Revised: December 7, 2010
Accepted: December 14, 2010
Published online: January 28, 2011
Pancreatic neuroendocrine tumors (PNETs) are an uncommon malignancy, accounting for a small percentage of all pancreatic malignancies. Due to their insidious course, most PNETs present with metastatic disease. Although reports in the literature describe PNET metastasis to the liver, lung and brain, to date there are no reports of stage IV disease involving the breast. Moreover, the lack of consensus regarding classification and treatment of this entity leaves practitioners without standards of practice or a firm base from which to formulate prognosis. In this report, the case of a previously healthy 51-year-old woman with stage IV PNET is examined. After combined neoadjuvant therapy with 5-fluorouracil, carboplatin, etoposide and radiation, surgical resection revealed metastatic PNET to the breast and lung, with no microscopic evidence of residual disease within the pancreas. An extensive analysis of the presentation, diagnosis, imaging modalities, treatment options, and prognosis is included in the discussion. As demonstrated by our review, there is a need for further studies to delineate inconclusive evidence with respect to subtype classification, treatment and prognosis of PNETs.
- Citation: Satahoo-Dawes S, Palmer J, III EWM, Levi J. Breast and lung metastasis from pancreatic neuroendocrine carcinoma. World J Radiol 2011; 3(1): 32-37
- URL: https://www.wjgnet.com/1949-8470/full/v3/i1/32.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i1.32
Neuroendocrine cells are widely distributed throughout the body. Common sites include the lung, gastrointestinal tract, adrenal gland and thyroid gland; any of which may give rise to malignant neoplasms. Pancreatic neuroendocrine tumors (PNETs) are thought to develop from embryonic neural crest cells[1] that later give rise to islet cell tissue. It is believed that these cells belong to the amine precursor uptake decarboxylase system[2-4]. PNETs are an uncommon malignancy, accounting for 1%-5% of all pancreatic malignancies[4-8]. The estimated incidence is 1-5 cases per million[5,7,9]. However, incidence rates of 1-1.5 cases per 100 000 population have been reported[8,10]. Abood et al[4] and You et al[8] have reported approximately 2500 cases per year in the United States, with a peak age of 30-60 years, and no gender preference. PNETs are classified as functioning or non-functioning depending on the presence of clinical manifestations secondary to increased hormonal secretion, with multiple hormones being common[11]. Non-functioning PNETs are the result of tumors that either produce inert hormones or subclinical concentrations of active hormones without symptoms. Non-functioning PNETs account for 15%-52% of all pancreatic endocrine tumors[2,4,6,7,12-14].
In 2000, the World Health Organization (WHO) classified gastroenteropancreatic neuroendocrine tumors based on histological and pathological characteristics into well-differentiated, poorly differentiated, and mixed endocrine-exocrine subtypes[2,15]. Other classifications grade PNETs based on behavior as low, intermediate, or high grade lesions[2,16]. Histologically, PNETs show features similar to small cell carcinoma of the lung, lymphoma, and neuroendocrine tumors of the stomach and colon. PNETs have a more indolent course than the more common pancreatic adenocarcinoma with a longer survival, although 50%-75% present with metastatic disease[2,4,6,9]. Common PNET metastases occur in lymph nodes, liver, spleen, and bone at 50%, 30%, 10% and 7%, respectively[17]. Solorzano et al[18] have described metastasis to lung and brain as well. The malignancy rate is often > 50%[2,4,7,9,19], with a mean survival of 6-8 years even in widely metastatic disease[2,10].
A literature search of Medline and Embase has revealed no cases of PNETs metastatic to the breast. Thus, the present case broadens the discussion because it describes the clinical course of a 51-year-old woman with a poorly differentiated pancreatic neuroendocrine carcinoma metastatic to the breast and lung. Additionally, this case illustrates the successful management of stage IV disease with neoadjuvant chemotherapy and radiotherapy, followed by radical surgical resection. The benefit of combined, aggressive medical and surgical management is highlighted, as well as an interesting parallel to the management of metastatic liver disease.
A previously healthy 51-year-old, Caucasian woman presented to her primary care physician with a 1-wk history of bloating and right abdominal discomfort, with radiation to the back. Initial imaging by abdominal ultrasound showed a pancreatic head mass with associated biliary dilatation, which was highly suspicious for primary pancreatic carcinoma. Further assessment by computed tomography (CT) revealed a 5 cm × 5 cm well-circumscribed pancreatic head lesion in the c-loop of the duodenum, which appeared to have a well-circumscribed edge or capsule. Of note, the lesion was not clearly cystic and had solid heterogeneous enhancement. Initial laboratory tests were significant for elevated amylase and lipase (598 IU/L and 1802 IU/L, respectively), along with elevated aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase (86 IU/L, 129 IU/L and 334 IU/L, respectively) and elevated total bilirubin at 2.9 mg/dL, despite normal prothrombin and partial thromboplastin time. Tumor markers at the time were significant for elevated visceral cancer markers: carbohydrate antigen (CA) 19-9 (81 IU/mL) and CA 125 (39 IU/mL). CA 27.29 and carcinoembryonic antigen (CEA) were within normal limits.
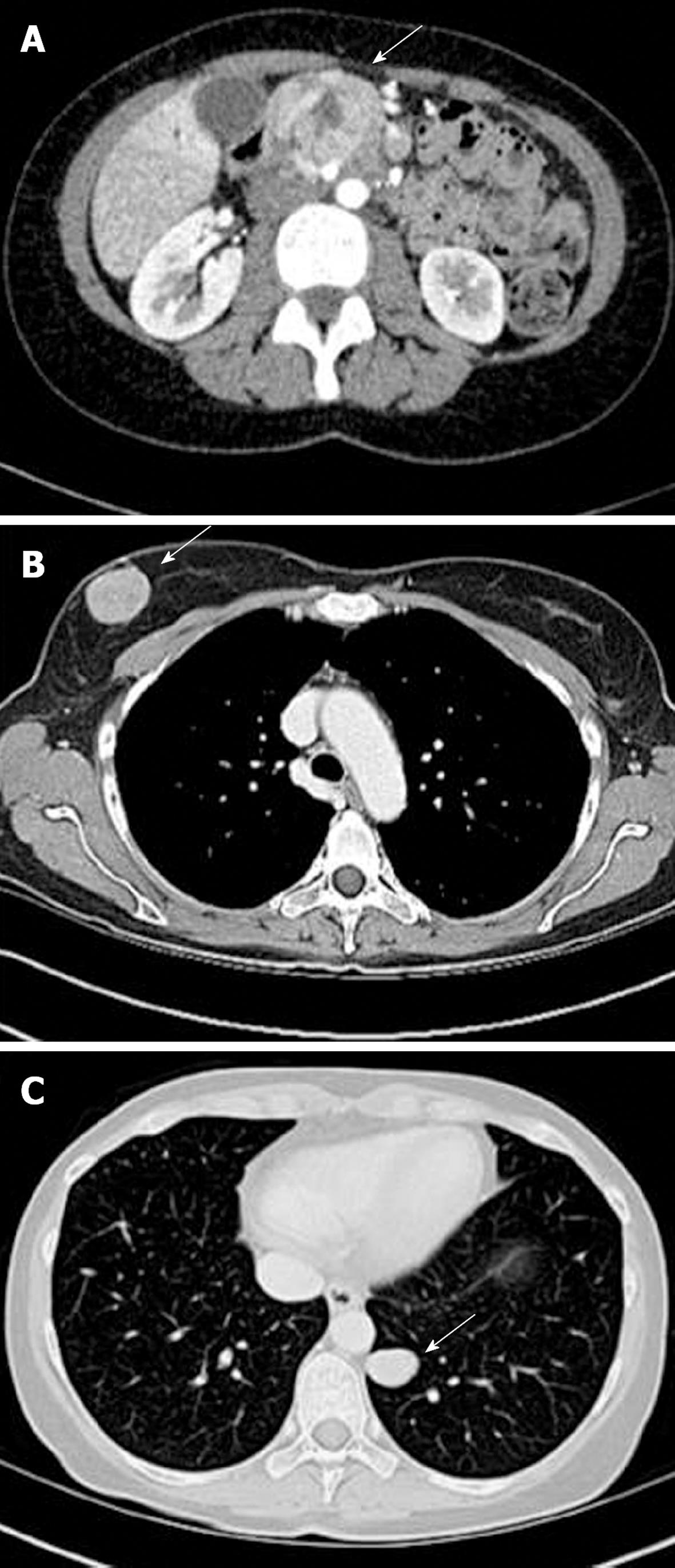
During the work-up of the pancreatic mass, the patient developed a stabbing breast pain and a new lump in the right breast. A diagnostic mammogram showed a new lobular mass in the right breast that was not present a year before. Positron emission tomography (PET)/CT was done to stage the disease process. There was abnormal activity corresponding to a large, ill-defined mass in the head of the pancreas, as well as within a 2.7-cm nodule in the left lower lobe of the lung and a 2.5-cm nodule in the superior aspect of the right breast (Figure 1). Both the lung and breast lesions were suspected to be metastatic nodules from the primary pancreatic mass. With these findings, stage IV carcinoma was diagnosed.
One month after the onset of symptoms, the patient underwent endoscopic retrograde cholangiopancreatography with stent placement to improve biliary obstruction. During the procedure, a single 40-mm region of stenosis was noted in the distal third of the main duct, with diffuse dilation in the middle third. Pancreatic biopsy revealed cellular evidence of poorly differentiated carcinoma, with non-contributory immunostaining. In addition, a right breast core biopsy demonstrated poorly differentiated carcinoma. Subsequent immunostaining for CD10 and epithelial membrane antigen (EMA) was positive, with stains for RCA and ER being negative. As such, the breast mass was considered to be immunohistochemically consistent with metastatic renal cell carcinoma. This pathology was later reviewed at an outside facility and described as a well-differentiated endocrine neoplasm with clear cell features. There was no prior clinical or radiographic evidence of renal involvement, therefore, renal cell carcinoma was unlikely. An octreotide scan, done 2 mo after the biopsy, was positive for uptake in the large pancreatic mass and right breast lesion. Given the results of this scan, a diagnosis of neuroendocrine carcinoma was favored by the consultant pathologist.
Shortly after the octreotide scan, the patient developed life-threatening gastrointestinal hemorrhage as the pancreatic tumor eroded into the duodenum. She subsequently underwent angiography and embolization of branched tumor vessels of the celiac, gastroduodenal and superior mesenteric arteries. She developed diarrhea and was started on 5-fluorouracil (5-FU) and octreotide (Sandostatin), for its postulated symptomatic relief and antitumor effect. She remained on Sandostatin LAR 30 mg once monthly for 6 mo. In an attempt to reduce tumor size, neoadjuvant chemotherapy and radiation were initiated. She underwent radiation for 3 mo and received three cycles of 5-FU followed by three cycles of carboplatin and etoposide (Appendix A). Bone marrow stimulation was administered via a combination of filgrastim, pegfilgrastim and darbepoetin alfa.
Surveillance CT scans were obtained at 5 mo post-presentation and again at 7 mo, just 3 wk before her first operation. During this 2-mo interval, there was progression of the right breast mass from 2.5 cm at presentation to 4.1 cm at 5 mo and 4.5 cm at 7 mo. The lesion in the left lung was reduced in size from 2.7 cm at presentation to 2.1 cm and remained relatively unchanged at 2.2 cm 7 mo later. Of note, there were small bilateral pulmonary nodules seen on the second scan at 5 mo. Finally, the mass at the head of the pancreas was reduced from 5 cm at presentation to 3.4 cm 7 mo later. A benign hemangioma of the left liver lobe was also noted on her 5-mo scan, which was the only liver finding.
Her first operation was a combined robotic video-assisted thoracoscopy with mini-thoractomy, resection of anterior basilar segment of the left lower lobe of the lung, and a right breast lumpectomy. There were no known post-surgical complications. The final pathology report of the right breast mass revealed a poorly differentiated carcinoma with clear cell features with positive margins, whereas the lung segment showed poorly differentiated malignant neoplasm with spindle and clear cell features with clear margins. Both specimens were thought to favor the diagnosis of poorly differentiated neuroendocrine carcinoma. Immunohistochemical staining of both specimens revealed some variability (Table 1). However, there was enough histological similarity to consider these two lesions to be from the same primary site.
| Staining | Breast | Lung |
| NSE | Positive | Positive |
| CD 56 | Positive | Positive |
| HMB-45 | Positive | Positive |
| CD10 | Positive | Positive |
| MIB-1 | High index | High index |
| EMA | Negative | Negative |
| S-100 | Negative | Negative |
| Melan A | Negative | Negative |
| Synaptophysin | Negative | Negative |
| Chromogranin | Negative | Negative |
| AE1-3 | Weakly reactive | Negative |
| CAM 5.2 | Weakly reactive | Negative |
Three weeks later, in preparation for her Whipple procedure (pancreatoduodenectomy), she underwent another CT scan of the chest, abdomen and pelvis. Imaging demonstrated that the previously visualized pulmonary nodule in the left lower lobe was resected, with no new pulmonary nodules. The liver had a cystic lesion that was consistent with a hemangioma, but no new lesions were seen. Additionally, there was still some enlargement of the pancreatic head mass, although it was smaller compared to a prior study (now 2.9 cm from 3.4 cm). The pancreas was also atrophic from mid-body to tail with no lymphadenopathy. Laboratory tests at that time were within normal limits, demonstrating resolution of previously elevated liver enzymes, lactate dehydrogenase, serotonin, uric acid, and chromogranin A.
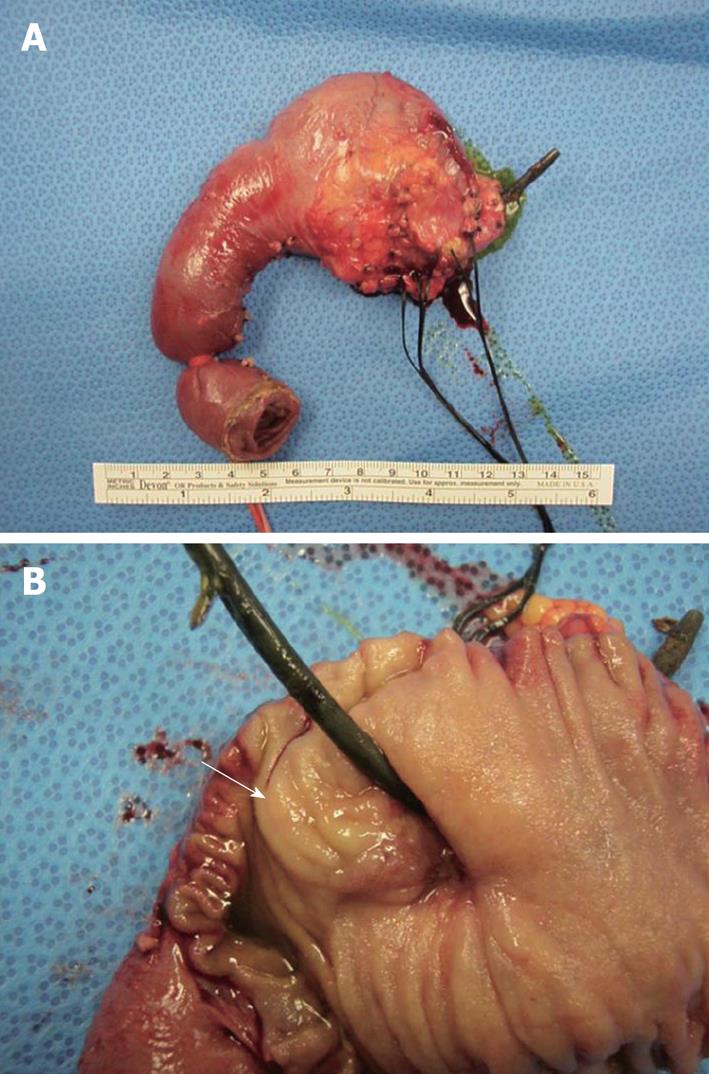
Nine months after presentation, the patient had a pylorus-sparing Whipple procedure and intraoperative ultrasound of the liver (Figure 2). Imaging showed a marked reduction in the size of her primary pancreatic head lesion, with atrophic body and tail. She also had a hemangioma in the left lobe of the liver and a cyst in the right lobe. The pancreatic specimen had no evidence of viable tumor, with clear margins and negative nodes.
The postoperative course was uncomplicated and the patient was discharged to home 1 wk later. One month after the operation, she is doing well and will undergo another round of chemotherapy with carboplatin and gemcitabine.
Most PNETs are diagnosed incidentally as a part of the workup for non-specific abdominal pain or mass effect leading to biliary or bowel obstruction[4]. General presenting symptoms include abdominal pain, weight loss, palpable mass and jaundice[6,8,16]. With lesions in the head of pancreas, there can be gastrointestinal bleeding secondary to erosion in the duodenum, as well as gastric or biliary outlet obstruction[18]. The patient presented with many of these symptoms including abdominal pain, weight loss, biliary obstruction and gastrointestinal bleeding.
As initial imaging, transabdominal ultrasound may be done. It is relatively inexpensive and widely available, but has a reported sensitivity of only 9%-64%[6]. Therefore, further imaging modalities may still be necessary. CT scanning also may be done as initial imaging to localize and stage the disease. It also can be used to assess liver metastases and pancreatic lymph node involvement[9]. Magnetic resonance imaging (MRI) also is helpful to evaluate metastatic disease, and to delineate the mass in relation to the pancreatic duct and major vessels[9]. MRI has been shown to be generally effective at detecting lesions > 1 cm, with 50% effectiveness in detecting lesions between 1 and 2 cm[6]. Somatostatin receptor scintigraphy or octreotide scanning has been deemed to be the most sensitive imaging modality[4] because it can effectively localize and stage disease by detecting primary and metastatic tumors > 1 cm[4,9]. It is especially good at detecting hepatic and bone metastases; however, it provides no information about tumor size or resectability[6]. Endoscopic ultrasound allows for visualization of small tumors in the head, uncinate and body of the pancreas[9]. It has been reported to be capable of detecting lesions as small as 0.5 cm[6]. It allows for visualization of lymph nodes, with a sensitivity of 58%[9]. Additionally, it is effective at visualizing the walls of the stomach, duodenum and adjacent structures[6]. Intraoperative ultrasound is used to evaluate non-palpable tumors, as well as the ducts, vessels, duodenal wall and lymph nodes[9]. Other modalities include PET, radiolabeled imaging selective venous sampling and selective pancreatic angiography. Imaging for this patient included many of these modalities: transabdominal ultrasound as initial evaluation, CT scan for initial and follow-up imaging, octreotide scan for localization, and intraoperative ultrasound for evaluation during resection. In this particular case, there was moderate concordance among these particular imaging modalities in determining the appearance and location of the primary cancer and its metastases.
Several studies have reported a difference in tumor size based on tumor type and stage. Hochwald et al[16] have reported the median size of non-functioning tumors to be 4.7 cm as opposed to 1.6 cm seen in functioning tumors (P = 0.005). O'Grady et al[6] have noted similar findings of 4.0 cm for non-functioning tumors vs 1.9 cm for functional tumors. Additionally, You et al[8] have reported that the average size of malignant tumors is 3.5 cm compared to 2.5 in well-differentiated tumors (P = 0.001). In consideration for metastatic disease, Bruzoni et al[2] have reported a median size of 7.3 cm vs 4.7 cm in patients without metastatic disease (P < 0.05). Additionally, the majority of these lesions are found in the pancreatic head[4,6,9]. These dimensions are consistent with this case because our patient presented with a 5-cm mass in the head of the pancreas.
PNETs have features similar to small cell cancer of the lung, as well as neuroendocrine tumors at other sites. Immunohistochemistry can help to differentiate between these tumors. PNETs commonly have granules that contain neuron specific enolase (NSE), synaptophysin and chromogranin[4]. As such, these tumors commonly stain positive for NSE and synaptophysin, with poor expression of chromogranin, sometimes staining negative in carcinomas[11]. Lloyd has suggested that thyroid factor-1 distinguishes lung from pancreatic primary tumor, and that stains for CD45, CD20 and CD45RO can help to distinguish pancreatic tumors from lymphomas[3]. Despite being negative for synaptophysin, immunohistochemical staining of the breast and lung lesions was positive for NSE, which is consistent with a PNET.
Serum markers also can be used to detect disease and monitor its course. They are particularly helpful in monitoring disease progression, relapse and burden. Chromogranin A has been considered to be the most sensitive marker for PNETs[10]. In fact, it is elevated in 60%-100% of patients[9]. Although the exact initial level of chromogranin A is unavailable, it was reported to be elevated in our patient, as expected. Of note, after octreotide therapy, the serum chromogranin level normalized and staining of the breast and lung specimens was negative. These were not unexpected findings because octreotide can affect the synthesis and release of chromogranin A in granules[20]. This effect on the granules could also explain the negative staining for synaptophysin. In addition to measuring these markers of PNETs, it is important to rule out other sites of primary disease. Markers such as CEA, CA 19-9, CA 125 and others should be measured.
The treatment of metastatic PNETs is under much debate with some studies recommending aggressive treatment, and others, observation. There are no published guidelines, and there is very little Level I evidence on a proper treatment plan for these tumors. Hence, treatment is approached on an individual basis with consideration of disease burden and comorbidity[2]. Treatment includes somatostatin analogs, interferon-α, site-directed radioablation, cytotoxic chemotherapy, and molecular targeted therapy. Somatostatin analogs, such as octreotide, have been shown to result in tumor stabilization and symptom relief in some patients[11]. Clancy et al[10] have indicated that analogs only rarely lead to tumor regression and that these drugs may lose efficacy over time. Octreotide is given two or three times per day for 2 wk; then the long-acting drug is dosed at 10, 20 or 30 mg monthly. Chemotherapy with streptozotocin and 5-FU or doxorubicin has been used as first-line treatment[11], with response rates varying between 39% and 69%[4,7]. However, in patients with more malignant disease, therapy with cisplatin and etoposide has shown a response in 41%-67% of patients with poorly differentiated PNETs[11,20] and thus is used as initial therapy in these patients. Despite metastatic disease, our patient had significant improvement with 5-FU in combination with carboplatin (which has similar pharmacology to cisplatin), etoposide and octreotide. As a result, she serves as a good example of the benefits of aggressive medical and surgical management. In continuation of therapy, given the history of small bilateral pulmonary nodules and microscopically positive breast lesion margins, she will also receive postoperative chemotherapy with carboplatin and gemcitabine in an attempt to increase survival in this patient with R0 resection of the primary tumor.
No clear prognostic factors have been determined. However, many researchers have presented a variety of factors that could be used to assess survival. Prognosis can be stratified based on criteria from the WHO, which takes into account stage- and grade-related factors: tumor size, distant metastasis, mitotic rate, necrosis, and patient age[20]. Similarly, an analysis using the National Cancer Database of 3851 patients with PNETs has concluded that age, tumor grade, and distant metastasis were the most significant predictors of survival[5]. A study of 137 patients described by Clancy et al[10] has confirmed age as a strong prognostic factor, as demonstrated by both univariate and multivariate survival analysis (P < 0.0003)[10]. Of interest, one article stated that age is not a significant prognostic factor if the patient is found to be medically fit for surgery[7]. The analysis done by Clancy et al[10] also showed that chromogranin A level > 500 ng/mL was associated with decreased survival from the date of evaluation, on univariate survival analysis (P < 0.030). Additionally, it was proposed that elevated alkaline phosphatase levels (> 127 U/L) could serve as a marker of prognosis, because they were associated with shorter survival by both univariate and multivariate survival analysis. Kaifi et al[12] have evaluated 63 patients to determine L1, a cell adhesion molecule, as a possible prognostic marker of poorly differentiated carcinoma. In this study, there was no difference between primary and metastatic specimens; also normal tissue did not stain positive, while 44% of poorly differentiated carcinoma specimens stained positive, as opposed to 1.9% in well-differentiated carcinomas. A study by Strosberg et al[21] has proposed that the mitotic rate and Ki-67 index (measured using MIB-1) could be related to tumor grade and thus could be used as prognostic factors. Using a Ki-67 index of 0%-2% as low grade, 2%-20% as intermediate grade, > 20% as high grade, there was an inverse relationship between Ki-67 index and mitotic rate with survival. This was supported by Hochwald et al[16] who have reported that MIB-1 index and necrosis show a difference in survival. The significance between functional and non-functional tumors is controversial because some studies have concluded no significance[2,6], whereas others have reported that non-functional tumors have decreased survival[10]. Additionally, extensive metastatic disease has been associated with a decrease in survival rates[2]. From a surgical standpoint, Solorzano et al[18] have concluded that the ability to resect the primary tumor and extent of metastatic disease are powerful predictors of outcome (P < 0.0001 for both variables). Given the variability of these factors, it would be difficult to determine prognosis in this relatively young asymptomatic patient with a high grade, metastatic, non-functioning malignancy that underwent R0 resection of the primary tumor, a high index of MIB-1, and elevated alkaline phosphatase. The previously mentioned study by Bilimoria et al[5] has formulated a prognostic score to be used to estimate 5-year survival. A score is determined by assigning points to each prognostic factor: age, grade and distant metastasis. Using this model, our patient had a prognostic score of 3, which was associated with a 5-year survival of 35.7%. However, this model does not account for the aggressive treatment that she received. Despite this aggressive treatment, it is important that our patient has long-term follow-up to monitor for further metastases, especially of the liver, and for disease recurrence.
From our literature review, there is a necessity for further investigations to understand fully the spectrum of gastroenteropancreatic neuroendocrine tumors, especially with regard to the determination of prognosis. Our case supports the proposed utility and effectiveness of the various imaging modalities. It also provides evidence for the benefit of aggressive medical and surgical management, as well as the response of poorly differentiated PNETs to combination treatment. Currently, the patient is doing well and she will be routinely followed to monitor her disease course.
Treatment was initiated with three cycles of 5-FU; the first dose being 360 mg/d × 3 d followed by 360 mg/d × 5 d, and finally, 360 mg/d × 4 d, each 1 wk apart. One month after completing 5-FU treatment, the patient received three cycles of carboplatin and etoposide; the first cycle was 900 mg/m2 of carboplatin and etoposide at 160 mg/m2 per day × 3 d. The remaining two cycles were completed 4 wk and 7 wk later with the regimen reduced to 720 mg/m2 of carboplatin and 130 mg/m2 per day × 3 d of etoposide. Filgrastim 273 μg every 3 d was started after the first round of carboplatin and etoposide for a total of four doses. At the end of both the second and third rounds of chemotherapy, a 6-mg dose of pegfilgrastim was received. Finally, 3 wk after completing the first round of carboplatin and etoposide, darbepoetin α was started with a total of three doses received; each 500 μg was received at 3-wk intervals, with the last dose being received at the completion of therapy.
Peer reviewer: Ian C Roberts-Thomson, Professor, Department of Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South, 5011, Australia
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Tonnhofer U, Balassy C, Reck CA, Koller A, Horcher E. Neuroendocrine tumor of the common hepatic duct, mimicking a choledochal cyst in a 6-year-old child. J Pediatr Surg. 2009;44:E23-E25. |
| 2. | Bruzoni M, Parikh P, Celis R, Are C, Ly QP, Meza JL, Sasson AR. Management of the primary tumor in patients with metastatic pancreatic neuroendocrine tumor: a contemporary single-institution review. Am J Surg. 2009;197:376-381. |
| 3. | Lloyd RV. Endocrine pathology: differential diagnosis and molecular advances. New Jersey: Hartman Press Inc 2004; 325. |
| 4. | Abood GJ, Go A, Malhotra D, Shoup M. The surgical and systemic management of neuroendocrine tumors of the pancreas. Surg Clin North Am. 2009;89:249-266, x. |
| 5. | Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490-500. |
| 6. | O'Grady HL, Conlon KC. Pancreatic neuroendocrine tumours. Eur J Surg Oncol. 2008;34:324-332. |
| 7. | Hodul PJ, Strosberg JR, Kvols LK. Aggressive surgical resection in the management of pancreatic neuroendocrine tumors: when is it indicated? Cancer Control. 2008;15:314-321. |
| 8. | You DD, Lee HG, Paik KY, Heo JS, Choi SH, Choi DW. The outcomes after surgical resection in pancreatic endocrine tumors: an institutional experience. Eur J Surg Oncol. 2009;35:728-733. |
| 9. | Mullan MH, Gauger PG, Thompson NW. Endocrine tumours of the pancreas: review and recent advances. ANZ J Surg. 2001;71:475-482. |
| 10. | Clancy TE, Sengupta TP, Paulus J, Ahmed F, Duh MS, Kulke MH. Alkaline phosphatase predicts survival in patients with metastatic neuroendocrine tumors. Dig Dis Sci. 2006;51:877-884. |
| 11. | Granberg D, Öberg K. Neuroendocrine tumours. Update Cancer Ther. 2006;1:75-84. |
| 12. | Kaifi JT, Zinnkann U, Yekebas EF, Schurr PG, Reichelt U, Wachowiak R, Fiegel HC, Petri S, Schachner M, Izbicki JR. L1 is a potential marker for poorly-differentiated pancreatic neuroendocrine carcinoma. World J Gastroenterol. 2006;12:94-98. |
| 13. | Kouvaraki MA, Solorzano CC, Shapiro SE, Yao JC, Perrier ND, Lee JE, Evans DB. Surgical treatment of non-functioning pancreatic islet cell tumors. J Surg Oncol. 2005;89:170-185. |
| 14. | Thompson GB, van Heerden JA, Grant CS, Carney JA, Ilstrup DM. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104:1011-1017. |
| 15. | Solcia E, Kloppel G, Sobin LH. Histological typing of endocrine tumours. Berlin: Springer 2000; . |
| 16. | Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633-2642. |
| 17. | Unger P. Pathology of Pancreatic Endocrine Neoplasia. Endocrine surgery. New York: Marcel Dekker Inc 2004; 500-520. |
| 18. | Solorzano CC, Lee JE, Pisters PW, Vauthey JN, Ayers GD, Jean ME, Gagel RF, Ajani JA, Wolff RA, Evans DB. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078-1085. |
| 19. | Gomez-Rivera F, Stewart AE, Arnoletti JP, Vickers S, Bland KI, Heslin MJ. Surgical treatment of pancreatic endocrine neoplasms. Am J Surg. 2007;193:460-465. |
| 20. | Kulke M. Management of metastatic gastroenteropancreatic neuroendocrine tumors. Waltham, MA: UpToDate 2009; . |
| 21. | Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262-1268. |