INTRODUCTION
Recent advances in digital technologies have resulted in remarkable developments in the field of imaging modalities[1]. Ultrasonography (US) is one of the diagnostic tools that have shown significant improvement within the last decade[2-7]. For the diagnosis of liver tumors, US examination has the advantages of real-time observation, simple technique and non-invasiveness[8-13]. This modality is being used worldwide with high frequency as a reliable method for the initial diagnosis of liver tumors[14-19]. Color Doppler and power Doppler have increased the sensitivity for hepatic lesion detection compared to that of gray-scale US[20-25]. Furthermore, the application of microbubble contrast agents provides details of the hemodynamics, which are useful for the detection and characterization of liver tumors[26-31].
Even if advances in imaging technology increase further, US findings are inevitably based on the pathological features. Therefore, knowledge of disease conditions and pathological features is essential to comprehend the findings on US images.
This paper reviews the diagnosis of hepatic malignancies contrasted between US images and pathological features.
HEPATOCELLULAR CARCINOMA
Disease conditions and pathological features
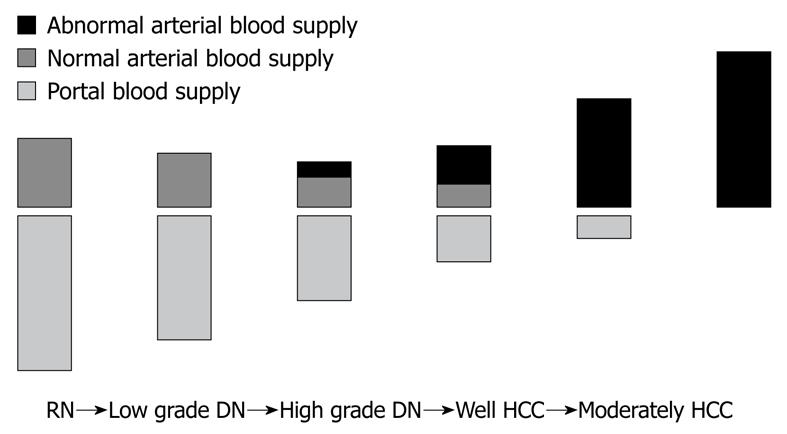
Hepatocellular nodules associated with liver cirrhosis are divided into six categories according to the classification proposed by the International Working Party of World Congress of Gastroenterology in 1994: namely, large regenerative nodule, low-grade dysplastic nodule (DN), high-grade DN, high-grade DN with malignant foci, well-differentiated hepatocellular carcinoma (HCC), and HCC with Edmondson II or higher, which is called classical HCC[32-36]. Among these nodules, two models of hepatocarcinogenesis are now hypothesized. One is de novo carcinogenesis, and the other is stepwise development from low-grade DN, high-grade DN, high-grade DN with malignant foci, and well-differentiated HCC, to classical HCC. The intranodular blood supply changes with the progression of human hepatocarcinogenesis from DN to overt HCC[32-36] (Figure 1). The portal tracts, including the portal vein and hepatic artery, decrease with the increasing grade of malignancy and are virtually absent in nodules. In contrast, abnormal arteries due to tumor angiogenesis develop in atypical adenomatous hyperplasia (high-grade DN) during the course of hepatocarcinogenesis, and are markedly increased in number in moderately differentiated HCCs. The intranodular vasculature changes in a stepwise manner as the grade of malignancy increases[32-36]. In the course of hepatocarcinogenesis, arterial and portal supply decreases (due to a decrease in the portal tracts), and arterial supply returns to a level equivalent (due to newly formed abnormal arteries) to the surrounding liver, while portal supply continues to decrease, and finally portal supply vanishes and only arterial blood (from newly formed abnormal arteries) supplies the lesion (classical HCC). Therefore, high-grade DN is usually hypodense relative to the surrounding liver. However, early-stage well-differentiated HCC often shows isodensity because the increased abnormal arterial supply compensates for the decreased normal hepatic arterial supply[34-36].
Figure 1 Changes in intranodular blood supply with the progression of hepatocarcinogenesis from dysplastic nodule to hepatocellular carcinoma.
RN: Regenerative nodule; DN: Dysplastic nodule; HCC: Hepatocellular carcinoma.
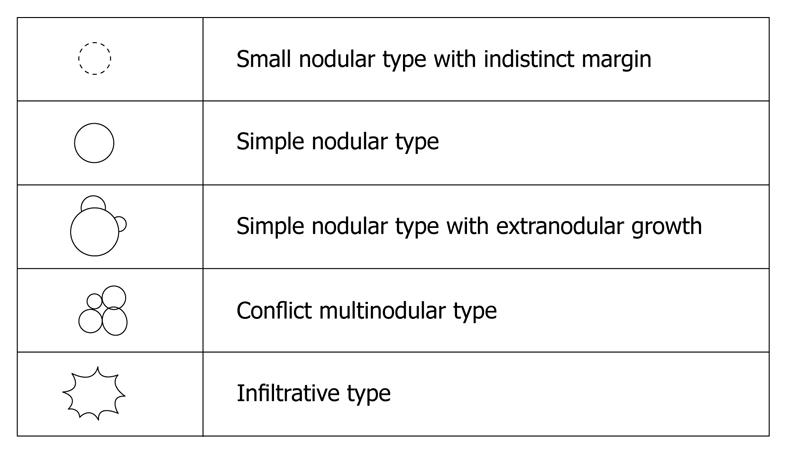
Macroscopic configurations of HCC
In the classification proposed by the Liver Cancer Study Group of Japan, macroscopic configurations of HCC are divided into five types, namely the small nodular type with indistinct margins, simple nodular type, simple nodular type with extranodular growth, confluent multinodular type, and infiltrative type[37,38] (Figure 2). The malignant potentials tend to change in accordance with the progression of macroscopic configurations[38].
Figure 2 Macroscopic configurations of hepatocellular carcinoma.
In the small nodular type with indistinct margins, approximately 85% of nodules consist of well-differentiated cancerous tissue with a replacing growth at the boundary, and many portal tracts are retained in the tumor with immature fibrous capsule formation. When such tumors reach 1.5-2.0 cm in diameter, moderately or poorly differentiated cancer tissues develop within the well-differentiated cancer tissue. When less differentiated cancerous tissues within the well-differentiated cancer nodules proliferate in an expansive fashion, a “nodule-in-nodule” appearance is frequently seen.
The simple nodular type exhibits a round or nearly round shape, and demonstrates a clear boundary with non-cancerous tissue, and it often has an obvious capsule. Some tumors appear intersected by thin fibrous septa, but these tumors are round, and have the appearance of a single expanding nodule. Moderately differentiated cancerous tissue accounts for approximately 75% of the simple nodular type of HCC, and approximately 20% show portal invasion on histology.
In the simple nodular type with extranodular growth, well-differentiated histological characteristics are present in only 12.5% of cases. The simple nodular type of HCC with extranodular growth is well developed in contrast to the delicate fibrous septa that are occasionally present in the small nodular type with indistinct margins. Most of the simple nodular type with extranodular growth demonstrates moderately differentiated HCC.
Confluent multinodular type tumor is formed by several small contiguous tumor nodules. In confluent multinodular type tumors, the margin of the whole tumor appears rugged because of the projection of each nodule. In addition, the internodular fibrous tissue of confluent multinodular type tumors is well developed. This unique appearance probably reflects the extension of tumor by growth replacement from one pseudolobule into its neighbors. Most of the confluent multinodular type is classified as moderately to poorly differentiated HCC.
The infiltrative type of tumor is classified as the poorly demarcated nodular type. The most important reason for classifying this tumor separately is that grossly the entire border is obscure. Histologically, the entire boundary between cancerous and non-cancerous parenchyma is composed of small nests of cancer cells that infiltrate the interlobular septa, similar to the infiltrative pattern of adenocarcinoma. Most infiltrative type tumor is diagnosed in poorly differentiated HCC or mixed HCC/cholangiocarcinoma.
US imaging of advanced HCC
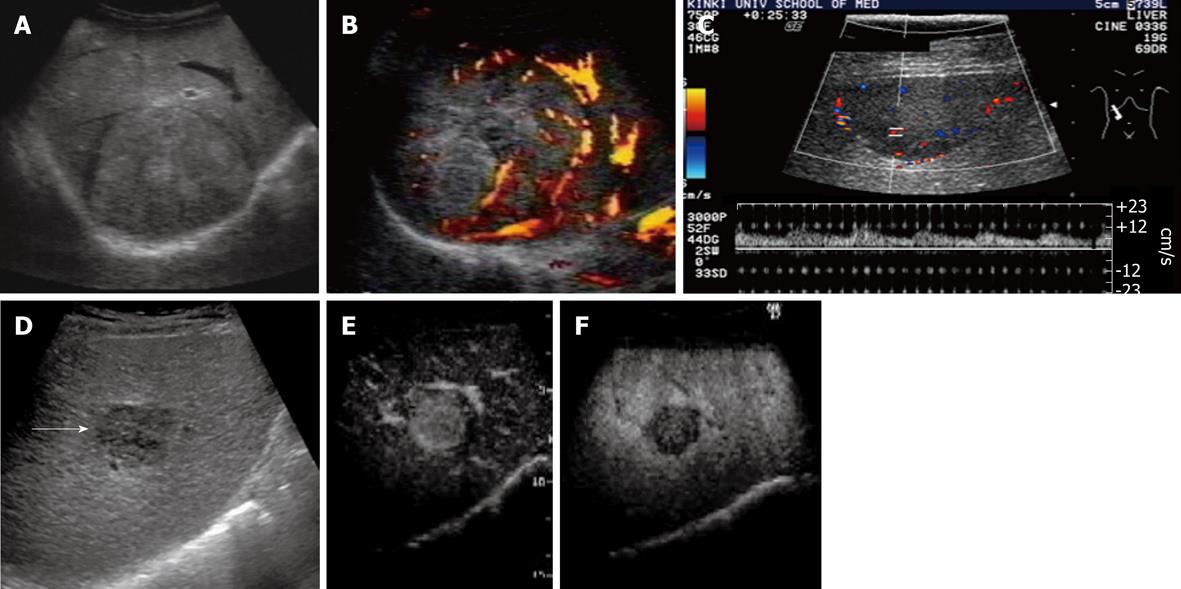
Typical US findings of advanced HCC are a mosaic pattern, septum formation, peripheral sonolucency (halo), lateral shadow produced by fibrotic pseudocapsule, posterior echo enhancement, arterial hypervascularity with dilated intratumoral blood sinusoids, and perinodular daughter nodule formation (Figure 3).
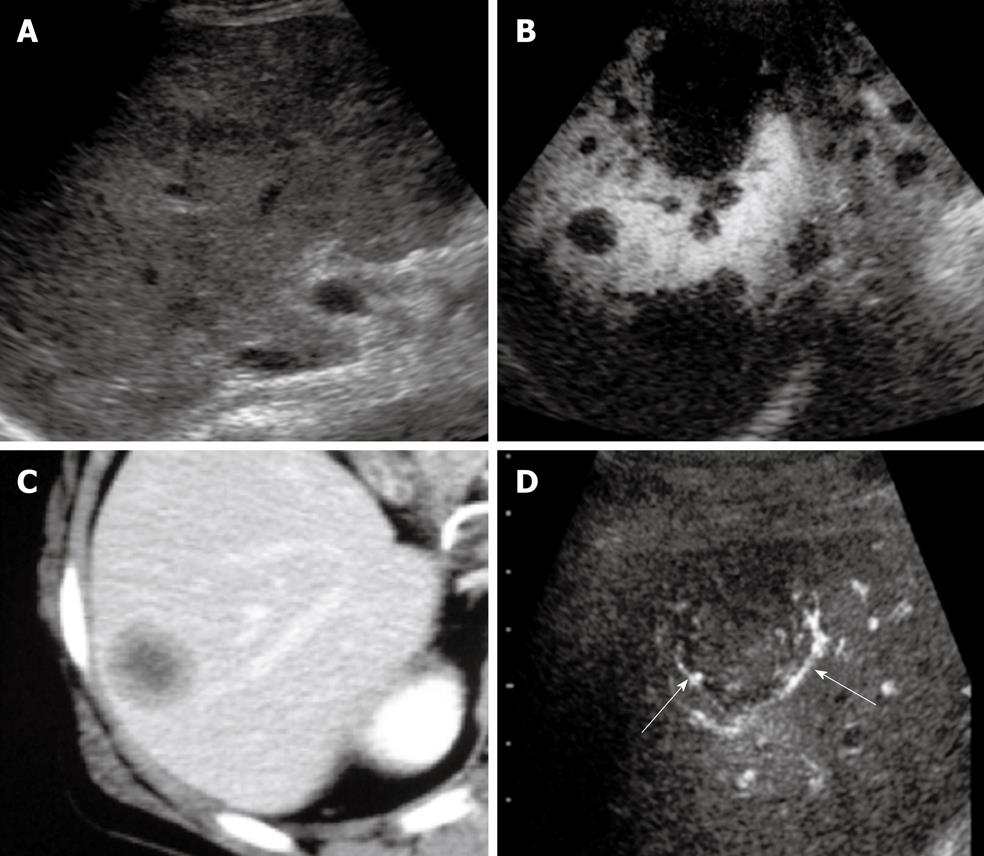
Figure 3 Advanced hepatocellular carcinoma.
A: The nodule had a halo image and mosaic pattern in segment 8 of the liver on B-mode ultrasonography; B: Power Doppler imaging showed hypervascularity of the tumor; C: Color Doppler imaging showed intratumoral blood flow. Arterial pulsatile waveforms could be detected by pulsed Doppler images; D: The image of a simple nodular type with extranodular growth (arrow) was obtained on B-mode ultrasonography (US); E: Contrast harmonic US showed enhancement of hepatocellular carcinoma in early vascular phase after administration of perfluorocarbon microbubbles; F: Contrast harmonic US depicted the defect image in the post-vascular phase.
Internal mosaic architecture and capsule formation are major macroscopic features of typical moderately differential HCCs[39-41]. The halo sign and lateral shadows correspond to the thin fibrous capsule of the HCC. Correspondence between sonographic halo sign and histological capsule has been reported as 90.1%, and that between the presence of extracapsular invasion on US and that on histology as 88.0%. The presence of extracapsular invasion on US is a predisposing factor for the development of tumor recurrence.
Posterior echo enhancement is due to the softness of the HCC[42]. However, posterior echo enhancement is not specific for HCC, as this finding is also observed with similarly frequency in hemangiomas.
The spread of HCC along the portal vein results in daughter nodule formation. In HCC, the hepatic artery is the feeding vessel and the portal vein serves mainly as an efferent vessel. Tumor cells invade efferent vessels by budding, extension to the vascular cavity, and then extending beyond the capsule to the portal vein branches. In more advanced cases of HCC, portal tumor thrombi, biliary invasion, and hepatic vein invasion are also observed, which strongly indicates a diagnosis of HCC.
A basket pattern on color Doppler images and/or power Doppler images has been described; this pattern represents a fine network of arterial vessels that surrounds the tumor nodules[43,44]. Typical color Doppler findings in advanced HCC are afferent pulsatile waveform signals, intratumoral pulsatile waveform signals associated with intratumoral continuous waveform signals, and efferent continuous waveform signals. Of the several parameters that can be obtained with Doppler spectral analysis, maximum flow velocity and pulsatility index (PI) are very important in the differential diagnosis of hepatic tumors. The PI in HCC is much higher than that in hemangioma.
Hepatic carcinogenesis is described as a multi-step process in which progressive arterialization and gradual loss of portal vessels are the principal features[45,46]. It is evident that vascular enhancement is related to the evolution of the lesion. Thus, during the arterial phase, DNs or early HCC usually appear hypo or isovascularized, while advanced HCCs are hypervascularized. Contrast-enhanced US can show selective enhancement in the arterial phase, which differentiates HCCs from regenerative nodules and DNs[4-6].
US imaging of early HCC
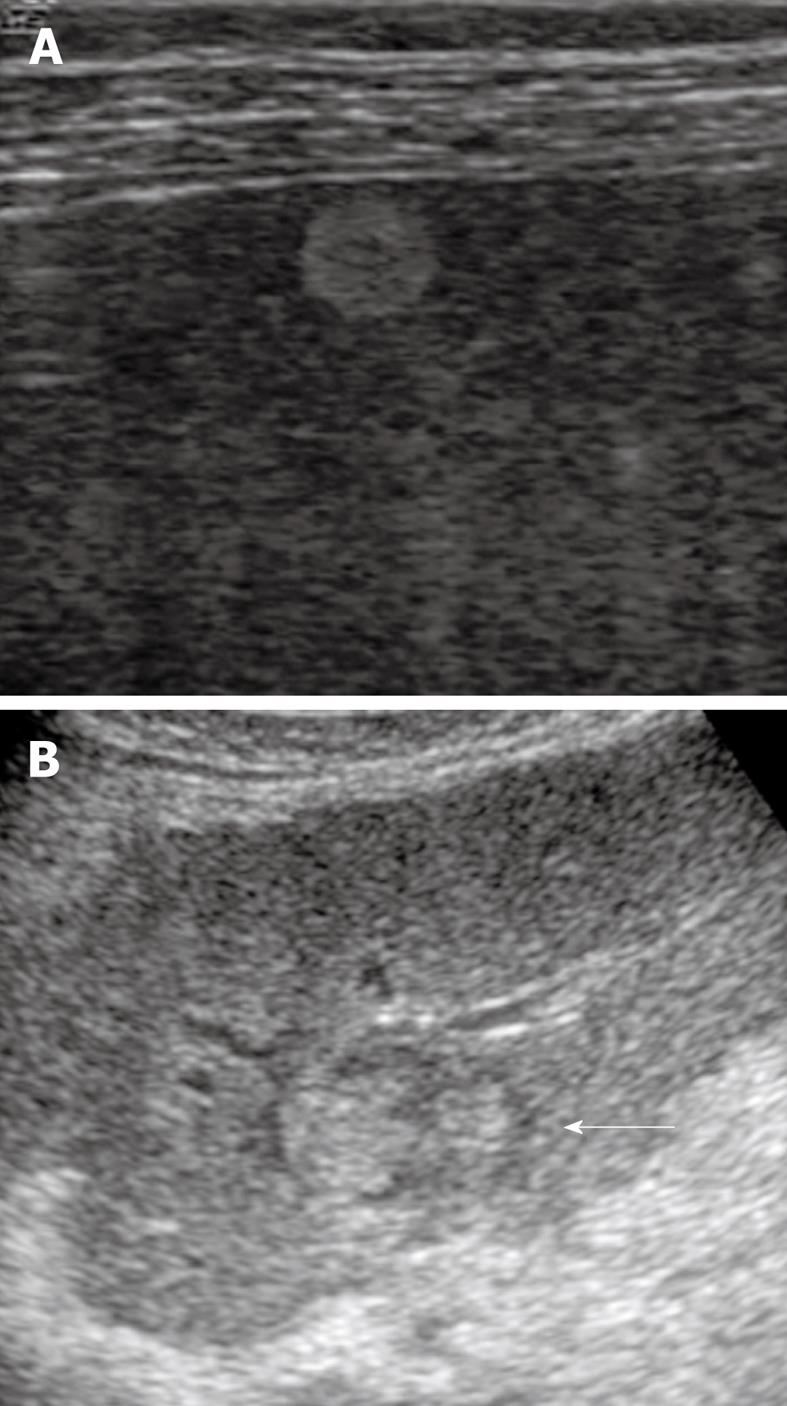
The imaging features of early HCC (highly well-differentiated HCC) are as follows: bright loop appearance, arterial hypovascularity and internal portal tracts or portal blood supply. Hypervascular foci in the nodule occasionally demonstrate a nodule-in-nodule appearance (Figure 4).
Figure 4 Early hepatocellular carcinoma.
A: A nodule that was 1.5 cm in diameter in segment 5 of the liver was shown as highly echoic because of fatty changes in the nodule; B: A nodule-in-nodule appearance (arrow) was demonstrated as a hyperechoic tumor within a hypoechoic nodule.
Bright loop appearance is defined as hypoechoic nodules in a hyperechoic tumor[47]. A nodule-in-nodule appearance might also appear as a hyperechoic tumor within a hypoechoic nodule[25,46]. Hyperechoic HCC nodules represent well-differentiated HCC with fatty changes, whereas an inner hypoechoic lesion represents moderately differentiated HCC without fatty changes. On US screening for HCC, these appearances are often observed in HCC nodules that measure 11-20 mm in diameter. Histological examination has demonstrated that bright loop appearance and nodule-in-nodule appearance of HCC on US are associated with tumor progression and dedifferentiation to moderately differentiated HCC within well-differentiated HCC with fatty changes.
Typical findings of early HCC are afferent continuous waveform signals, which reflect a feeding portal flow, which is rarely associated with pulsatile wave-form signals[48]. Thus, with afferent blood flow on Doppler US imaging, constant waveform signals that reflect portal inflow are a characteristic finding in DNs and early well-differentiated HCC.
Half of the well-differentiated HCCs wash out slowly during the late phase on contrast-enhanced US and the average washout time was significantly different from that of moderately to poorly differentiated HCCs[17,21,23,46]. Microbubbles continuously infusing the tumor through the portal vein could be the pathological basis of slow washout. Furthermore, well-differentiated HCCs consist of a trabecular pattern of cell cords and abundant sinusoids that may cause stagnation and slow clearance of microbubbles. Conversely, less differentiated HCCs contain fewer sinusoids and are mainly fed by the hepatic artery, which could cause the difference in washout time compared with that of well-differentiated HCCs.
LIVER METASTASIS
Disease conditions and pathological features
The liver is the organ second most commonly affected by metastatic disease. The most common primary sites are the gastrointestinal (GI) tract, lung, breast and head and neck[49-52]. Therefore, liver metastases vary in size, shape, vascularity, and growth pattern. However, most liver metastases are multiple and show the so-called “cluster sign”. In 77% of patients with liver metastases, both lobes are involved, whereas metastasis is solitary in only 10% of cases[53]. Most metastatic tumors of the liver are expansive or infiltrative. Although most metastases are generally hypovascular, metastases often show the same degree of vascularity as the primary tumor[54]. Calcification can be seen in metastases from colon, stomach, breast, and other organs. Fundamentally, liver metastases occur in patients without liver cirrhosis[54].
In metastases from the GI tract, intratumoral fibrous septum and capsule formation are macroscopically rare. Hypervascular metastases are uncommon, however, arterial vascularity of metastases develops finely near the border. Large metastases often outgrow their blood supply and subsegment hypoxia causes a necrotic region at the center of the tumor[54].
In addition, metastatic carcinoma from the breast or pancreas induces an intense fibrous or sclerosing reaction around the tumor, which leads to fibrous scar formation[55]. In 7%-15% of patients, tumor thrombi occlude the portal vein, the hepatic vein, or both. In the presence of mucin secretion, necrosis, and phosphate activity, metastases can develop calcification that is detectable radiographically.
US imaging
US imaging features of liver metastases from the GI tract are as follows: Bull’s eye appearance, multiple masses, absence of liver cirrhosis, irregular tumor border, arterial rim-like enhancement, and hypoenhancement in the late vascular phase (Figure 5).
Figure 5 Liver metastasis.
A: Multiple masses were seen in the liver by B-mode ultrasonography (US); B: Multiple defects were seen by Sonazoid-enhanced US in the post-vascular phase; C: Portal phase dynamic scan detected a hypoenhanced nodule in segment 6 of the liver; D: Peripheral enhancement of the nodule (arrows) was obtained by Sonazoid-enhanced ultrasonography in the early vascular phase.
Bull’s eye or target lesion is a common presentation of metastases from the GI tract[56]. Sonography also shows multiple round and/or hypoechoic masses with irregular borders[57]. A Bull’s eye appearance represents histological findings of an area that shows central coagulative necrosis surrounded by a zonal area of viable tumor. The surrounding zonal area appears thick, and reflects a layer of viable cells. Calcified metastases might demonstrate shadows when they are densely echogenic. Then, colon cancer is the most likely cause in a patient with unknown primary tumor when calcified liver metastases are demonstrated by US.
Color or power Doppler US can show intratumoral vascularity in the peripheral hypoechoic zone, in which viable tumor cells are proliferating[58]. Actually, these signals appear to be poor because the density of tumor vasculature is lower than that of moderately differentiated HCC. However, in patients with metastasis from renal cell carcinoma or sarcoma, intratumoral flow can be demonstrated because of its hypervascularity.
Contrast-enhanced US of the liver with SonoVue provides a significantly higher sensitivity in the detection of liver metastases compared to that of unenhanced sonography, and identifies up to 40% more metastases[59-61]. It has been reported that the presence of rim-like enhancement with peripheral tumor vessels (sensitivity, 88.1%; specificity, 100%) is the typical pattern[62]. Contrast-enhanced US in the late phase provides a marked improvement in the detection of hepatic metastases as areas of hypoenhancement, and can be advantageous in detecting small metastases compared with computed tomography and magnetic resonance imaging[63-65].
INTRAHEPATIC CHOLANGIOCARCINOMA
Disease conditions and pathological features
Intrahepatic cholangiocarcinoma is a slow-growing ductal adenocarcinoma in the liver; it is relatively rare and comprises 3%-7% of malignant liver tumors[66-69]. The bile ducts are dilated because of obstruction by tumors[70,71]. Intrahepatic cholangiocarcinoma, unlike HCC, is not usually related to liver cirrhosis. However, hepatitis C virus infection has also been reported to be a risk factor for cholangiocarcinoma[72]. The Liver Cancer Study Group of Japan has proposed a classification of intrahepatic cholangiocarcinoma based on macroscopic features: mass-forming, periductal infiltrating, and intraductal, or mixed mass-forming, and periductal infiltrating[73,74]. More than half of intrahepatic cholangiocarcinomas are classified as the mass-forming type.
A mass-forming intrahepatic cholangiocarcinoma is usually large. On gross specimens, the tumor is firm and whitish gray because of its large amount of fibrous stroma[75]. The margin is well circumscribed and wavy or lobulated. Central necrosis might be present. Multicentricity, especially around the main tumor, is common, probably because of the propensity of the tumor to invade the adjacent peripheral branches of the portal vein[75-77]. It easily spreads to the lymph nodes. Most of the mass-forming intrahepatic cholangiocarcinomas are poorly or moderately differentiated[75].
US imaging
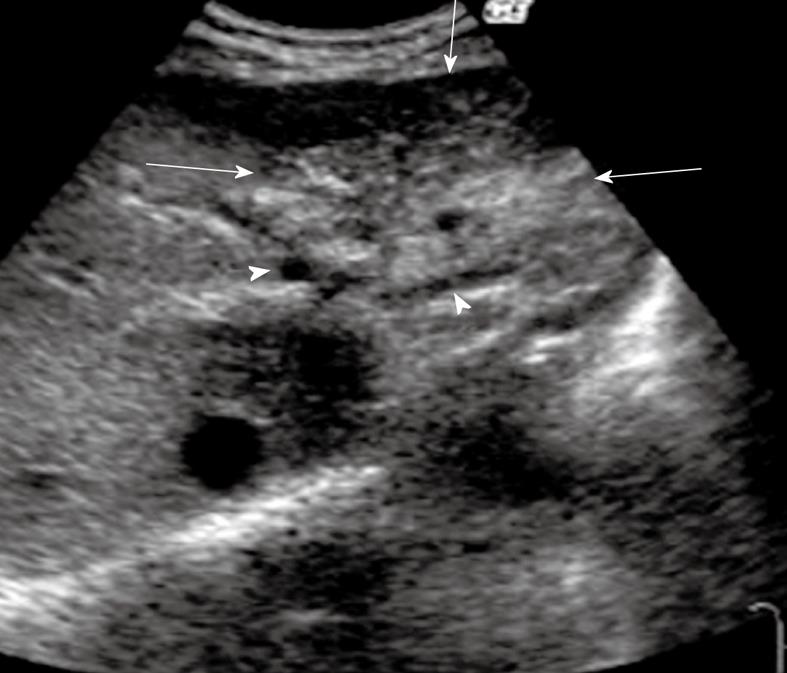
US imaging features of mass-forming cholangiocarcinoma are as follows: peripheral bile duct dilatation, absence of liver cirrhosis, irregular tumor border, arterial enhancement due to minute intratumoral blood sinusoids, and hypoenhancement in the late vascular phase (Figure 6).
Figure 6 Intrahepatic cholangiocarcinoma (mass-forming type).
B-mode ultrasonography showed mixed echogenicity with irregular borders (arrows) in the left lateral lobe. The intrahepatic bile duct peripheral to the tumor was dilated (arrowheads).
The bile ducts peripheral to the tumor are usually dilated because of obstruction by the tumor, however, the US findings of intrahepatic cholangiocarcinoma are fundamentally very similar to those described in liver metastases[71]. Mass-forming cholangiocarcinomas can be hypoechoic, hyperechoic, or have mixed echogenicity, with irregular borders. Peripheral cholangiocarcinoma can appear as a solitary mass or as diffusely abnormal liver echotexture. Because of their nonspecific symptomatology, mass-forming lesions are generally far advanced when detected by US. In addition, mass-forming lesions might mimic HCC or metastases on B-mode US[70].
Color Doppler US typically shows a poor color signal in cholangiocarcinoma compared with HCC, which is hypervascular. Hence, color Doppler US is helpful in differentiating vessels from dilated ducts and can provide information regarding the status of vessels. It is considered highly accurate in detecting neoplastic involvement of the portal vein. In the study by Neumaier et al[78], the sensitivity of color Doppler US for portal vein occlusion was 100% and that for portal vein infiltration was 83%, with 100% specificity. However, there was poor sensitivity in detecting infiltration of the hepatic artery (43%) and metastases to regional lymph nodes (37%), liver (66%), and peritoneum (33%)[78].
Although the imaging findings of peripheral cholangiocarcinoma showed certain characteristics on low-mechanical index (MI) contrast-enhanced sonography, contrast-enhanced US findings are fundamentally similar to those described for liver metastases. It has been reported[79] that all peripheral cholangiocarcinomas show inhomogeneous enhancement during the arterial phase, such as irregular, peripheral, rim-like hyperenhancement (44.4%), inhomogeneous hyperenhancement (11.1%), or inhomogeneous hypoenhancement (44.4%). However, all cholangiocarcinomas show hypoenhancement in the late vascular phase.