. 1997;204:43-46. |
| 7. | Coleman J, Brennan MF, Alektiar K, Russo P. Adult spermatic cord sarcomas: management and results. Ann Surg Oncol. 2003;10:669-675. |
| 8. | Ou SM, Lee SS, Peng YJ, Sheu LF, Yao NS, Chang SY. Production of beta-HCG by spermatic cord leiomyosarcoma: a paraneoplastic syndrome? J Androl. 2006;27:643-644. |
| 9. | Alberghini M, Zanella L, Bacchini P, Maltarello MC, Maraldi NM, Bertoni F. Leiomyosarcoma of the spermatic cord: a light and ultrastructural description of one case. Pathol Res Pract. 2004;200:487-491. |
| 10. | Fagundes MA, Zietman AL, Althausen AF, Coen JJ, Shipley WU. The management of spermatic cord sarcoma. Cancer. 1996;77:1873-1876. |
| 11. | Dangle P, Basavaraj DR, Bhattarai S, Paul AB, Biyani CS. Leiomyosarcoma of the spermatic cord: case report and literature review. Can Urol Assoc J. 2007;1:55-58. |
| 12. | Cardenosa G, Papanicolaou N, Fung CY, Tung GA, Yoder IC, Althausen AF, Shipley WU. Spermatic cord sarcomas: sonographic and CT features. Urol Radiol. 1990;12:163-167. |
| 13. | Kyle VN. Leiomyosarcoma of the spermatic cord: a review of the literature and report of an additonal case. J Urol. 1966;96:795-800. |
| 14. | Stein A, Kaplun A, Sova Y, Zivan I, Laver B, Lurie M, Lurie A. Leiomyosarcoma of the spermatic cord: report of two cases and review of the literature. World J Urol. 1996;14:59-61. |
| 15. | Watanabe J, Soma T, Kawa G, Hida S, Koisi M. Leiomyosarcoma of the spermatic cord. Int J Urol. 1999;6:536-538. |
| 16. | Secil M, Kefi A, Gulbahar F, Aslan G, Tuna B, Yorukoglu K. Sonographic features of spermatic cord leiomyosarcoma. J Ultrasound Med. 2004;23:973-976; quiz 977-978. |
| 17. | Fisher C, Goldblum JR, Epstein JI, Montgomery E. Leiomyosarcoma of the paratesticular region: a clinicopathologic study. Am J Surg Pathol. 2001;25:1143-1149. |
| 18. | Woodward PJ, Schwab CM, Sesterhenn IA. From the archives of the AFIP: extratesticular scrotal masses: radiologic-pathologic correlation. Radiographics. 2003;23:215-240. |
| 19. | Kim W, Rosen MA, Langer JE, Banner MP, Siegelman ES, Ramchandani P. US MR imaging correlation in pathologic conditions of the scrotum. Radiographics. 2007;27:1239-1253. |
| 20. | Garriga V, Serrano A, Marin A, Medrano S, Roson N, Pruna X. US of the tunica vaginalis testis: anatomic relationships and pathologic conditions. Radiographics. 2009;29:2017-2032. |