INTRODUCTION
Fertility is a two-person phenomenon and successful conception depends on a complex set of interactions between the male and the female reproductive tracts. Infertility is defined as failure to conceive after 1 year of unprotected intercourse. Infertility is a relatively common problem that affects approximately 15% of the reproductive age range population[1]. More couples now seek infertility evaluations, which may reflect the increased availability of infertility-related services and an increased media focus on medical advances in reproductive technology. Male and female factors coexist in about one third of cases, whilst one third of cases are secondary to male factors only[2]. Therefore the man should be evaluated concurrently with the woman, since a male factor is the primary or contributing cause in 40% to 60% of cases. In addition to detecting treatable abnormalities, evaluation of the infertile man is critical to uncover life-threatening problems associated with the symptom of infertility, as well as genetic conditions associated with male infertility that could be transmitted to offspring with assisted reproduction. New diagnostic tests have been developed and surgical techniques refined resulting in improved treatment results and patient care[3].
A large body of literature describes the causes, investigations, and treatment of infertility. This paper reviews the causes of infertility in men with obstruction of the seminal tract, describes medical imaging investigations, and suggests guidelines for referral to infertility specialists.
CAUSES OF MALE INFERTILITY
Male infertility has many causes, which may be pre-testicular, testicular, and post-testicular. From the practical point of view, abnormalities that cause testicular failure and impaired spermatogenesis cannot be corrected while obstructive processes involving the sperm transport system are potentially correctable. Post-testicular causes include obstruction of the sperm delivery route (male factor obstructive infertility) anti-sperm antibodies and retrograde ejaculation[4]. Obstruction can occur at any level either proximal, affecting the epididymis or distal, affecting the ejaculatory duct[5,6].
It is important to distinguish non-obstructive azoospermia from obstructive azoospermia, because infertile men with obstructive azoospermia may be amenable to surgical or interventional correction. On the other hand, in those with primary testicular failure, it may be reasonable to proceed directly to an advanced assisted reproductive technique such as intracytoplasmic sperm injection[7]. Obstruction of the seminal tract represents 6% of cases[3]. Men with obstructive azoospermia typically have normal-sized testes, possible epididymal fullness, and a normal serum follicle stimulating hormone (FSH). Men with non-obstructive azoospermia present frequently with small or soft testes and an elevated FSH[8-10].
Seminal tract obstruction can be classified according to the level of obstruction, into proximal seminal tract obstruction, including epididymis and scrotal portions of the vas deferens and distal seminal tract obstruction including inguinal, pelvic and ampullary portions of the vas deferens, and ejaculatory ducts. Pathology from complete ejaculatory duct obstruction (EDO) occurs in < 1% of infertile men, whereas the frequency of incomplete obstructive pathologies is reportedly 4.4%[11]. Seminal tract obstruction may be congenital or acquired. Congenital causes include atresia or stenosis as well as midline prostatic cystic lesions, e.g. utricular, Müllerian and ejaculatory duct cysts. Acquired causes may be of inflammatory or traumatic origin, including calculus formation and stenosis after transurethral resection of the prostate[5].
Fortunately, fifty percent of the causes of male infertility are potentially correctable[12]. The main purpose of imaging evaluation in cases of male infertility is to identify these correctable causes. Treatment of correctable male-factor pathology is cost effective, does not increase the risk of multiple births, and can spare the woman invasive procedures and the potential complications associated with assisted reproductive technologies[13].
MEDICAL IMAGING IN MALE INFERTILITY
Various imaging modalities are available to evaluate men with obstructive infertility such as scrotal ultrasonography, transrectal ultrasound (TRUS), vasography, magnetic imaging resonance, seminal vesicle aspiration, seminal tract washout (STW), and seminal vesiculography. The imaging and analysis of infertility in males has become more common in recent years. The practicing radiologist should be familiar with the evaluation of the infertile man and the common radiologic findings and disease processes associated with infertility[14-16].
Scrotal ultrasound
Ultrasound (US) is a widely used and well tolerated imaging modality for evaluation of pathologic conditions in male factor infertility. Recent technical advances in US applications and post processing developments have enabled new aspects in the structural and functional analysis of testicular tissue, varicocele and seminal tract. US is being used with increased frequency in the evaluation of the infertile male. Scrotal US is considered the primary imaging modality for the evaluation of scrotal abnormalities[17-19]. Scrotal US can be helpful in determining whether azoospermia is non-obstructive or obstructive, because it can directly detect abnormalities in the testis, mediastinum testis, epididymis, and the proximal vas deferens.
Scrotal US findings in non obstructive Azoospermia: Testicular pathologies causing infertility include; cryptorchidism, atrophy, torsion/infarction, inflammation,mumps, tuberculosis, neoplasm, trauma, microlithiasis, hydrocele, and varicocele. Varicoceles are the most frequent physical finding in infertile men; indeed, they may be responsible for nearly one-third of cases of male infertility[20]. Scrotal US is a good diagnostic tool for diagnosis of varicocele (sensitivity 97%, specificity 94%)[21]. The commonly accepted color Doppler US criterion for varicocele with a maximal vein diameter of 3 mm or greater had a sensitivity of 53% and specificity of 91% compared to physical examination[22]. Varicocele management, however, has always been a controversial issue because very few randomized, controlled studies have been performed to examine varicocelectomy as an infertility treatment. Significant evidence suggests that varicoceles have a harmful effect on the testis and that varicocelectomy can not only prevent progressive decline in testicular function but also reverse the damage[23-25].
However, the degree to which varicocele repair improves pregnancy rates and the success of assisted reproductive technology remains controversial. Varicoceles are associated with infertility but the significance of this relationship is uncertain; surgical or radiological repair of varicoceles is not recognized as appropriate treatment for infertility[20,26-29].
Scrotal US is used to examine the testes in at least two planes, the transverse and longitudinal; the size is measured and the echotexture is evaluated[30]. Recent technical advances in US applications and post processing developments have enabled new aspects in the structural and functional analysis of testicular tissue and therefore male fertility[31].
Testicular volume measured by testicular US correlates significantly with testicular function. Increased resistive index and pulsatility index of capsular branches of the testicular arteries on unenhanced color Doppler US examination may be an indicator of impaired testicular microcirculation[19]. Also, if a testis is non-palpable, scrotal US can determine whether it is congenitally absent, cryptorchid, atrophic, or ectopic[18].
Scrotal US findings in obstructive Azoospermia: Scrotal US can directly demonstrate abnormalities in the proximal portion of the seminal duct and can also depict secondary changes of the proximal seminal duct caused by obstruction in the distal part of the seminal duct. Evaluation of the proximal genital duct and measurement of testicular volume with scrotal US are helpful in distinguishing obstructive azoospermia from nonobstructive azoospermia in infertile men. Testicular volume measured at scrotal US is higher for obstructive azoospermia than for nonobstructive azoospermia. The median testicular volume in obstructive azoospermia was 11.6 mL (range, 7.7-25.8 mL) and that in nonobstructive azoospermia was 8.3 mL (range, 1.2-16.4 mL) (P < 0.05)[7].
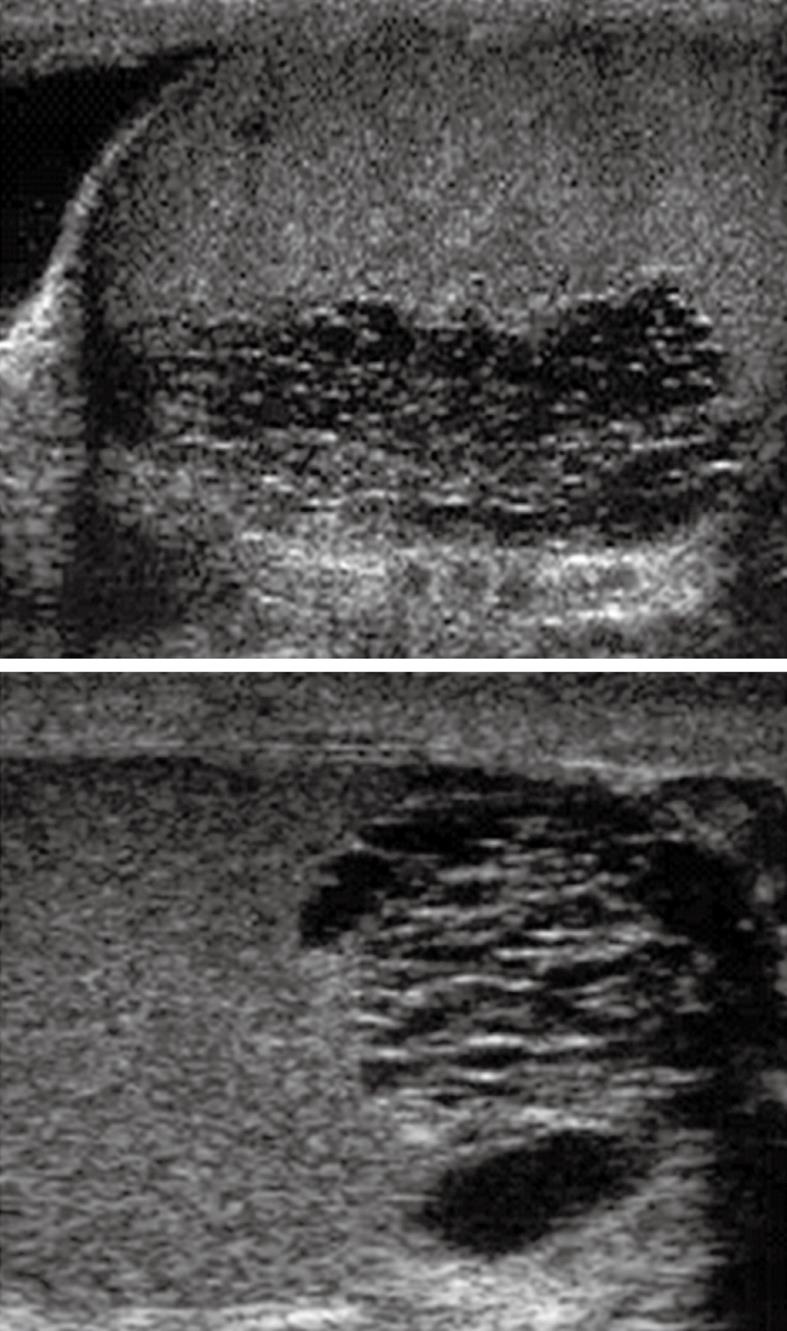
Scrotal US also can diagnose obstruction in an azoospermic patient by directly demonstrating dilatation in the proximal seminal duct (mediastinum testis, epididymis, and intrascrotal portion of the vas deferens) as seen in Figure 1. The epididymal abnormalities depicted with scrotal US are significantly associated with obstructive azoospermia (P = 0.001)[7]. Scrotal US may also depict secondary changes of the proximal genital duct caused by distal genital duct obstruction (terminal vas deferens, ampulla of the vas deferens, seminal vesicle, and ejaculatory duct)[26]. Thus, evaluation of the epididymis and testicular volume with scrotal US are important in distinguishing obstructive azoospermia from nonobstructive azoospermia in infertile men. Sensitivity, specificity, and accuracy of scrotal US for differentiation of obstructive from nonobstructive azoospermia were 82.1%, 100% and 87.5%, respectively[7].
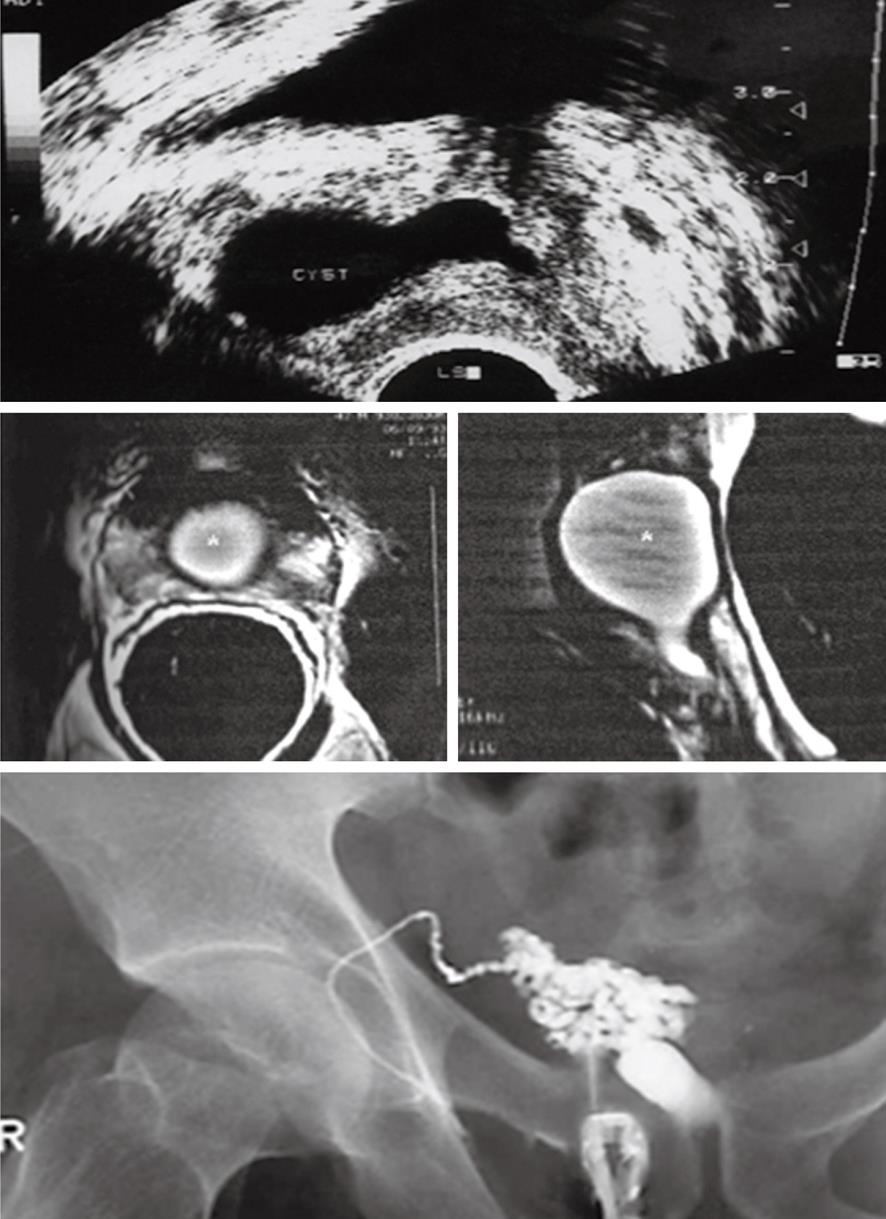
Figure 1 Ultrasound of both testes (sagittal images) demonstrates ectasia of the testes with formation of intratesticular cysts.
These finding are suggestive of a seminal tract obstructive etiology which should be managed by epididymo-vasotomy.
TRUS
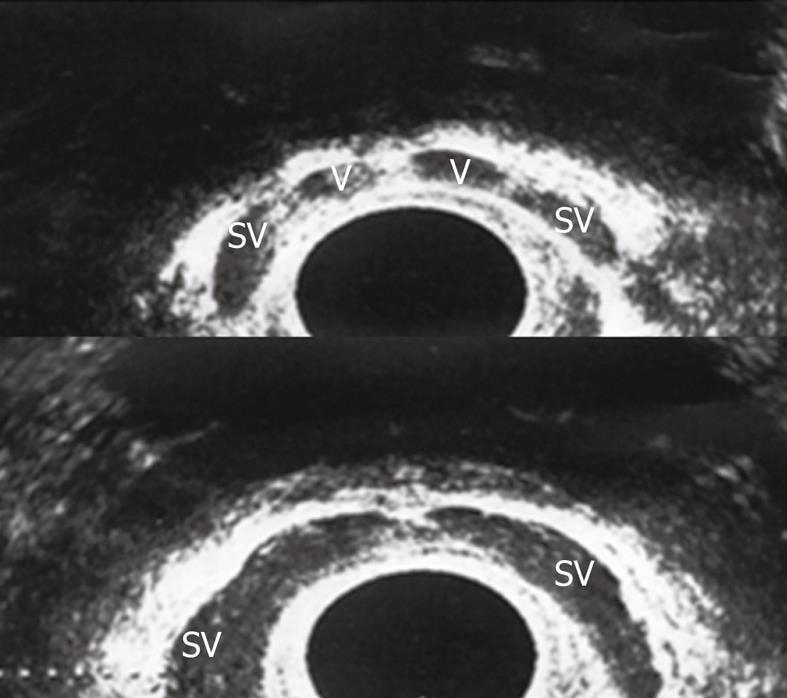
TRUS can clearly visualize the distal genital tract as vassal ampullae, seminal vesicles and ejaculatory ducts (Figure 2). Seminal vesicles are thought to be normal when > 25 mm in length, hypoplastic when > 16 mm but < 25 mm and atrophic when < 16 mm. TRUS proved to be a reliable diagnostic tool in men with obstructive infertility, especially when combined with seminal analysis. TRUS is most commonly performed if the diagnosis of distal seminal tract obstruction is being considered[5,32]. The role TRUS is now firmly established in diagnosing post testicular causes of infertility.
Figure 2 Transrectal ultrasound (TRUS) in the axial plane showed normal both vasal ampullae (V) and seminal vesicles (SV).
Pathologic findings were detected in 75% of patients with azoospermia on TRUS. However TRUS did not reveal any pathologies in 64.7% of patients with nonazoospermia. The incidences of hypoplastic/atrophic seminal vesicles and vasal agenesis were significantly higher in the azoospermic subgroup (P < 0.002)[33].
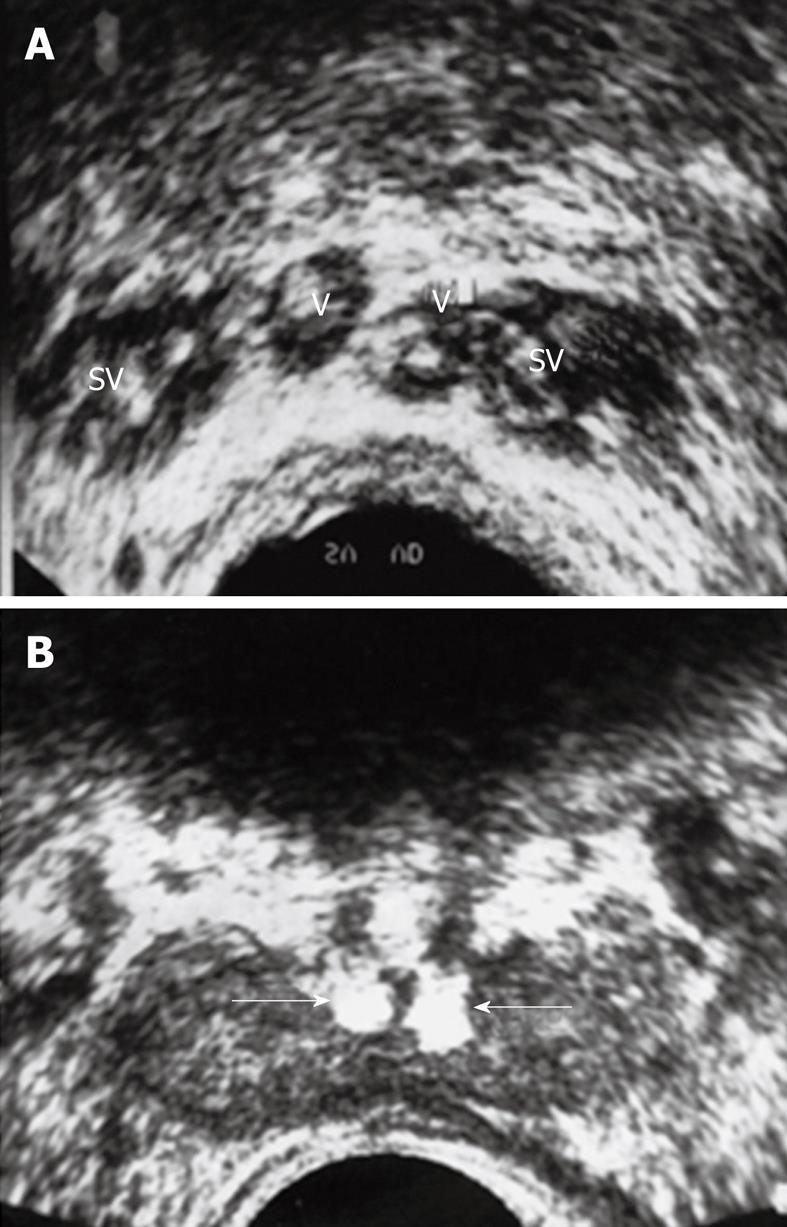
Currently, the most important indication for TRUS to assess for obstruction and the absence or hypoplasia of the seminal vesicles and the ejaculatory ducts, is low ejaculate volume. TRUS has proved invaluable in visualizing and evaluating the patency of the ductal system. The advantage of TRUS is that it is non invasive, well accepted by patients and aids in visualizing the normal and abnormal seminal vesicles, the vasa deferentia, ejaculatory ducts and the prostate. Congenital abnormalities of the vas are the most common finding on TRUS in men with azoospermia and low ejaculate volume. Agenesis of the vas deferens is reported to occur in between 1.0%-2.5% of cases. It may be partial, complete, unilateral or bilateral. Other vasal abnormalities that may be seen on TRUS in infertility are: echogenic vas (fibrosis, calcification), cysts of the vas deferens and calculi (Figure 3). Obstructive findings are also seen in seminal vesicles secondary to midline urogenital cysts as utricle and Mullerian duct cysts. Seminal vesicles are thought to be absent when no tissue is identified. Seminal vesicular cysts are commonly congenital rather than acquired. The obstructed ejaculatory duct as seen as a hypoechoic tubular structure, is best seen in the saggital plane. The US features of EDO are: ejaculatory duct cyst, calcification, dilatation and seminal vesicular dilatation[17]. Transrectal ultrasonography is the initial investigation method used to visualize and locate the presence of a cyst or calcifications that may contribute to the obstruction. Transurethral resection of the ejaculatory ducts (TURED) represents the best treatment modality, resulting in marked improvement in the semen parameters and pregnancy rate in well selected cases[34].
Figure 3 Twenty five years infertile man with azospermia.
A: Multiple calculi within the SV and V; B: Bilateral echogenic calculi impacted within the ejaculatory ducts (arrows).
Vasography
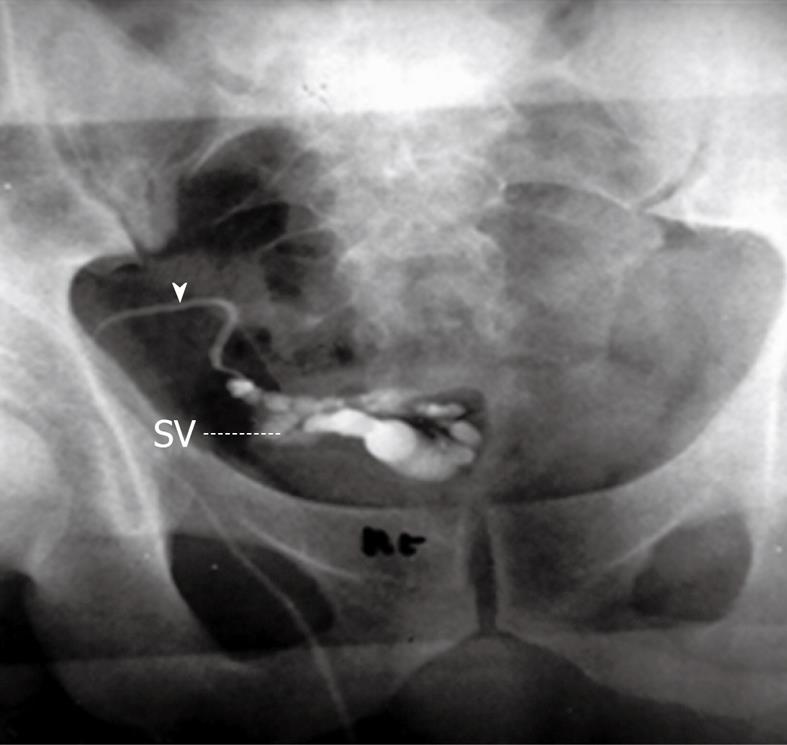
Traditionally vasography (vesiculodeferentography) has been considered the gold standard radiological imaging modality used for evaluation of the patency of the seminal tract (Figure 4). It can clearly demonstrate the site of obstruction along the seminal tract (Figure 5). Unfortunately, it has the complication of possible iatrogenic vasal stricture[35]. This complication has narrowed its clinical use in obstructive infertility. Vasography has been considered the reference standard for the evaluation of the distal genital duct, but is an invasive procedure which carries the risk of genital duct scarring. Vasography was used in the past to evaluate suspected cases of obstruction of the seminal ducts. Over the years, numerous attempts have been made to improve the technique used to perform this examination and to render it less invasive. Bilateral dilatations of the seminal vesicles and/or dilated ejaculatory ducts with no contrast flow into the urethra are the common findings of complete EDO on vasography. In contrast, vasography may not always confirm a diagnosis of partial EDO, as the contrast medium used in this method may pass into the bladder on partial obstruction, similar to the flow seen in patients with no obstruction[36,37]. Currently, the use of vasography is indicated in selected cases, where it is combined with functional studies like STW and followed by immediate interventions to correct the obstruction[38].
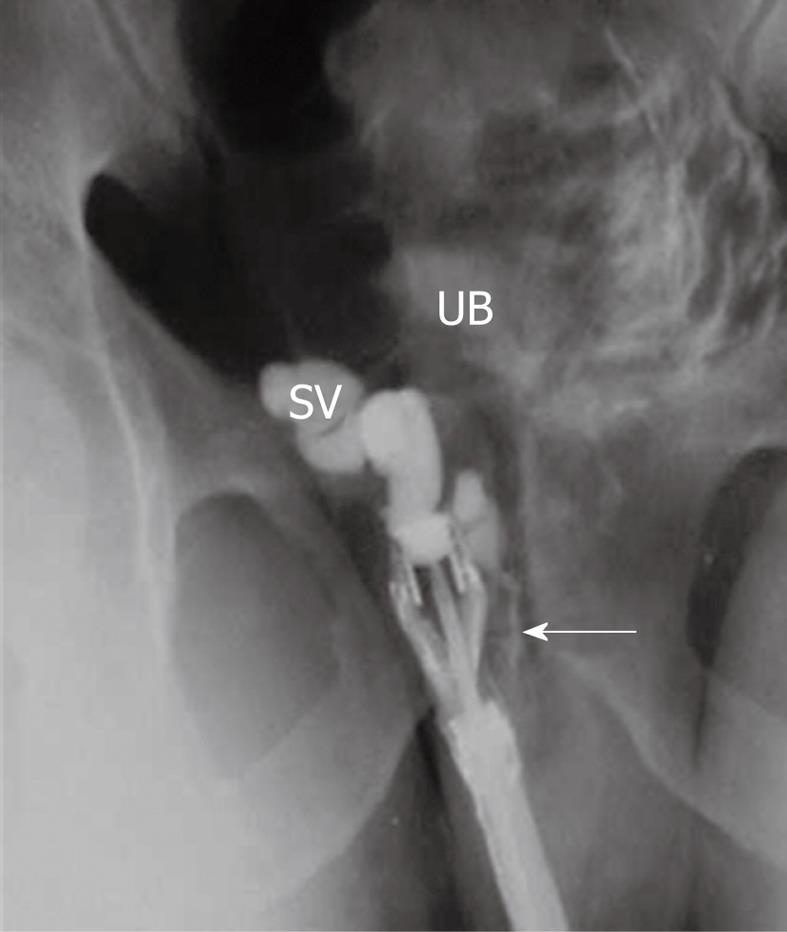
Figure 4 Percutaneous vasography shows normal right sided vasogram with opacification of the right vas (arrowhead), SV and ejaculatory duct (arrow) with retrograde opacification of the urinary bladder (UB).
Figure 5 Percutaneous right vasography in a patient with obstructed infertility shows complete obstruction of the ejaculatory duct with retention of the dye in the vas (arrowhead) and SV and non opacification of the urinary bladder.
STW
In cases of distal seminal tract obstruction, there are retained spermatozoa within the seminal tract anywhere downstream of the epididymis. In these instances the technique of STW may be useful. This technique involves cannulation of the vas deferens and subsequent antegrade washing of the vas with collection of sperm from the bladder[36-40]. The advent of TRUS has greatly facilitated the accurate diagnosis of distal seminal tract obstruction being less invasive. TRUS can provide information about the exact location of any cyst, or the level of obstructed ejaculatory duct, which is very helpful during TURED[32].
Endorectal magnetic resonance imaging
In patients with male infertility, endorectal surface coil magnetic resonance imaging (MRI) is superior to TRUS in delineating the anatomy of the prostate and distal seminal tract (vassal ampullae, seminal vesicles and ejaculatory ducts) due to its high soft tissue contrast and multiplanar capability. A MR image serves as a “detailed map” for guiding interventional diagnostic or corrective procedures. Pitfalls in the interpretation of MR images can be avoided by familiarity with normal and abnormal findings in patients with male infertility[41,42].
Causes of male infertility like Wolffian duct abnormalities include agenesis of the kidney, vas deferens, or seminal vesicle and cysts of the vas deferens, seminal vesicle, or urogenital sinus-ejaculatory duct. Müllerian duct abnormalities such as müllerian duct cysts and utricle cysts can be easily diagnosed by MRI. MRI can clearly demonstrate the level and cause of EDO (Figure 6). However, endorectal MR imaging is expensive and less available than TRUS and should be reserved for selected patients in whom results of TRUS are not conclusive[34]. TRUS is a good method for initial evaluation of infertile patients especially those with complete obstruction. Endorectal MR imaging should be reserved for selected patients in whom results of TRUS are not conclusive[42]. Recently, with the increased awareness of functional obstruction of ejaculatory ducts, reports have been focusing on the diagnosis of partial or functional obstruction and abnormalities of the ejaculatory duct related to infertility by increased use of magnetic resonance imaging[43]. Pathologic findings were detected in 61% of patients with azoospermia by MR imaging. MR imaging did not reveal any pathologies in 59.1% of patients with nonazoospermia[33].
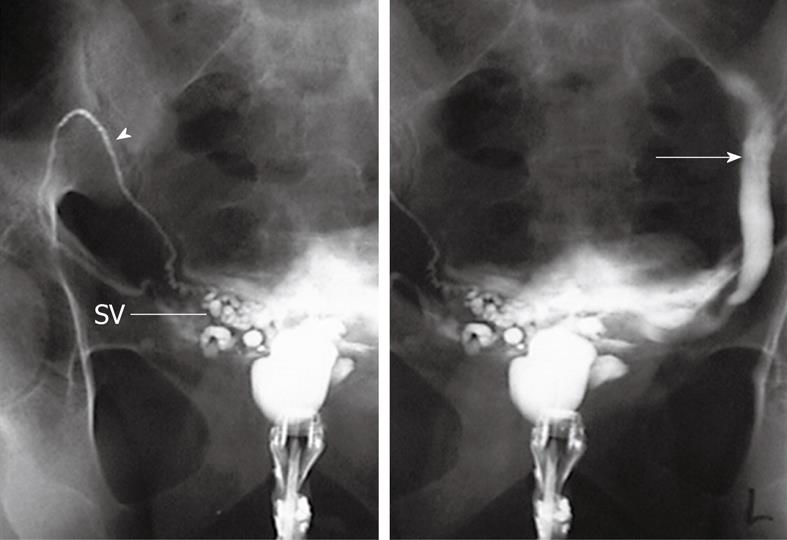
Figure 6 TRUS-right seminal vesiculography shows normal opacification of right SV and ejaculatory duct (arrow).
The injected dye is seen in the posterior urethra and UB denoting patency of the right ejaculatory duct.
TRUS-guided seminal vesiculography
TRUS-guided seminal vesiculography is a technique that couples US with radiography to evaluate male-factor infertility. Seminal vesiculography is performed after needle puncture of the seminal vesicle to inject contrast material for radiography (Figure 7). Seminal vesiculography has helped imaging of the distal male reproductive tract (vas deferens, seminal vesicles, and ejaculatory ducts)[39,44]. TRUS has also been used to guide aspiration of seminal vesicles to diagnose EDO. The presence of sperm in the aspirated fluid documents the presence of obstruction, confirms the presence of intact spermatogenesis, and rules out more proximal obstruction. Normal fertile men do not have significant numbers of motile sperm in the seminal vesicles immediately after ejaculation but, in the presence of an anatomical or functional distal obstruction, sperm reflux may occur and sperm can be detected within the seminal vesicles. The presence of more than three motile sperm per high-power field in the seminal vesicle aspirate obtained immediately after ejaculation indicates obstruction[39,45]. A the comparative analysis done by Purohit et al[32], compared the four common diagnostic tests used to evaluate patients with distal seminal duct obstruction which are TRUS and three dynamic tests (chromotubation, seminal vesicle aspiration and seminal vesiculography). They concluded that TRUS alone has poor specificity for diagnosis of distal seminal duct obstruction as obstruction on TRUS was confirmed in only 52%, 48% and 36% of vesiculography, seminal vesicle aspiration and duct chromotubation studies, respectively. Thus incorporation of dynamic tests into the algorithm for diagnosis of distal seminal duct obstruction may decrease unnecessary duct resection procedures and improve the success of the resection procedures that are indicated. Some urogenital cysts communicate with the seminal tract and if TRUS-guided aspiration is done for these patients it reveals the presence of sperm (Figures 7 and 8). In other patients the ejaculatory ducts are compressed and obstructed by virtue of the urogenital cysts (Figure 9) and after cyst aspiration, the compression is released and EDO is relieved[46].
Figure 7 A 29-year-old man with primary obstructive infertility.
TRUS (upper image) and endorectal magnetic resonance imaging (middle image) show a well-defined midline urogenital cyst with intra-and extraprostatic components. TRUS-seminal vesiculography (lower image) shows the seminal vesicle is communicating with the urogenital cyst with non opacification of the urethra or urinary bladder denoting complete distal obstruction (N.B. the left vas and seminal vesicles were absent). Trans-urethral incision of the cyst lead to improvement of sperm count.
Figure 8 A 33-year-old man with primary infertility.
TRUS-guided contrast opacification of midline prostatic cyst shows the presence of a large cyst communicating on the right side with the right vas (arrowhead) and right SV. On the left side the cyst is communicating with a blind tubular structure (arrow), which proved to be an ectopic short ureter of a hypoplastic left kidney.
Figure 9 A 27-year-old with primary infertility.
A: TRUS shows a 3 cm × 2 cm thin walled midline intraprostatic urogenital cyst; B: TRUS-guided contrast opacification of the cyst revealed that the cyst was blind with no communication with the seminal tract. Semen analysis showed improvement of the sperm count 3 d after complete cyst aspiration.
TRUS alone is not a reliable tool for the diagnosis of EDO. For this reason, seminal vesicle aspiration should be used as an adjunctive technique in patients with seminal vesicle dilation or a prostatic midline/ED cyst to confirm the diagnosis before surgery. Engin et al[33], studied 70 patients with suspected EDO; they found 55 patients (78.6%) had evidence of EDO on diagnostic TRUS. However, obstruction on TRUS was confirmed in 49.1% (27 of 55) of the patients with seminal vesicle aspiration.
TREATMENT OF MALE OBSTRUCTIVE INFERTILITY
Management of patients with obstructive disorders of the seminal tract is one of the most rapidly growing fields in medicine in recent years. Numerous changes in diagnostic and therapeutic decision-making strategies for these patients have been made. Seminal tract obstruction is one of the correctable causes of male infertility.Treatment for proximal seminal tract obstruction is by vasoepididymostomy anastomosis operation to overcome the obstruction. Vasoepididymostomy has a lower cost burden per birth than does intracytoplasmic sperm injection, and natural pregnancy initiated with surgical correction may filter some chromosomal or genetic abnormalities. Moreover, epididymal damage that can happen during sperm retrieval could be prevented[7].
The treatment of choice for distal seminal tract obstruction is TURED. Approximately half of the men undergoing this procedure for EDO show improvement of their semen parameters and half of the men who improve achieve a subsequent pregnancy[11]. Although the assisted reproduction technique (ART) has been considered the cutting edge in management of male infertility in recent years, men should investigate the cause of their male factor infertility for many reasons. In addition to detecting treatable abnormalities, evaluation of the infertile man is critical to uncover life-threatening problems associated with the symptom of infertility, as well as genetic conditions associated with male infertility that could be transmitted to offspring with assisted reproductive techniques[3]. Analysis of the genetic factors that impact male factor infertility will provide valuable insights into the creation of targeted treatments for patients and the determination of the causes of idiopathic infertility. Novel technologies that analyze the influence of genetics from a global perspective may lead to further developments in the understanding of the etiology of male factor infertility through the identification of specific infertile phenotype signatures[47,48].
CONCLUSION
Thorough evaluation of infertile men is mandatory to identify patients with potentially correctable defects such as obstructive infertility from patients with nonobstructive azoospermia to eliminate unnecessary investigations and interventions.
In men with the clinical and laboratory findings of suspected seminal tract blockage, scrotal sonography should be the initial diagnostic procedure performed. If they have findings of non obstructive azoospermia such as varicocele or testicular pathology they will be managed according to the standard protocol for management of these pathologies. If the patients have findings of proximal obstructive azoospermia they can be managed surgically by vasoepididymostomy. If the scrotal US is normal or they have findings of distal obstructive azoospermia, they should be evaluated by TRUS. According to the TRUS results, the patients are classified into patients with sonographic criteria of obstructive infertility without urogenital cysts where TRUS-guided aspiration of the seminal vesicles is performed and TURED will be the management of choice. If a urogenital cyst is seen by sonography, TRUS-guided cyst aspiration and contrast opacification are performed. If the cyst is communicating with the seminal tract, management will be transurethral incision of the cyst. If the cyst is not in communication, obstructive infertility may be relieved after cyst aspiration. If the obstruction is not cured, TURED will be the management of choice. Also, sperm harvested during TRUS-guided aspiration may be stored and used for the ART which is the cutting edge in management of male infertility. If the results of TRUS are inconclusive or doubtful, endorectal MRI should be performed to serve as a “detailed map” for guiding interventional diagnostic or corrective procedures.