Published online Aug 28, 2010. doi: 10.4329/wjr.v2.i8.323
Revised: June 7, 2010
Accepted: June 14, 2010
Published online: August 28, 2010
Tracheobronchial balloon dilation and stent placement have been well used in the treatment of patients with benign and/or malignant diseases. Balloon dilation is the first option in the treatment of benign airway stenosis. Although balloon dilation is simple and fast, recurrence rate is high. Stent placement promptly relieves acute airway distress from malignant extraluminal and intraluminal airway obstruction. Temporary stent placement may be an alternative for benign airway strictures refractory to balloon dilation. This article reviews the indications, pre-procedure evaluation, technique, outcomes and complications of balloon dilation and stent placement with regard to benign and malignant tracheobronchial stenoses.
- Citation: Shin JH. Interventional management of tracheobronchial strictures. World J Radiol 2010; 2(8): 323-328
- URL: https://www.wjgnet.com/1949-8470/full/v2/i8/323.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i8.323
Tracheobronchial obstructions arising from either benign or malignant diseases result in dyspnea, stridor, and obstructive pneumonia, and can occasionally be life-threatening due to suffocation. Even in the absence of parenchymal lung disease, ventilatory failure frequently occurs if the obstruction is not relieved. Tracheobronchial obstructions are challenging to manage, particularly in patients unsuitable for curative surgery, with endobronchial intervention having an increasing role in their management[1]. Endobronchial intervention can be performed under local anesthesia by fluoroscopic guidance and/or fiberoptic bronchoscopy, or under general anesthesia using rigid bronchoscopy. Although aggressive endobronchial interventions for tissue destruction (Nd YAG laser, argon plasma coagulation, cryotherapy, or electrocautery) can be performed using rigid bronchoscopy, balloon dilation and stent placement can be performed using fluoroscopic guidance and/or flexible bronchoscopy.
Bronchoscopically or fluoroscopically guided balloon dilation is an accepted initial therapy for patients with benign bronchial strictures, primarily because balloon dilation is associated with lower morbidity and mortality rates than corrective surgery. The balloon dilates the stenotic trachea or bronchus by stretching and expanding the bronchial wall, making balloon dilation appropriate for the treatment of cicatric annular strictures. Balloon dilation has been extended to the treatment of tracheobronchial stenoses due to, for example, post-intubation tracheal stenosis, postoperative anastomotic stenosis, granulomatous stenosis (tuberculosis, histoplasmosis), radiation therapy, mediastinal fibrosis, congenital stenosis, bronchial trauma and bronchial artery embolization[2-5]. Before balloon dilation, the site, severity, proximal and distal extent, and characteristics of the stricture should be evaluated by conventional radiography, computed tomography scans including three-dimensional reconstructions, and/or bronchoscopy.
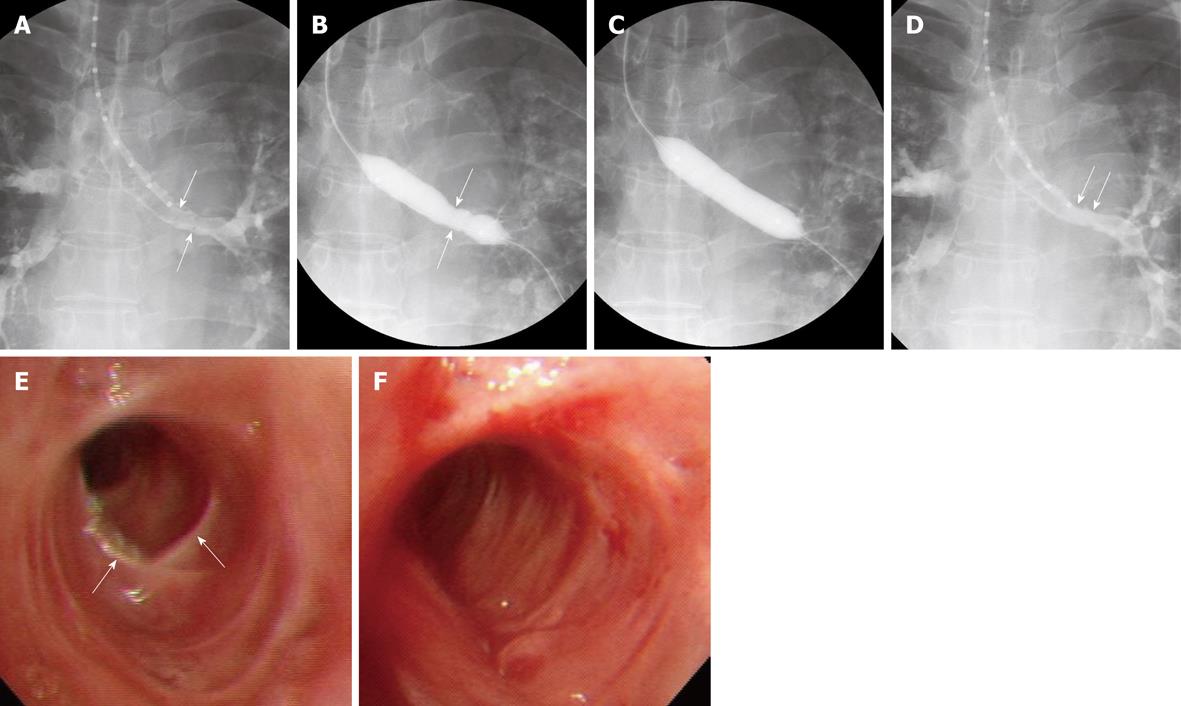
In our center, the pharynx and larynx are topically anesthetized using an aerosol spray 3-5 min before the procedure, followed by conscious sedation by intravenous administration of midazolam. A 0.035-inch angled exchange guide wire (Radifocus M; Terumo, Tokyo) is inserted through the bronchoscope and positioned across the stenosis. If a bronchoscope is not available, the guide wire can be inserted across the stenosis under fluoroscopic guidance. After removing the bronchoscope, the stricture is measured by passing a graduated sizing catheter over the guide wire to the distal part of the stricture. The degree and length of the stricture are evaluated in detail by selective tracheobronchography, by passing approximately 5 mL of water-soluble nonionic contrast medium mixed 1:1 with lidocaine through the sizing catheter (Figure 1). An angioplasty balloon catheter is then passed over the guide wire to dilate the stricture. In children, 6-mm diameter balloon catheters are used in the bronchi and tracheae, whereas in adults, 10-12-mm diameter balloon catheters are used in bronchi and 14-20-mm diameter balloon catheters are used in tracheae (Figure 1). If the stenosis is too narrow to allow passage of a balloon catheter > 10 mm in diameter, a 6-mm diameter balloon catheter is used first to provide a passage for the larger balloon catheter. Using diluted water-soluble nonionic contrast medium, the balloon is inflated at pressures up to 16 atm, as determined by a pressure-gauge monitor. After the procedure, selective tracheobronchography is performed to evaluate lumen dilation.
A review of several representative studies, each including 21-59 patients[2,3,5-7], found that the technical success rate was 100% and that all patients achieved initial symptomatic improvement. In some of these studies, however, up to 80% of patients required adjuvant treatment, including stent placement and laser therapy[2,3,5-7].
Complications associated with balloon dilation include chest pain during dilation, bronchospasm, atelectasis after dilation, superficial or deep mucosal laceration, pneumomediastinum, and massive bleeding have been reported[3,8-10]. In one large series, bronchial lacerations occurred during 64 of 124 (52%) tracheobronchial balloon dilation procedures, but none of these progressed to transmural laceration[10]. The median cumulative airway patency period was significantly longer in patients with than in those without lacerations (24 mo vs 4 mo), indicating that laceration secondary to balloon dilation may improve patency outcomes[10].
Recently, cutting balloon dilation has shown much better patency, approximately 60% at 2 years, for the treatment of benign bronchial strictures resistant to conventional balloon dilation[4]. Endobronchial brachytherapy may be used to treat benign bronchial strictures resistant to conventional balloon dilation or as an adjuvant treatment to treat granulation tissue formation after airway restoration[5,11].
Tracheobronchial balloon dilation is a simple, rapid, and safe method of restoring airway lumen, providing immediate symptom relief. This simple procedure may be a first option in the treatment of benign airway stenosis. If restenosis occurs, however, adjuvant endobronchial therapy, consisting of stent placement or laser treatment, will be necessary.
Up to 30% of patients with lung cancer have been reported to develop central airway obstruction secondary to endoluminal disease or external compression by a hilar tumor or bulky lymphadenopathy[12]. Recent technological advances have increased the popularity of tracheobronchial stents with interventional radiologists and chest physicians, particularly because stenting is effective for both extraluminal and intraluminal lesions and promptly relieves acute airway distress from airway obstruction. Expandable metallic stents are better tolerated than, and preferred to, non-expandable silicone stents because the former are more flexible and can be used in smaller delivery systems.
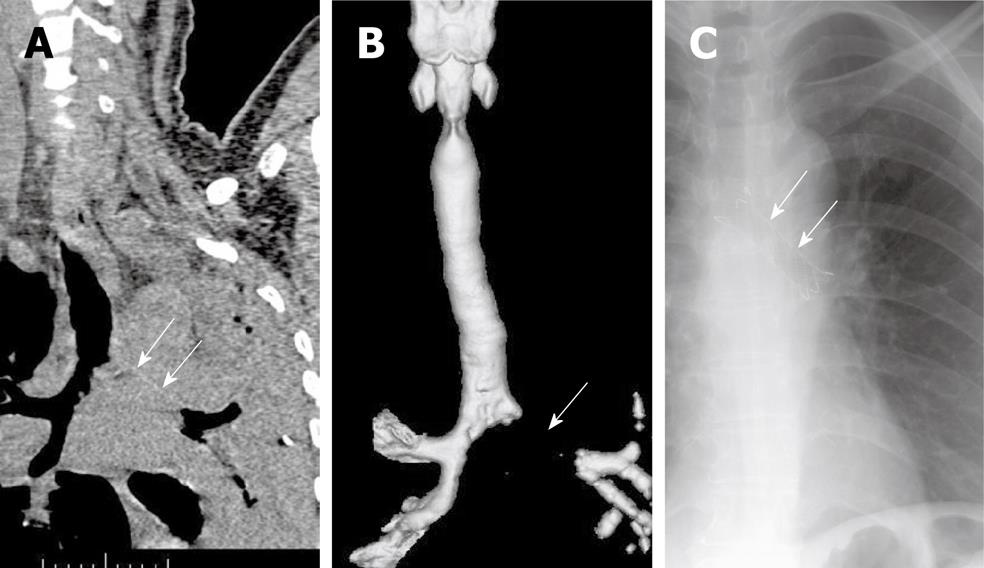
Stent placement is indicated for patients with submucosal and extraluminal pathology or tracheobronchomalacia, as well as for patients with intraluminal pathology (Table 1 and Figure 2). Tracheobronchomalacia is a special entity denoting functional airway obstruction with destruction of the surrounding airway cartilage. In patients with malignancies, the most common indication for stent placement is bronchogenic carcinoma, which can present as extraluminal compression with or without an intraluminal lesion. Tracheobronchial stenting is the only immediate treatment for unresectable extraluminal compression, promptly stabilizing a threatened airway while the primary tumor is treated with radiation or chemotherapy.
| Malignant intraluminal or extraluminal obstructive pathology |
| Benign inflammatory obstructive pathology such as tuberculosis |
| Benign post-intubation tracheal stenosis |
| Benign postoperative anastomotic stenosis |
| Tracheobronchomalacia |
| Compression by esophageal stents |
| Esophagorespiratory fistula |
The only definitive contraindication for placement of an airway stent is in patients with external compression of the airway by a vessel. Stent placement in these patients was associated with unacceptably high rates of erosion, hemorrhage, and death[12].
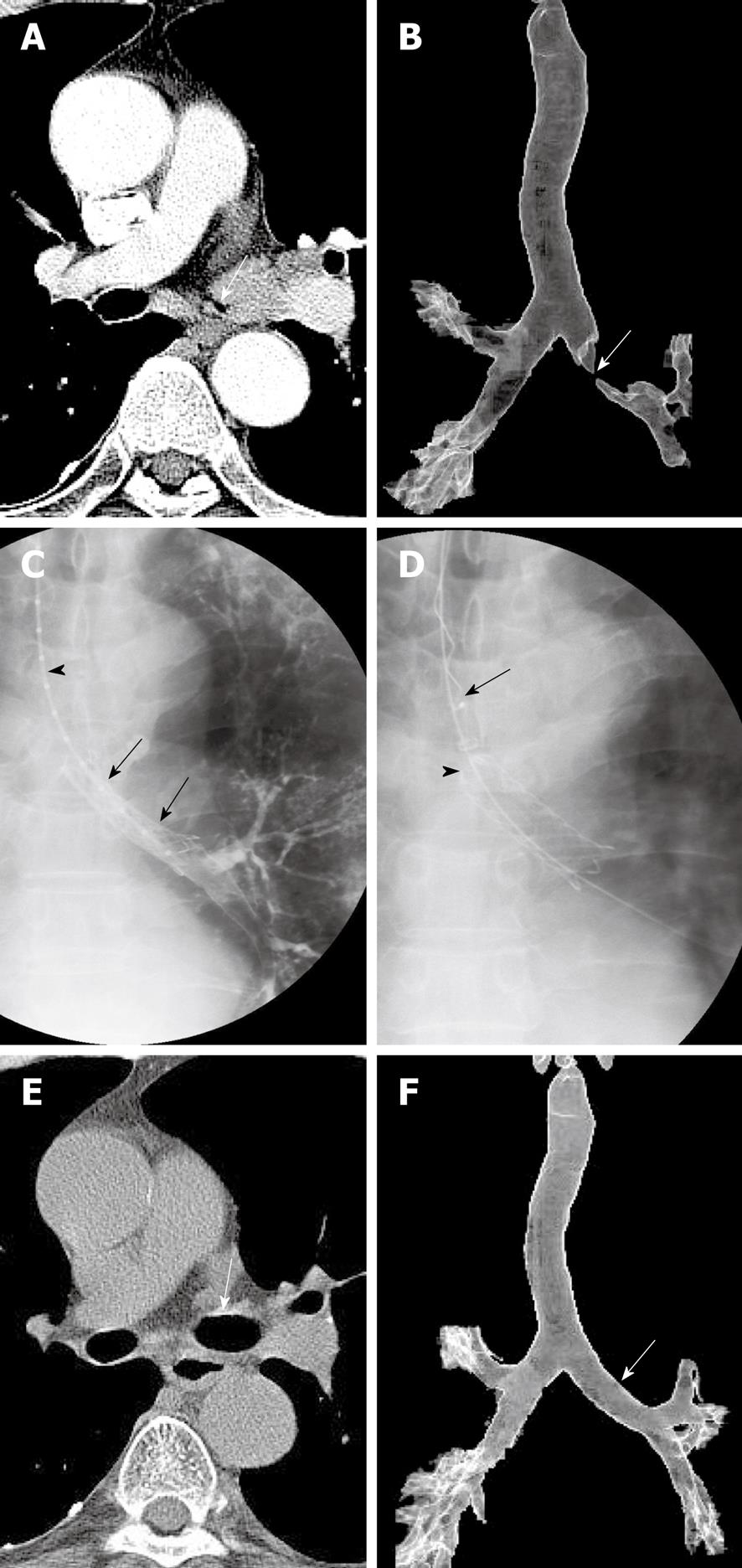
At present, two types of stents are available, silicone and metallic. The advantages of metallic stents include their favorable ratios of wall to inner diameter and their ability to be placed using flexible bronchoscopy and/or fluoroscopy. Metallic stents can be classified as balloon-expandable and covered and uncovered self-expandable metallic stents. Silicone stents have been associated with high rates of migration, resulting in reocclusion, adherence of secretions due to the impairment of mucociliary clearance, and unfavorable wall to inner diameter ratios. Among the advantages of silicone stents are their ability to be repositioned or removed as many times as needed, which is especially important for benign tracheobronchial stenoses and for slowly growing, frequently recurring stenoses[12]. General anesthesia and the use of a rigid bronchoscope are mandatory for the placement of silicone stents. Among the advantages of balloon-expandable stents, including Strecker and Palmaz stents, and of uncovered self-expandable metallic stents, including Gianturco Z, Ultraflex, and Polyflex stents, are their lower rates of migration and interference with mucociliary clearance. Among their disadvantages are difficult removal and the growth of tumors or granulation tissue through the stent meshes. Because balloon-expandable metallic stents require an additional step, balloon dilation, and sometimes require rigid bronchoscopes, they are less commonly used. Uncovered and covered self-expandable metallic stents have become increasingly popular due to their relative ease of placement. These stents can be compressed into a small delivery device, and once deployed, are embedded into the surrounding tissue via a radial force. Whereas uncovered self-expandable metallic stents cannot be removed or exchanged easily, covered self-expandable metallic stents, such as Ultraflex, Wall, and Alveolus stents, can be removed or exchanged relatively easily. Therefore, temporarily placed retrievable, covered self-expandable metallic stents can be used to treat benign airway strictures as well as to treat malignant airway strictures in combination with radiation therapy and/or chemotherapy (Figure 3)[13,14]. In addition, covered self-expandable metallic stents can be used to seal esophagorespiratory fistulae. Recently introduced barbed retrievable covered metallic stents have shown particularly low migration rates[15].
Expandable metallic stents can be inserted only under fluoroscopic guidance and only by radiologists with patients under topical anesthesia. Due to the importance of bronchoscopic evaluation immediately before and after stent placement, bronchoscopic assistance is valuable. Moreover, it is relatively easy to insert a guide wire across the stricture into the distal portion of the trachea or bronchus through the working channel of the bronchoscope.
The techniques for providing topical anesthesia, introducing the guide wire and catheter into the tracheobronchial tree, and obtaining selective tracheobronchography are the same as for balloon dilation. Subsequently, the location of the narrowed lumen can be marked on the patient’s skin using radiopaque markers. With the patient in a supine position and with the neck fully extended, the delivery system, the proximal part of which is lubricated with jelly, is passed over the guide wire into the trachea and is advanced until the distal tip reaches beyond the stricture. When the stricture is severe (i.e. more than two-thirds of the lumen is narrowed), the stenotic portion is dilated with an angioplasty balloon catheter. The stent should be at least 10 mm longer than the stricture, so that the proximal and distal parts of the stent rest on the upper and lower margins of the stricture, respectively.
Following stent placement, its patency and location are evaluated by bronchoscopy. It is important to avoid inexact stent deployment that results in partial obstruction of a bronchial orifice or incomplete coverage of a tumor stenosis. If this occurs, the stent should be repositioned using bronchoscopic biopsy forceps or it should be removed and its placement reattempted.
Early investigators used forceps/rotation techniques to remove uncovered expandable metallic stents under general anesthesia[16,17]. In patients in whom the stent was tightly welded to the tracheobronchial wall, however, this removal procedure carried potential risks of mucosal bleeding and airway occlusion during the procedure. Our removal technique uses a hook-like device and has been reported to be safe and easy to perform because the stents (Song Airway Stent, S&G Biotech, Seongnam, Korea) are completely covered and designed for optimal removal[13-15,18]. To make the stent removable, a nylon loop is hooked inside each bend of its proximal end and two nylon threads are passed through each loop. To remove these stents, a hooked wire is introduced into the sheath and passed through it into the stent lumen. The sheath containing the hook is then pulled out of the stent so that the hook grasps the drawstring. When this occurs, the hook wire is withdrawn through the sheath, collapsing the proximal stent. The sheath, hook wire, and stent are then pulled out of the trachea.
In benign tracheobronchial stenosis, technical success rates of 100% and clinical success rates of 88-100% have been reported in 46 patients across three representative studies using uncovered self-expandable stents[19-21]. The most common causes of stenosis in these patients were post-lung transplantation strictures and tuberculosis. Stent fracture was the most commonly observed complication, occurring in seven patients (15%), followed by granulation tissue formation (7%) and stent migration (2%). Four patients (9%) required stent removal due to stent fracture or migration, but stent removal was difficult because the wire mesh was embedded in the airway walls. These findings indicate that the placement of permanent stents may not be ideal due to the formation of granulation tissue. We have placed 30 covered retrievable expandable metallic stents into 24 patients[13], resulting in technical and short-term clinical success rates of 100%. All stents were successfully removed electively, either 2 (n = 12) or 6 (n = 12) months after placement or when complications occurred (n = 6). The 6-mo stenting group showed a lower recurrence rate (41.7% vs 83.3%, P = 0.045) and a better mean maintained patency (39.7 ± 7.8 mo vs 9.4 ± 5.4 mo, P = 0.001) than the 2-mo stenting group. Although stent migration and tissue hyperplasia at either end of the stent was observed in 13% and 37% of these patients, respectively, stent removal was easy and safe.
Four representative studies of stent placement, two using uncovered[22,23] and two using covered[18,24] stents, in 133 patients with malignant tracheobronchial stenosis showed technical success rates of 98%-100% and clinical success rates of 82%-92%. Covered metallic stents were associated with much higher rates of stent migration (12%-17%) and sputum retention (20%-38%) than uncovered metallic stents (0% and 9%, respectively). In contrast, tumor ingrowth into the stent lumen occurred more often with uncovered (21%-23%) than with covered (0%) metallic stents because the former do not contain covering material between the wire mesh. In patients with malignant bronchial obstructions, involvement of the lower-lobe segmental bronchus has been associated with lower rates of radiologic and clinical improvement following stent placement[25].
In patients with benign disease, stent migration is more likely when there is no substantial extrinsic compression maintaining the stents in place and when short stents are placed in conical stenoses. In patients with malignancies, migration can be expected after tumor shrinkage from radiation or chemotherapy. Granulation tissue is more likely to form at the proximal and distal ends of the stent, and excessive granulation tissue can lead to obstruction of the airway. This occurs more commonly with metallic stents, especially uncovered stents (up to 7%), than with silicone stents because metal stents are more rigid and have multiple edges, therefore causing more irritation. The incidence of tumor overgrowth at the tip of the covered metallic stent depends on the follow-up period and extent of the malignancy at the time of stent placement and has been reported to occur in 6%-28% of patients[18,24]. Because airway obstruction by tumor ingrowth/overgrowth can be life-threatening, patients with new symptoms or radiographic findings should undergo further diagnostic evaluation. Sputum retention is more likely to occur after placement of silicone and covered expandable metallic stents than after placement of uncovered expandable metallic stents due to impaired mucociliary clearance in the former. Lack of mucociliary clearance can lead to obstruction and infection.
In summary, balloon dilation is an accepted initial therapy for benign airway stricture. It is easy to perform, but is plagued by a high recurrence rate. While, stent placement can improve life quality by dramatic resolution of dyspnea in malignant airway stricture. For benign airway strictures refractory to balloon dilation, temporary placement of airway stent could be considered.
Peer reviewer: James Chow, PhD, Radiation Physicist, Radiation Medicine Program, Princess Margaret Hospital, 610 University Avenue, Toronto, ON, M5G 2M9, Canada
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Ranu H, Madden BP. Endobronchial stenting in the management of large airway pathology. Postgrad Med J. 2009;85:682-687. |
| 2. | Shitrit D, Kuchuk M, Zismanov V, Rahman NA, Amital A, Kramer MR. Bronchoscopic balloon dilatation of tracheobronchial stenosis: long-term follow-up. Eur J Cardiothorac Surg. 2010;38:198-202. |
| 3. | Lee KH, Ko GY, Song HY, Shim TS, Kim WS. Benign tracheobronchial stenoses: long-term clinical experience with balloon dilation. J Vasc Interv Radiol. 2002;13:909-914. |
| 4. | Kim JH, Shin JH, Song HY, Ko GY, Gwon DI, Yoon HK, Sung KB. Cutting balloon treatment for resistant benign bronchial strictures: report of eleven patients. J Vasc Interv Radiol. 2010;21:748-752. |
| 5. | Rahman NA, Fruchter O, Shitrit D, Fox BD, Kramer MR. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg. 2010;5:2. |
| 6. | Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J. 2004;24:345-347. |
| 7. | Mayse ML, Greenheck J, Friedman M, Kovitz KL. Successful bronchoscopic balloon dilation of nonmalignant tracheobronchial obstruction without fluoroscopy. Chest. 2004;126:634-637. |
| 8. | Ferretti G, Jouvan FB, Thony F, Pison C, Coulomb M. Benign noninflammatory bronchial stenosis: treatment with balloon dilation. Radiology. 1995;196:831-834. |
| 9. | Kato R, Kakizaki T, Hangai N, Sawafuji M, Yamamoto T, Kobayashi T, Watanabe M, Nakayama M, Kawamura M, Kikuchi K. Bronchoplastic procedures for tuberculous bronchial stenosis. J Thorac Cardiovasc Surg. 1993;106:1118-1121. |
| 10. | Kim JH, Shin JH, Song HY, Shim TS, Ko GY, Yoon HK, Sung KB. Tracheobronchial laceration after balloon dilation for benign strictures: incidence and clinical significance. Chest. 2007;131:1114-1117. |
| 11. | Kim JH, Shin JH, Song HY, Shim TS, Oh YM, Oh SJ, Moon DH. Liquid (188)Re-filled balloon dilation for the treatment of refractory benign airway strictures: preliminary experience. J Vasc Interv Radiol. 2008;19:406-411. |
| 12. | Chin CS, Litle V, Yun J, Weiser T, Swanson SJ. Airway stents. Ann Thorac Surg. 2008;85:S792-S796. |
| 13. | Kim JH, Shin JH, Song HY, Shim TS, Yoon CJ, Ko GY. Benign tracheobronchial strictures: long-term results and factors affecting airway patency after temporary stent placement. AJR Am J Roentgenol. 2007;188:1033-1038. |
| 14. | Kim JH, Shin JH, Song HY, Ohm JY, Lee JM, Lee DH, Kim SW. Palliative treatment of inoperable malignant tracheobronchial obstruction: temporary stenting combined with radiation therapy and/or chemotherapy. AJR Am J Roentgenol. 2009;193:W38-W42. |
| 15. | Kim YH, Shin JH, Song HY, Kim JH. Tracheal stricture and fistula: management with a barbed silicone-covered retrievable expandable nitinol stent. AJR Am J Roentgenol. 2010;194:W232-W237. |
| 16. | Filler RM, Forte V, Chait P. Tracheobronchial stenting for the treatment of airway obstruction. J Pediatr Surg. 1998;33:304-311. |
| 17. | Nicolai T, Huber RM, Reiter K, Merkenschlager A, Hautmann H, Mantel K. Metal airway stent implantation in children: follow-up of seven children. Pediatr Pulmonol. 2001;31:289-296. |
| 18. | Shin JH, Kim SW, Shim TS, Jung GS, Kim TH, Ko GY, Song HY. Malignant tracheobronchial strictures: palliation with covered retrievable expandable nitinol stent. J Vasc Interv Radiol. 2003;14:1525-1534. |
| 19. | Eisner MD, Gordon RL, Webb WR, Gold WM, Hilal SE, Edinburgh K, Golden JA. Pulmonary function improves after expandable metal stent placement for benign airway obstruction. Chest. 1999;115:1006-1011. |
| 20. | Orons PD, Amesur NB, Dauber JH, Zajko AB, Keenan RJ, Iacono AT. Balloon dilation and endobronchial stent placement for bronchial strictures after lung transplantation. J Vasc Interv Radiol. 2000;11:89-99. |
| 21. | Choi YW, Kim YS, Jeon SC, Hahm CK, Choi CS. Treatment of tracheobronchial stenosis with a self-expandable metallic stents. J Korean Radiol Soc. 1994;31:35-41. |
| 22. | Miyazawa T, Yamakido M, Ikeda S, Furukawa K, Takiguchi Y, Tada H, Shirakusa T. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest. 2000;118:959-965. |
| 23. | Sawada S, Tanigawa N, Kobayashi M, Furui S, Ohta Y. Malignant tracheobronchial obstructive lesions: treatment with Gianturco expandable metallic stents. Radiology. 1993;188:205-208. |
| 24. | Monnier P, Mudry A, Stanzel F, Haeussinger K, Heitz M, Probst R, Bolliger CT. The use of the covered Wallstent for the palliative treatment of inoperable tracheobronchial cancers. A prospective, multicenter study. Chest. 1996;110:1161-1168. |
| 25. | Shin JH, Song HY, Kim KR, Kim JH, Kim SW, Lee DH, Hong SB. Radiologic and clinical outcomes with special reference to tumor involvement pattern after stent placement for malignant bronchial obstructions. Acta Radiol. 2009;50:1011-1018. |