INTRODUCTION
It is important to localize prostate gland tumors to evaluate the transcapsular spread and staging in order to plan treatment protocols and avoid positive anterior surgical margins during radical prostatectomy. Prostate cancer arises from the peripheral zone (PZ) in 75%-85% of patients[1]. Cancers arising from the transition zone (TZ) represent 40% of autopsy series and 25%-30% of radical prostatectomy series[1]. The utility of magnetic resonance (MR) imaging in prostate cancer is currently under investigation, and it has been shown to be an excellent technique for evaluating prostate cancers, particularly PZ cancers[2,3]. As TZ cancers are less frequent than PZ cancers, MR imaging in TZ cancers has not been widely used. However, recent studies attempting to identify MR characteristics of the TZ, by means of emerging techniques, have shown that MR can be used to delineate TZ cancers accurately[4-7]. Herein, the MR imaging features of TZ tumors, the role of MR imaging in detection and staging, and recent advanced MR techniques in the evaluation of TZ cancers will be discussed including a review of literature.
ANATOMY AND MR IMAGING OF THE PROSTATE GLAND
According to zonal anatomy, the prostate is composed of anterior fibromuscular stroma, periurethral glandular tissue, the TZ, central zone (CZ) and PZ. The TZ is the inner prostate and forms 5% of the gland. It surrounds the anterior and lateral parts of the proximal urethra. In younger men this zone is small, however, with aging it enlarges and compresses the CZ due to hyperplastic changes. The CZ is the outer prostate forming approximately 25% of the gland in young men[8]. It is less clearly distinguished histologically from the PZ. The PZ is the outer prostate and forms 70% of the gland[8]. Radiologically, the prostate has been divided into two parts: the PZ and the central gland which is composed of the PZ, TZ and CZ[9]. In young men, the gland is mainly composed of the CZ. With aging, the TZ is enlarged due to benign prostatic hyperplasia (BPH) which commonly arises from the TZ[10].
MR imaging enables differentiation between the PZ, CZ and TZ. In young adults, normal prostate is homogenous, whereas with aging the differentiation between the PZ and the central gland is more clearly depicted. T1-weighted (T1W) images distinguish between the prostatic parenchyma and the surrounding periprostatic fat and vascular plexus. On T1W images, the homogenous gland has an intermediate-to-low signal intensity, and zonal differentiation can not be identified[11]. Post-biopsy hemorrhage has high signal-intensity on T1W images. On T2-weighted (T2W) images, better tissue differentiation is achieved and zonal anatomy is better depicted[12]. As the glandular components are more prominent in the PZ, it has a homogeneously high signal intensity and is surrounded by a capsule which is seen as a thin, hypointense rim on T2W images. Both the CZ and TZ are hypointense compared to the PZ because of their stroma which consists of compact muscle fiber bundles. MR also enables multiplanar imaging of the prostate (Figure 1).
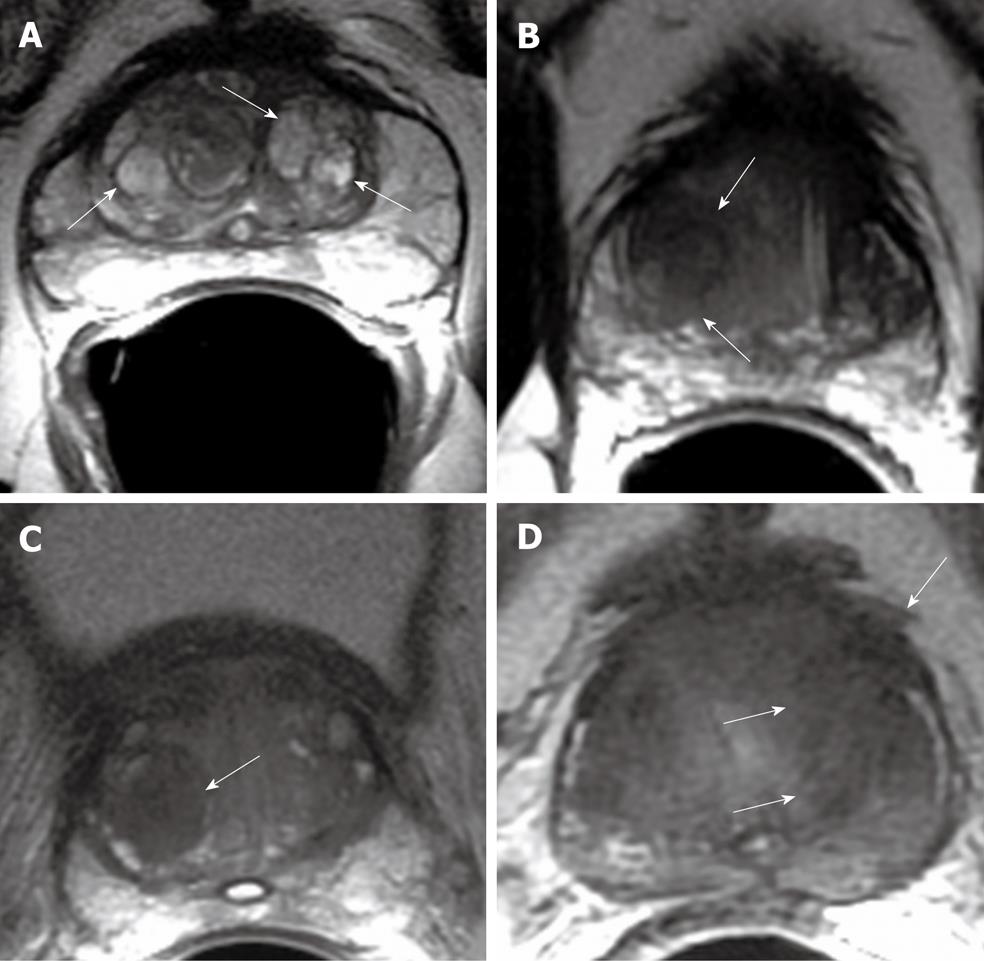
Figure 1 Magnetic resonance (MR) images demonstrating zonal anatomy of prostate gland.
A: Axial T2-weighted (T2W) MR image depicts the central gland and peripheral zone (PZ). Central gland is hypointense compared to hyperintense PZ; B. Coronal T2W MR image shows hyperintense PZ and hypointense central gland.
MR imaging has been increasingly used in the evaluation of prostate cancer[13-18]. It enables multiplanar imaging and is superior to ultrasound and computed tomography in anatomic and volumetric evaluation of the gland[19]. It is more accurate than digital rectal examination and transrectal ultrasound (TRUS)-guided biopsy for cancer detection and localization. In a recent study, the detectability of prostate cancer using MR imaging prior to TRUS-guided biopsy was determined by calculating the sensitivity and positive predictive value of TRUS, T2W imaging, diffusion weighted imaging (DWI), apparent diffusion coefficient (ADC) map and biopsy[20]. The relationship between the detectability on each sequence and cancer location, Gleason score, and the short and long axis diameter of the tumor were also evaluated. The sensitivities were 26.9%, 41.2%, 56.7%, 57.7% and 75.1%, respectively. The sensitivity of each sequence increased as the Gleason score and the short- and long-axis diameters of the tumors increased. It was stated that MR imaging prior to biopsy has a high detectability for prostate cancer. MR imaging is used to guide targeted biopsy when prostate cancer is clinically suspected and previous ultrasound-guided biopsy results are negative. MR imaging also enables the localization and staging of prostate cancer. The high soft tissue resolution of MR imaging helps to show extracapsular extension and seminal vesicle invasion. It may be used in planning a roadmap for therapeutic approaches and for residual or locally recurrent cancer after treatment. MR imaging has mainly been used as a diagnostic tool for the detection of PZ cancers[18-21]. It is considered insufficient for evaluating the TZ, as BPH, which causes a heterogenous signal intensity, especially in elderly men, also originates from the TZ leading to conspicuous findings on T2W images[2,22,23]. Recent studies using MR imaging of TZ cancers have shown that it can be used in the detection of TZ tumors that are not sampled during TRUS-guided biopsy and also for localization and staging[4].
MR IMAGING OF TZ CANCERS
Prostate cancer begins as a small focus of carcinoma within the gland which grows very slowly[24]. Approximately 75%-85% of cancers arise from the PZ, 25% arise from the TZ and 10% arise from the CZ[1,25,26]. As there is no clear demarcation between the CZ and the PZ, most pathologists do not routinely recognize tumors as originating from the CZ. For that reason, comparison is generally focused on the distinctions between PZ and TZ cancers. TZ tumors are located anteriorly, far from the rectum and they are more difficult to detect compared to PZ tumors. These tumors can be of a large volume and are associated with high serum prostate specific antigen (PSA) levels but they are confined to the gland[27]. They are mostly low grade and relatively nonaggressive. Most TZ tumors are found incidentally in resection specimens. It is important to accurately distinguish TZ cancers to guide biopsy and to avoid positive anterior surgical margins at radical prostatectomy.
Currently, the PZ is the primary target in most biopsies[28]. However, in patients with elevated PSA levels with negative biopsy results, it should be kept in mind that the tumor focus may be in the central gland. Therefore, it has been suggested that TZ-targeted biopsy should be performed in patients with multiple negative biopsy results. As a result, although tumor zonal origin is not an independent determinant of biochemical failure, it is helpful in predicting the route of cancer spread. If the zonal origin can be determined preoperatively, the cure rate may be increased by modification of the surgical approach.
The central gland has a heterogeneously variable signal intensity appearance in older men due to the presence of BPH or other coexisting benign diseases. BPH nodules occur almost exclusively in the TZ. As hypertrophied TZ tissue might also show metabolic heterogeneity similar to BPH nodules, it may be difficult to differentiate them from carcinoma. Discrimination between BPH and central gland tumors is important for staging. BPH is an enlargement of the TZ (central gland) which gives a heterogeneous appearance on MR imaging[29,30]. BPH nodules may be seen as hypointense, isointense or hyperintense on T2W images, depending on the ratio of glandular to stromal tissue[31]. It has been shown that, high signal intensity is due to hyperplastic glandular elements which are filled with secretion and the presence of cystic ectasia (Figure 2A). Low signal intensity is due to the presence of prominent sclerotic, fibrous or muscular elements[22,29] (Figure 2B).
Figure 2 Axial T2W MR image.
A: Multiple, well defined hyperintense glandular benign prostatic hyperplasia (BPH) nodules in central gland (arrows); B: Well defined, amorphous, hypointense TZ tumor (arrows); C: Hypointense stromal BPH nodule in the right transition zone (TZ) (arrow); D: Hypointense TZ tumor with extracapsular extension (arrows).
TZ cancers tend to have uniform low intensity on T2W imaging, but their diagnosis is not certain in the presence of coexisting benign disease[31,32] (Figure 2C and D). It has been shown that, unless cancers in the TZ are of a large dimension, their detection on MR imaging is very difficult[33]. Akin et al[4] determined the accuracy of MR imaging in detection and local staging in 148 patients. Features indicative of TZ cancers were defined as: homogenous low T2 signal intensity, ill defined margins, lack of capsule, lenticular shape, and invasion of anterior fibromuscular stroma. For identification of patients with TZ cancer, the sensitivity of MR imaging was 75%-80% and the specificity was 78%-87%. The area under the receiver operating characteristic curve was 0.75 for detection and localization of tumor. For detection of extraprostatic extension, the sensitivity and specificity of MR imaging were 28%-56% and 93%-94%, respectively. Li et al[5] determined the conventional MR findings of TZ lesions in 86 patients, of which 53 were cancers and 33 were benign, by comparing T2W and contrast-enhanced T1W images. Lesions were classified as uniform, low signal intensity on T2W images, lesions with homogeneous contrast enhancement and lesions with irregular margins on both gadolinium enhanced T1 and T2W images. Sensitivity, specificity and accuracy for cancer were 50%, 51% and 51%, respectively, for the uniform low T2 signal intensity criterion; 68%, 75% and 71% for homogeneous gadolinium enhancement; 60%, 72% and 65% for irregular margins on both T2W and gadolinium enhanced images.
ADVANCED MR TECHNIQUES
TZ cancers are difficult to diagnose particularly in the presence of BPH. Even in the PZ, some cancers such as those with a more permeative pattern can not be detected. Moreover, focal prostatic atrophy or prostatitis may also mimic cancer and may cause false-positive results. To increase the accuracy of MR imaging and to improve the detection of prostate cancer at an earlier stage, special techniques such as DWI, dynamic contrast-enhanced MR imaging (DCE-MRI), MR spectroscopy (MRS) and high-field-strength (3.0-T) MR imaging have been increasingly used. It has also been shown that these techniques may play a role in the detection of prostate tumor foci in patients with persistently elevated PSA levels and prior to negative random TRUS-guided biopsy[34].
DWI
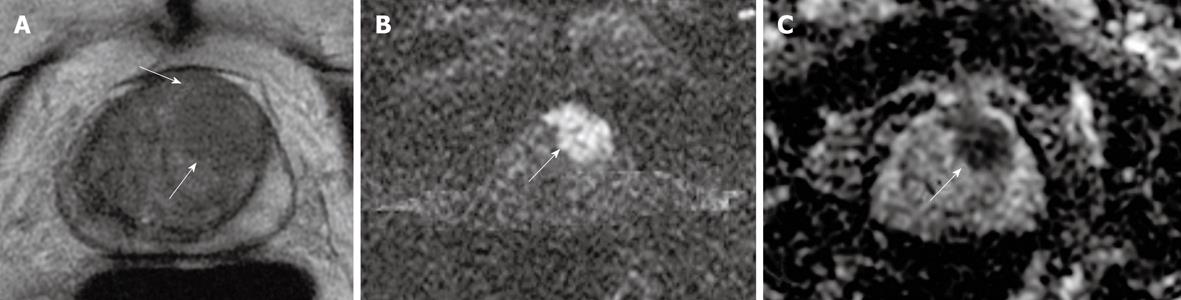
DWI is a technique sensitive to molecular translation of water in biologic tissues due to the random thermal motion of molecules. The rapid changes in the movement of water in tissues and the measurement of the flow of water molecules can be identified by calculating the ADC[35]. When the flow of water or diffusion is restricted, ADC is decreased. If ADC values are increased, there is no restriction in water flow. The ADC has been determined for tumor growth. It has been shown that, in proliferating cells, cellular density increases and extra- as well as intracellular space decreases leading to decreased ADC[36]. In recent years, an increased number of studies have evaluated the utility of DWI in prostate cancer diagnosis[37-44]. It has been shown that cancer tissues show higher signal intensity on DWI and thus a lower ADC compared with BPH nodules and normal tissue due to replacement of normal tissue (composed of water rich acinar structures) with densely packed malignant epithelial cells. TZ tumors have also been shown to have lower ADC values than the surrounding tissue[37] (Figure 3). Namiki et al[45] stated that different b factors may effect the detection of tumors. Noworolski et al[41] showed that glandular-ductal tissues (glandular BPH) had lower peak enhancement and higher ADC values than the stromal-low ductal tissues (stromal BPH and central gland). Oto et al[46] showed significant ADC differences between tumor, stromal BPH and glandular BPH (lowest in tumor, highest in glandular BPH). These authors stated that there were differences between the perfusion parameters of tumor, stromal and glandular BPH, with the exception of the k-trans values between tumor and glandular BPH. Tamada et al[47] compared the ADC values in peripheral and transitional zones between normal and malignant prostatic tissues. Mean ADC values were significantly lower in both the PZ and TZ than in the corresponding normal regions. Ren et al[48] investigated the diagnostic value of DWI and ADC values in normal and pathologic prostate tissues. They showed that BPH nodules had a lower and non-homogenous signal intensity than the PZ. Prostate cancer showed high signal intensity while prostate cyst showed low intensity. ADC values of BPH nodules were larger than prostate cancer foci and normal central gland. They stated that DWI and ADC values for normal central gland, PZ, prostate cyst, BPH nodules and cancer foci showed significant differences and could be used in the differential diagnosis of diseases of the prostate gland. Yoshizako et al[6] determined the clinical value of DWI and DCE-MRI in combination with T2W images, for the diagnosis of TZ tumors. They found that adding DWI to T2W images improved the sensitivity, specificity, accuracy and positive predictive value of diagnosing TZ tumors. In a recent study, the need for biexponential signal decay modeling for prostate cancer diffusion signal decays with b-factor over an extended b-factor range was evaluated. The researchers found that the fast and slow ADC values of cancer were significantly lower than those of the TZ and PZ, and the apparent fraction of the fast diffusion component was significantly smaller in cancer than in the PZ. It was stated that biexponential diffusion decay functions were required for prostate cancer diffusion signal decay curves when sampled over an extended b-factor range, enabling specific tissue characterization of prostate cancers[49].
Figure 3 Tumor in the left mid prostate gland demonstrated by MR.
A: Axial T2W image shows ill defined, amorphous, hypointense tumor (arrows); B: Diffusion weighted imaging (DWI) reveals focal area of bright signal consistent with tumor(arrow); C: Apparent diffusion coefficient (ADC) map reveals clear focal mass with dark signal consistent with decreased ADC (arrow).
DCE-MRI
DCE-MRI was introduced to effectively visualize the pharmacokinetics of gadolinium uptake in the prostate gland. It depicts the physiological function of the tumor microcirculation. There is a relationship between contrast material uptake and microvascular structures in tumors, in which tumor angiogenesis is correlated with the parameters of signal intensity-time curves. As the reliability of T2W MR imaging in distinguishing prostate cancer of the PZ and TZ is limited, several studies have been performed to delineate the enhancement characteristics of prostate cancer to achieve more accurate information[2,50-53]. In a recent study, the accuracy of T2W and DCE-MRI for cancer detection in 18 prostate cancer patients were compared prior to prostatectomy[54]. The accuracy of DCE-MRI for cancer detection was calculated by a pixel-by-pixel correlation of quantitative DCE-MRI parameter maps and pathology. It was shown that DCE-MRI was more sensitive than T2W images for tumor localization (50% vs 21%) and more specific (85% vs 81%). The researchers stated that due to its higher sensitivity and specificity, DCE-MRI could be used to guide radiotherapy boosts in prostate cancer patients. Due to increased microvessel density (MVD) in carcinomatous tissue, the enhancement curve of prostate tumors was shown to be different when compared to the PZ and BPH. Engelbrecht et al[55] found that in both the PZ and TZ, the relative peak enhancement was the optimal parameter when compared to other parameters such as onset time, time to peak, peak enhancement and wash-out. Yoshizako et al[6] stated that the addition of DCE-MRI to T2W images and DWI improved the specificity and positive predictive value of diagnosing TZ cancer (93.8% and 94.7%, respectively). Turnbull et al[2] found significant differences in amplitude of the initial enhancement and wash-out patterns between carcinoma and BPH. In both the PZ and the central gland, relative peak enhancement was the optimal parameter. The combination of relative peak enhancement with other dynamic parameters (onset time, time to peak, peak enhancement, and washout) did not yield a significant gain in discriminatory performance. Ogura et al[56] demonstrated a sensitivity, specificity and accuracy rate of 37%, 97% and 63%, respectively, for the detection of TZ cancer. In another study, it was shown that the glandular-ductal tissues had lower peak enhancement than the stromal-low ductal tissues suggesting that gadolinium-DTPA does not enter healthy prostatic tissues[2]. Ren et al[57] examined DCE-MRI parameters in 21 patients with prostate cancer and 29 patients with BPH by means of signal intensity-time curves and angiogenesis. Prostate cancer showed stronger enhancement with an earlier peak time, higher enhancement and enhancement rate. The vascular endothelial growth factor (VEGF) and MVD expression levels in cancer were higher than in BPH. They found a negative correlation between peak time and the expression levels of VEGF and MVD, however, the degree of enhancement and enhancement rate showed positive correlations.
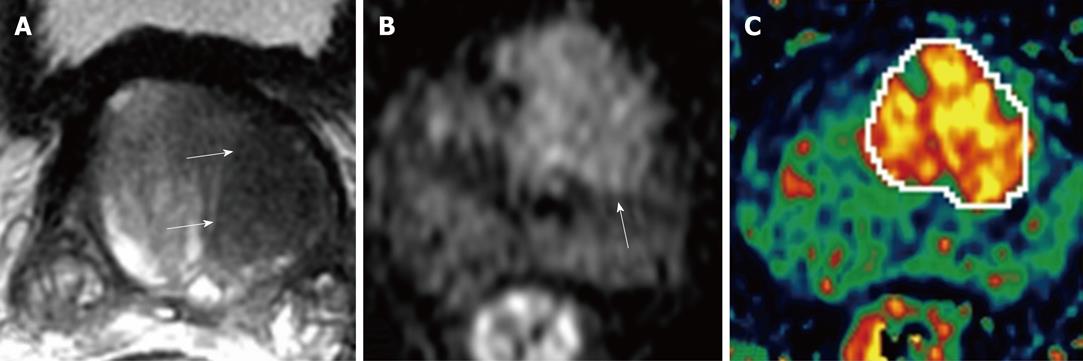
In cancerous tissues, there is uncontrolled angiogenesis and the permeability of vascular structures is markedly increased resulting in significantly different pharmacokinetics compared to surrounding normal tissue. Pharmacokinetic parameter mapping clearly identifies pathologic areas in heterogeneously enhanced prostate. K-trans maps enable the identification of tumor within heterogeneously enhanced PZ and can reveal the extent of extra-glandular involvement. These maps may also be useful in providing a biopsy target and in revealing intra-tumoral heterogeneity (Figure 4).
Figure 4 Left TZ tumor of prostate gland demonstrated by MR.
A: Axial T2W image depicts ill defined, round, homogenous hypointense tumor (arrows); B: DWI depicts focal area of bright signal on left mid gland (arrow); C: K-trans map in dynamic contrast-enhanced MR imaging (DCE-MRI) clearly localizes the tumor and reveals some internal heterogeneity.
MRS
MRS imaging is an emerging technique used in combination with MRI in the evaluation of prostate cancer[58-63]. This technique allows the metabolites within tissues to be identified and provides information on the biochemical and metabolic environment of tissues. As prostate is composed of different types of glands and tissues, it is difficult to study the gland using MRS. However; there are sophisticated chemical shift filtering techniques and three dimensional chemical shift imaging which allow examination of the entire prostate at one time and the selection of particular chemicals for diagnosis[59,64]. It has been shown that stromal and glandular tissue have the same resonances with different relative peak height intensities[65]. In addition, it has been stated that citrate is produced by glandular epithelial cells and the amount of glandular elements can affect tissue citrate levels. Glandular BPH has higher levels of citrate than stromal BPH[66]. It has also been stated that citrate levels show the degree of tissue differentiation, in that poorly differentiated tumors have lower citrate levels than well differentiated tumors[67]. Healthy PZ is known to have high citrate content, whereas in cancer tissues, the resonance signal from citrate is reduced or even absent. Adenocarcinomatous tissue in the prostate gland also shows a similar spectrum to adenocarcinoma in other organs (except for citrate)[68], which show elevated choline relative to creatine due to the increased cell proliferation associated with malignant tumors[69]. In their series performed in 40 patients, Zakian et al[7] studied the mean values of choline + creatine/citrate, choline/creatine and choline/citrate in TZ cancer and normal tissue, in which a significant difference was found. It was shown that 56% of patients had tumor voxels with at least one detectable choline peak, while control voxels showed only choline peaks.
3.0-T MR imaging
High-field-strength MR imaging has recently been investigated in prostate imaging. The introduction of 3.0-T MR scanners has resulted in an increase in the in-plane resolution of anatomic T2W imaging due to higher signal to noise ratio. Higher magnetic field strengths have been shown to enable structural imaging of the prostate with improved spatial resolution leading to improved detection and staging of PZ tumors[70-72]. Moreover, functional imaging such as DWI, DCE-MRI or MRS at high field strength is thought to improve the detection of CZ and TZ cancers, prevent false-positive diagnoses and help less experienced readers to improve their local staging performance[73,74].
CONCLUSION
TZ cancers demonstrate similar imaging features to BPH and are therefore more difficult to diagnose on MR imaging. However, certain imaging features (alone or in combination) on multi-parametric MR imaging can help in the differentiation between cancerous and benign TZ tissue. MR imaging can also provide reliable local staging of TZ cancers. By the addition of emerging MR techniques, such as DWI, DCE-MRI, MRS and high-field-strength (3.0-T) MR imaging to standard T2W images, MR imaging has now become a promising technique in the evaluation of TZ tumors.
Peer reviewers: James Chow, PhD, Radiation Physicist, Radiation Medicine Program, Princess Margaret Hospital, 610 University Avenue, Toronto, ON, M5G 2M9, Canada; Chan Kyo Kim, MD, Assistant Professor, Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon-dong, Kangnam-gu, Seoul 135-710, South Korea
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM