Published online Nov 28, 2010. doi: 10.4329/wjr.v2.i11.434
Revised: July 20, 2010
Accepted: July 27, 2010
Published online: November 28, 2010
AIM: To study the peripheral dose (PD) from high-energy photon beams in radiotherapy using the metal oxide semiconductor field effect transistor (MOSFET) dose verification system.
METHODS: The radiation dose absorbed by the MOSFET detector was calculated taking into account the manufacturer’s Correction Factor, the Calibration Factor and the threshold voltage shift. PD measurements were carried out for three different field sizes (5 cm × 5 cm, 10 cm × 10 cm and 15 cm × 15 cm) and for various depths with the source to surface distance set at 100 cm. Dose measurements were realized on the central axis and then at distances (1 to 18 cm) parallel to the edge of the field, and were expressed as the percentage PD (% PD) with respect to the maximum dose (dmax). The accuracy of the results was evaluated with respect to a calibrated 0.3 cm3 ionization chamber. The reproducibility was expressed in terms of standard deviation (s) and coefficient of variation.
RESULTS: % PD is higher near the phantom surface and drops to a minimum at the depth of dmax, and then tends to become constant with depth. Internal scatter radiation is the predominant source of PD and the depth dependence is determined by the attenuation of the primary photons. Closer to the field edge, where internal scatter from the phantom dominates, the % PD increases with depth because the ratio of the scatter to primary increases with depth. A few centimeters away from the field, where collimator scatter and leakage dominate, the % PD decreases with depth, due to attenuation by the water. The % PD decreases almost exponentially with the increase of distance from the field edge. The decrease of the % PD is more than 60% and can reach up to 90% as the measurement point departs from the edge of the field. For a given distance, the % PD is significantly higher for larger field sizes, due to the increase of the scattering volume. Finally, the measured PD obtained with MOSFET is higher than that obtained with an ionization chamber with percentage differences being from 0.6% to 34.0%. However, when normalized to the central dmax this difference is less than 1%. The MOSFET system, in the early stage of its life, has a dose measurement reproducibility of within 1.8%, 2.7%, 8.9% and 13.6% for 22.8, 11.3, 3.5 and 1.3 cGy dose assessments, respectively. In the late stage of MOSFET life the corresponding values change to 1.5%, 4.8%, 11.1% and 29.9% for 21.8, 2.9, 1.6 and 1.0 cGy, respectively.
CONCLUSION: Comparative results acquired with the MOSFET and with an ionization chamber show fair agreement, supporting the suitability of this measurement for clinical in vivo dosimetry.
- Citation: Vlachopoulou V, Malatara G, Delis H, Theodorou K, Kardamakis D, Panayiotakis G. Peripheral dose measurement in high-energy photon radiotherapy with the implementation of MOSFET. World J Radiol 2010; 2(11): 434-439
- URL: https://www.wjgnet.com/1949-8470/full/v2/i11/434.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i11.434
With radiotherapy treatment modalities there is an increase in tumor cure rates, and additionally, there are a significant number of patients who are irradiated for benign diseases[1]. During radiotherapy treatment with high-energy photon beams, a small fraction of the delivered dose is absorbed a few centimetres away from the irradiated field[2]. This dose is known as peripheral dose (PD) and, compared to higher doses, the associated cancer risk is likely to be much lower but not insignificant[3].
The risk for secondary cancer associated with low doses of ionizing radiation, especially appearing in long-term surviving patients, is gaining new interest every day[4]. Dörr et al[1] showed that the majority of secondary tumors within the margin region of the treatment volume (from 2.5 cm inside to 5 cm outside the margin of the planning target volume) received a dose less than 6 Gy. Brenner et al[5] reported that there is a 40% increase in solid tumors in the lung after radiotherapy of the prostate, where the lung is receiving doses in the range of 0.5-1.0 Gy. Since there is no dose that is regarded as safe, assessment of PDs to radiosensitive tissue/organs, such as the breast, the gonads and the thyroid, is essential to determine the possible risk of late effects, such as secondary cancers that could appear in long-term surviving patients (e.g. pediatric patients)[6,7]. In general, it is of extreme importance to calculate the PD down to the level of 0.1% of the central axis maximum dose (dmax)[8] and its determination has been the subject of extensive investigation[2,9-20]. Metal oxide semiconductor field effect transistor (MOSFET) is used as a clinical dosimeter for radiotherapy beams, and mobileMOSFET seems to be the appropriate dose verification system[21-30], since due to its small size it can be positioned very easily on the patient’s skin, and can evaluate the delivered dose both at the target and at organs at risk[21].
This paper aims to assess the PD in high-energy photon beam radiotherapy as a function of the distance from the edge of the field, the depth, the field size and the energy of the photon beam, while the overall accuracy has been investigated by comparing the derived experimental results to corresponding ones obtained with an ionization chamber. Additionally, the paper aims to investigate the reproducibility of the mobileMOSFET dose verification system with respect to the low PDs.
The present study was carried out in the Radiotherapy Department of the University Hospital of Patras, where the measurements were made with 6 MV and 18 MV X-ray beams of an ELEKTA SLI linear accelerator. Thomson Nielsen’s mobileMOSFET Dose Verification System (TN-RD-70-W) with standard sensitivity MOSFET dosimeter (TN-502 RD) were used for the dose measurements.
MobileMOSFET (TN-RD-70-W) is Best Medical Canada Ltd’s mobile, battery-operated dose verification system. The system consists of PC-based user-interface software and one mobileMOSFET reader module. The mobileMOSFET reader was set up in the treatment room, connected to the dosimeters and the measurement circuitry was arranged. The measurement procedure was controlled by a PC using the mobileMOSFET software through an RS-232C cable or the provided wireless transceiver. The software provides a console on screen for the operator to perform all required actions in the dose measurements, such as reading, displaying, saving and printing results. MOSFET dosimeters were placed in a water phantom. The dose measurements were carried out by clicking the “start” and “read” buttons on the dose measurement screen[31].
The MOSFET dosimeter was set at the high bias in order to achieve the highest sensitivity at lower doses. The first step of the study consisted of the calibration procedure, measuring the radiation sensitivity of the dosimeter under known conditions. Each dosimeter was placed on the central axis of the 6 MV and 18 MV photon beam at 10 cm depth inside a water phantom, with the irradiated field size set at 10 cm × 10 cm. The dosimeter received a nominal dose of 200 cGy and the output voltage of the reader was compared to the set level. The ratio of the measured voltage difference value (ΔV) to the radiation dose delivered determines the calibration factor (CF), also known as sensitivity. To improve the accuracy of the calibration procedure, the measurement was repeated four times and the average value of the CF was calculated.
The radiation dose absorbed by the MOSFET was calculated by the threshold voltage shift (ΔV) attributed to the irradiation, according to the formula: dose = CR ×ΔV/CF.
Where CR is the manufacturer’s Correction Factor which is equal to 1.
The threshold voltage shift is proportional to the dose deposited in the active volume of the MOSFET and increases linearly up to an inherent functional limit of 20 V, beyond which the voltage change is no longer proportional to the dose.
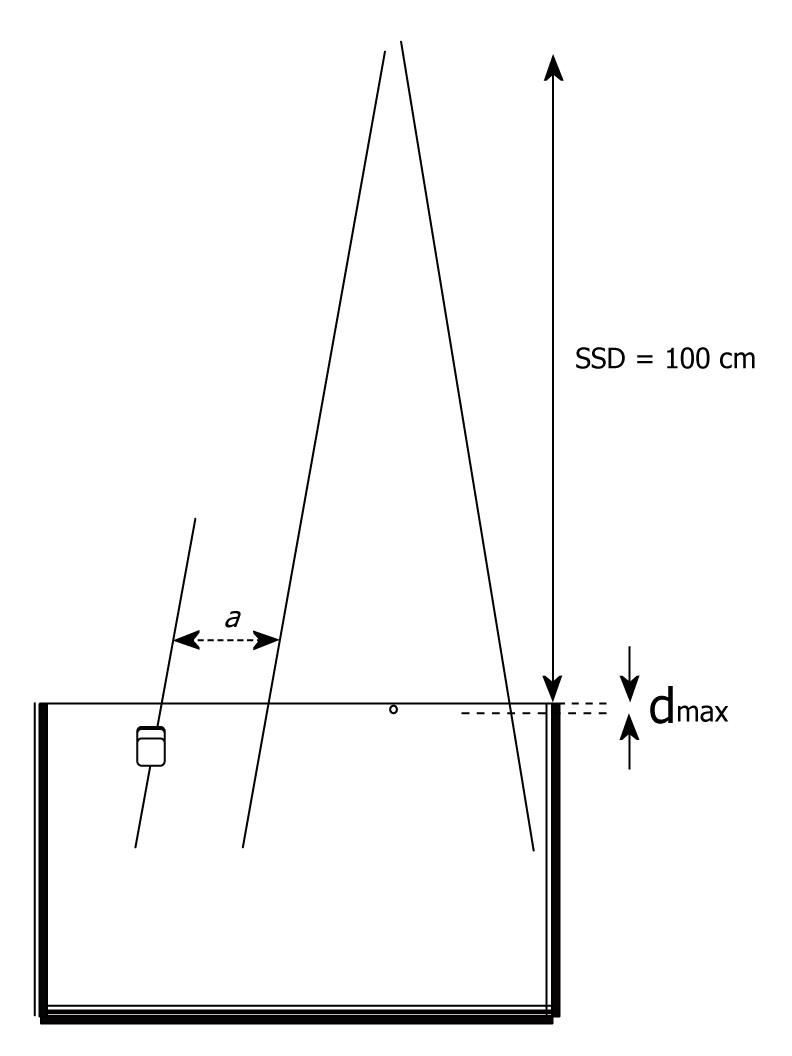
PD measurements were made in a water phantom with dimensions 54 cm × 52 cm × 30 cm. The field sizes at the surface of the phantom were set at 5 cm × 5 cm, 10 cm × 10 cm and 15 cm × 15 cm and the source to surface distance was set at 100 cm. A representative experimental arrangement is shown in Figure 1.
Dose measurements were realized on the central axis and then in peripheral regions at several distances (1 to 18 cm) from the edge of the primary geometric field, and for various depths. All the results are expressed as the percentage PD (% PD) with respect to the dmax of each field. Note that since the dimensions of the irradiated field increase with depth, due to the cone beam geometry, the axis of the PD measurements points (were the MOSFET dosimeter was placed) was not parallel to the central axis, but parallel to the edge of the field in order to keep the distance a from the edge of the primary field constant for all depths (Figure 1).
The % PD from the edge of the field was studied with respect to the field size and depth for 6 and 18 MV beams, and the results were evaluated for their accuracy against corresponding values obtained with a PTW 0.3 cm3 Unidos ionization chamber.
Each MOSFET was read immediately after exposure to minimize the potential effects of charge recombination and annealing and in order to achieve electronic stabilization[27,28].
The reproducibility of the dose measurements, which is a critical indicator for any dose verification system, was estimated. For different dose levels (4 for the early stage, and 6 for the late stage) the same measurement was repeated 6 times, and the shift voltage was measured. The standard deviation (s) which represents the reproducibility of each measurement, as well as the coefficient of variation (CV), were deduced according to the following formulae:
Where N is the number of measurements, xi the dose measurement and x the mean value of the measurements.
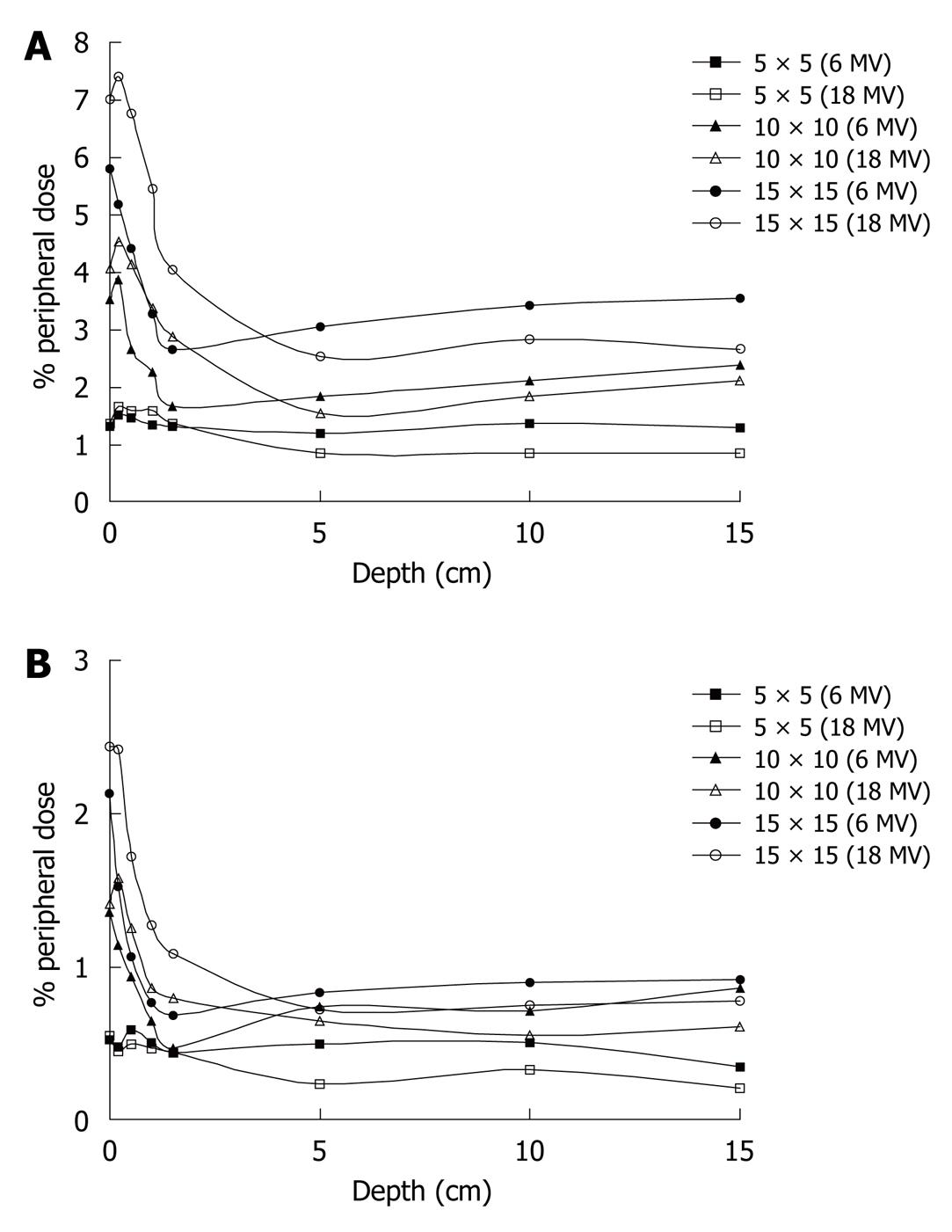
% PDs at distances of 5 and 15 cm from the field edge are presented as functions of the depth for both photon energies and for various field sizes (5 cm × 5 cm, 10 cm × 10 cm and 15 cm × 15 cm) in Figure 2A and B. From the curves it is obvious that the dose is higher near the surface and drops to a minimum at the depth of dmax, and then the % PD tends to become constant with depth.
The variations of the % PD with distance from the field edge from 1 to 18 cm as a function of the field size and depth (0, 1.5 and 10.0 cm) for both energies are shown in Table 1.
| Depth (cm) | |||||||||
| 0 | 1.5 | 10 | |||||||
| Distance (cm) | 1 | 5 | 10 | 1 | 5 | 10 | 1 | 5 | 10 |
| 5 × 5 (6 MV) | 3.22 | 1.30 | 0.79 | 3.88 | 1.32 | 0.59 | 4.64 | 1.36 | 0.49 |
| 5 × 5 (18 MV) | 3.32 | 1.29 | 0.67 | 5.87 | 1.57 | 0.74 | 7.59 | 0.79 | 0.38 |
| 10 × 10 (6 MV) | 6.84 | 3.53 | 1.75 | 6.03 | 1.65 | 0.89 | 7.59 | 2.11 | 1.28 |
| 10 × 10 (18 MV) | 8.18 | 3.82 | 2.00 | 11.81 | 3.19 | 1.36 | 7.32 | 1.74 | 0.73 |
| 15 × 15 (6 MV) | 10.52 | 5.80 | 3.48 | 7.23 | 2.64 | 1.10 | 9.52 | 3.41 | 1.66 |
| 15 × 15 (18 MV) | 12.90 | 6.60 | 3.72 | 12.94 | 4.28 | 3.41 | 8.17 | 2.66 | 1.11 |
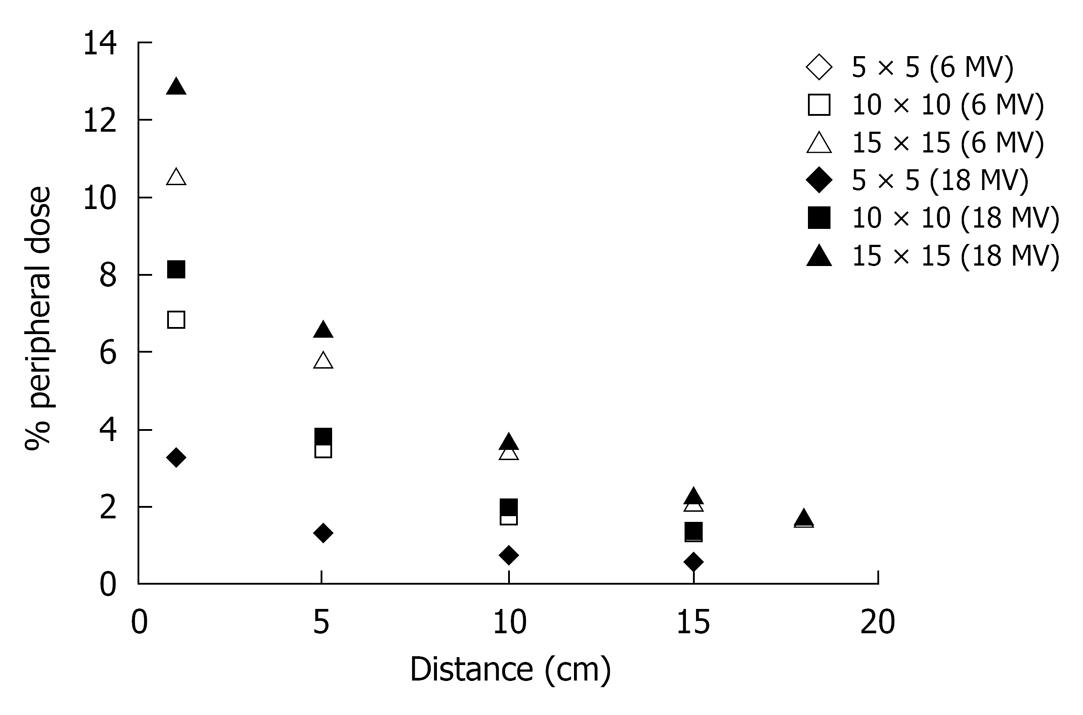
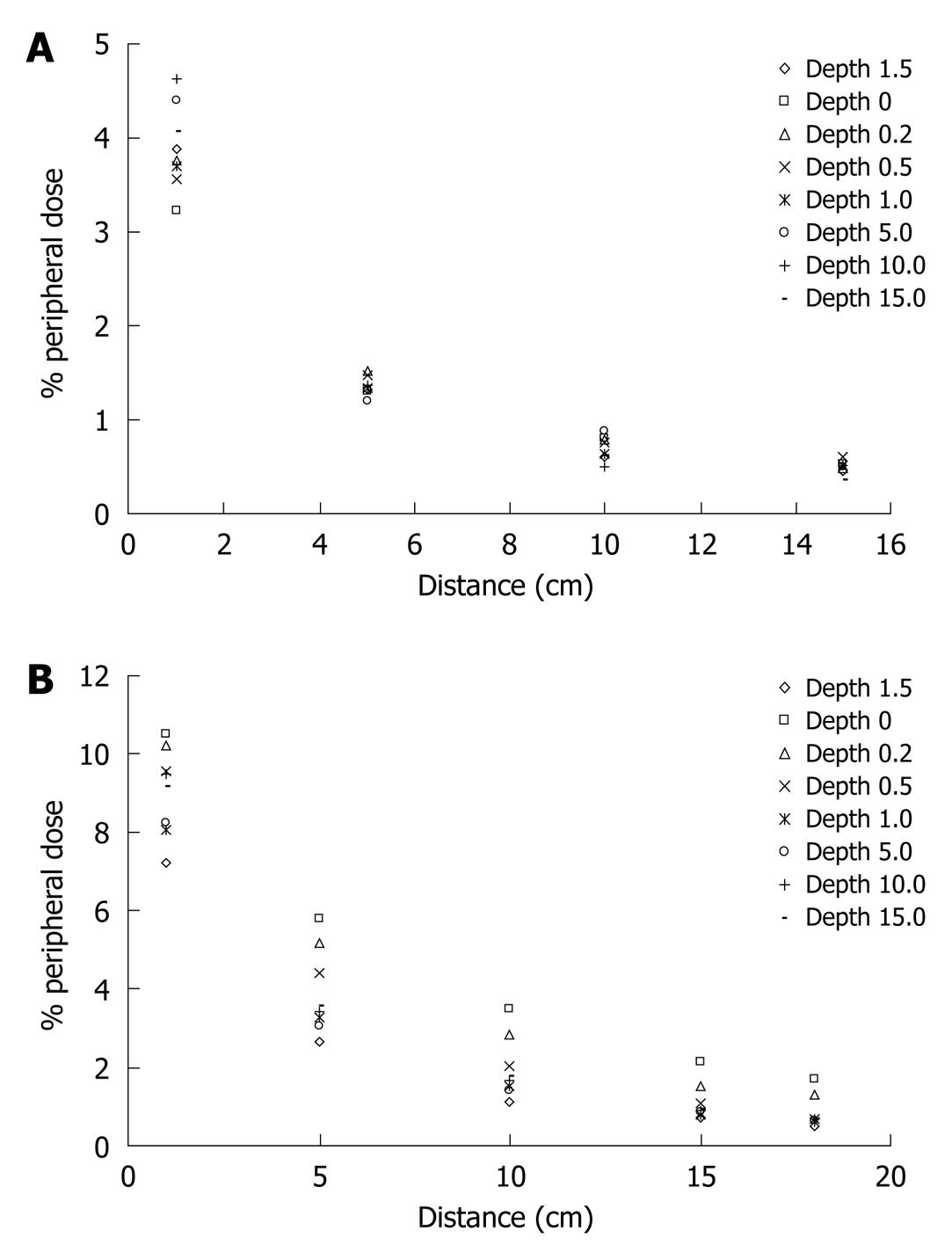
The % PD decreases almost exponentially with the increase of distance. Figure 3 shows this behavior for the three field sizes and both energies (6 and 18 MV) while the same behavior of the % PD as a function of the depth (0, 0.2, 0.5, 1.0, 1.5, 5.0, 10.0 and 15.0 cm) can be noted in Figure 4.
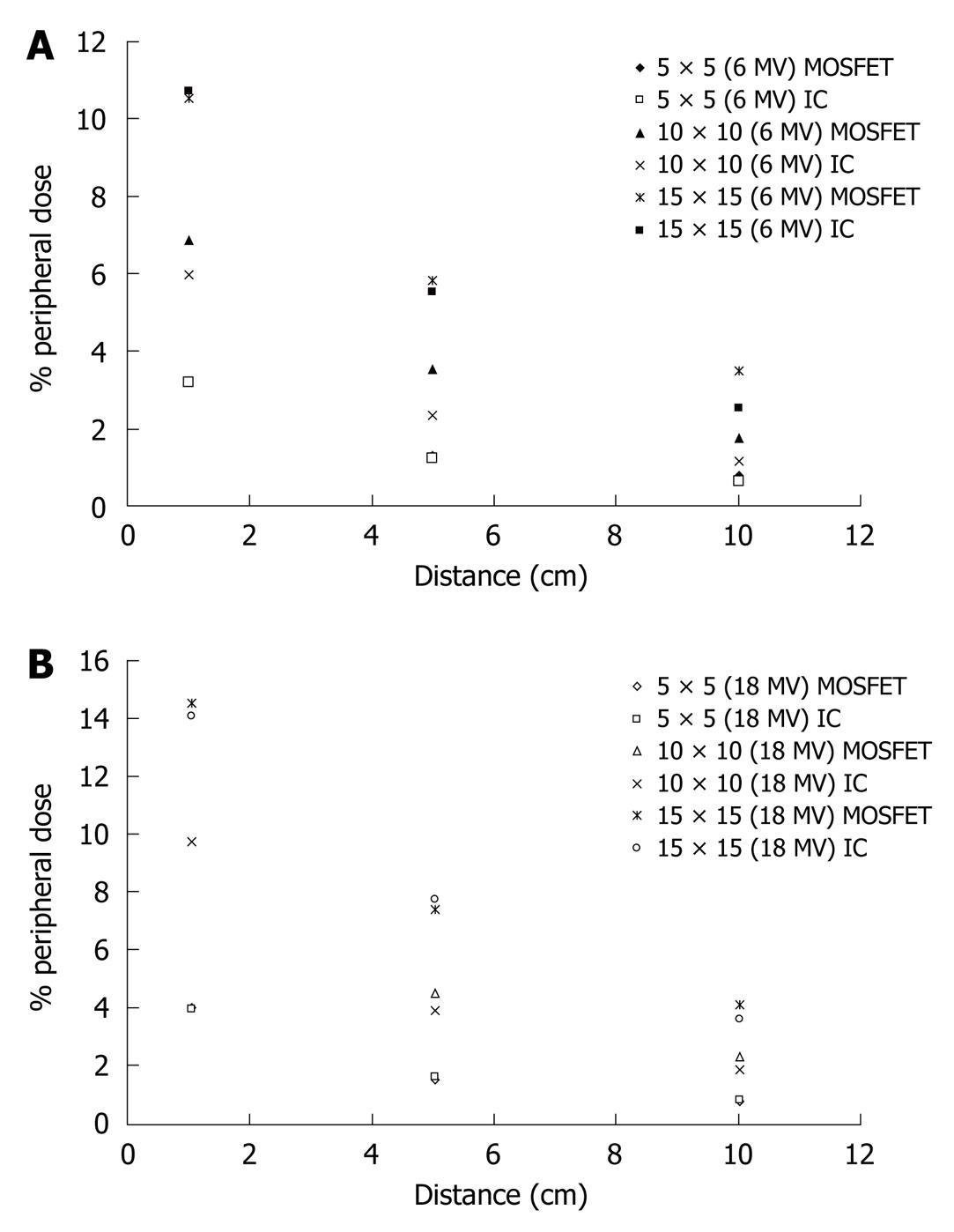
Finally, the accuracy of the mobileMOSFET dose verification system was evaluated by comparing PD measurements with corresponding measurements obtained with a PTW 0.3 cm3 ionization chamber and the results are represented in Figure 5A and B.
The MOSFET system, in high sensitivity mode, and in the early stage of its life (0-4000 mV) has a dose measurement reproducibility of within 1.8%, 2.7%, 8.9% and 13.6% for 22.8, 11.3, 3.5 and 1.3 cGy dose assessments, respectively. In the late stage of MOSFET life (> 18 000 mV) the corresponding values change to 1.5%, 4.8%, 11.1% and 29.9% for 21.8, 2.9, 1.6 and 1.0 cGy, respectively (Figure 6).
Calibration Factor (CF) in the early stage of MOSFET life (0-4000 mV) has the value of 3.09 mV/cGy, in the middle stage of its life has the value of 2.90 mV/cGy and finally at the end of its lifetime the value is 2.80 mV/cGy.
From the curves of Figure 2A and B it is obvious that the PD is higher near the surface and drops to a minimum at the depth of dmax, and then the % PD tends to become constant with depth. Internal scatter radiation is the predominant source of PD and the depth dependence is determined by the attenuation of the primary photons[14]. This is more evident away from the field edge. This might seem to be in disagreement with Fraass et al[20], who report that after dmax the PD increases with depth. However, in our measurements, as the field size increases with depth, the distance from the field edge is also displaced from the central axis, in order to stay constant with depth.
Closer to the field edge, where internal scatter from the phantom dominates, the PD increases with depth, because the ratio of the scatter to primary increases with depth. The fact that at dmax the % PD is minimized can also be observed from the measurements presented in Table 1, for the 6MV photon energy.
A few centimeters away from the field, where collimator scatter and leakage dominate, the PD decreases with depth, due to the attenuation by the water. This is in agreement with Francois et al[9].
The % PD decreases almost exponentially with the increase of distance. Figure 3 shows this behavior for the three field sizes and for both energies. The decrease of the % PD is more than 60% and can reach up to 90% as the measurement point departs from the edge of the field. For a given distance, the % PD is significantly higher for larger field sizes, due to the increase of the scattering volume.
The same behavior of the % PD as a function of the depth can be noted in Figure 4, where also the influence of the increase of the field size on the % PD can be observed. Figure 5A shows that the measured PD obtained with MOSFET is higher than that obtained with the ionization chamber in the case of the 6 MV beam at the surface, at three different distances (1.0, 5.0 and 10.0 cm) from the edge of the field, with percentage differences between corresponding values of the two dosimetric systems being from 0.6% to 34.0%. However, comparing with the central dmax this difference is less than 1%, which is in agreement with the findings of Butson et al[25]. Similar results arise for measurements obtained with the 18 MV beam, as shown in Figure 5B.
MOSFET reproducibility results as shown in Figure 6 are comparable to that obtained by Cheung et al[23]. Finally, from the measurements of Calibration Factor (CF) we conclude that after a large accumulated dose the detector requires larger doses for the same potential change.
The most important advantages of the mobileMOSFET dosimeter are its small size, as it can be easily placed on the patient’s skin, and the almost direct estimation of the dose during exposure. Additionally, comparative results acquired with the MOSFET and with an ionization chamber show fair agreement, supporting the suitability of this measurement technique for clinical in vivo dosimetry, allowing radiation oncologists to evaluate and further optimize radiation treatment. Moreover, its sensitivity and reproducibility make it suitable for measurements of low PDs.
Regarding PD, these measurements show that a significant amount of the treatment dose reaching up to 14% of the central axis dmax, can be delivered to critical organs outside the treatment field at points near the primary field edge. Further studies are necessary to evaluate the clinical effects of this amount of dose and to develop appropriate solutions.
With radiotherapy treatment modalities there is an increase in tumor cure rates and additionally, there are a significant number of patients who are irradiated for benign diseases. During radiotherapy treatment with high energy photon beams, a small fraction of the delivered dose is absorbed a few centimeters away from the irradiated field. This dose is known as peripheral dose (PD) and compared to higher doses, the associated cancer risk is likely to be much lower but not insignificant.
Many investigations have been carried out in order to measure PD in radiotherapy treatment modalities. In vivo dosimetry for radiotherapy patients often requires dose measurements not only in the treatment area, but also in the peripheral regions, so that doses to critical organs can be recorded and if possible minimized. For such measurements, there is a need for dosimeters with ability to measure low doses accurately and with tolerance to the variations of the spectral quality of the beam. Metal oxide semiconductor field effect transistor (MOSFET) features the ability to integrate dose measurements and to provide immediate dose readout. This, in combination with a very small sensing volume, makes the MOSFET dosimetry system advantageous over the other systems used in radiotherapy.
This study aims to assess the PD in high energy photon beam radiotherapy as a function of the distance from edge of the field, the depth, the field size and the energy of the photon beam using the mobileMOSFET dose verification system. The results are compared to corresponding ones obtained with an ionization chamber. Additionally, the reproducibility of the mobileMOSFET dose verification system is investigated, with respect to the low PDs. The small size, the radio-transparency, the high sensitivity and the immediate read make MOSFET dosimeters an excellent choice for dosimetry in radiotherapy, and when physical constraints, concerns over shadowing, or issues of scattered doses are important, then MOSFET dosimeters have clear advantages over both diodes and thermoluminescence dosimeters.
Since there is no dose that is considered as safe, assessment of PDs to radiosensitive tissue/organs, such as the breast, the gonads and the thyroid, is essential to determine the possible risk of late effects, such as secondary cancers that could appear in long-term surviving patients after the radiotherapy treatment. It is of extreme importance to calculate the PD down to a level of 0.1% of the central axis maximum dose and its determination has been the subject of extensive investigation.
PD is the small fraction of the delivered radiotherapy dose that is absorbed a few centimeters away from the irradiated field MOSFET dosimeter is a MOSFET made of Si/SiO2. It is used as a clinical dosimeter for radiotherapy beams. The MOSFET dosimeter is direct reading with a very thin (less than 2 mm) active area. Calibration factor is the ratio of the recorded voltage difference value over the corresponding radiation dose delivered to the dosimeter.
The paper can be published if some questions mentioned can be revised.
Peer reviewer: Igor Meglinski, PhD, Head of Bio-Photonics and Bio-Medical Imaging, Cranfield Health, Cranfield University, Cranfield, Bedfordshire, MK43 0AL, United Kingdom
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
| 1. | Dörr W, Herrmann T. Cancer induction by radiotherapy: dose dependence and spatial relationship to irradiated volume. J Radiol Prot. 2002;22:A117-A121. |
| 2. | van der Giessen PH. Peridose, a software program to calculate the dose outside the primary beam in radiation therapy. Radiother Oncol. 2001;58:209-213. |
| 3. | Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761-13766. |
| 4. | Tubiana M, Aurengo A, Averbeck D, Masse R. Recent reports on the effect of low doses of ionizing radiation and its dose-effect relationship. Radiat Environ Biophys. 2006;44:245-251. |
| 5. | Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398-406. |
| 6. | Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178-186. |
| 7. | Brenner DJ, Sachs RK. Estimating radiation-induced cancer risks at very low doses: rationale for using a linear no-threshold approach. Radiat Environ Biophys. 2006;44:253-256. |
| 8. | Reissland JA. BEIR III 'The effects on populations of exposure to low levels of ionising radiation'. J Soc Radiol Prot. 1981;1:17-22. |
| 9. | Francois P, Beurtheret C, Dutreix A. Calculation of the dose delivered to organs outside the radiation beams. Med Phys. 1988;15:879-883. |
| 10. | McParland BJ, Fair HI. A method of calculating peripheral dose distributions of photon beams below 10 MV. Med Phys. 1992;19:283-293. |
| 11. | Sherazi S, Kase KR. Measurements of dose from secondary radiation outside a treatment field: effects of wedges and blocks. Int J Radiat Oncol Biol Phys. 1985;11:2171-2176. |
| 12. | van der Giessen PH. Calculation and measurement of the dose at points outside the primary beam for photon energies of 6, 10, and 23 MV. Int J Radiat Oncol Biol Phys. 1994;30:1239-1246. |
| 13. | Van der Giessen PH. A simple and generally applicable method to estimate the peripheral dose in radiation teletherapy with high energy x-rays or gamma radiation. Int J Radiat Oncol Biol Phys. 1996;35:1059-1068. |
| 14. | van der Giessen PH. Collimator-related radiation dose for different cobalt machines and linear accelerators. Int J Radiat Oncol Biol Phys. 1996;35:399-405. |
| 15. | Van der Giessen PH. Measurement of the peripheral dose for the tangential breast treatment technique with Co-60 gamma radiation and high energy X-rays. Radiother Oncol. 1997;42:257-264. |
| 16. | Van der Giessen PH, Bierhuizen HW. Comparison of measured and calculated peripheral doses in patients undergoing radiation therapy. Radiother Oncol. 1997;42:265-270. |
| 17. | Starkschall G, St George FJ, Zellmer DL. Surface dose for megavoltage photon beams outside the treatment field. Med Phys. 1983;10:906-910. |
| 18. | van der Giessen PH, Hurkmans CW. Calculation and measurement of the dose to points outside the primary beam for CO-60 gamma radiation. Int J Radiat Oncol Biol Phys. 1993;27:717-724. |
| 19. | Diallo I, Lamon A, Shamsaldin A, Grimaud E, de Vathaire F, Chavaudra J. Estimation of the radiation dose delivered to any point outside the target volume per patient treated with external beam radiotherapy. Radiother Oncol. 1996;38:269-271. |
| 21. | Consorti R, Petrucci A, Fortunato F, Soriani A, Marzi S, Iaccarino G, Landoni V, Benassi M. In vivo dosimetry with MOSFETs: dosimetric characterization and first clinical results in intraoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:952-960. |
| 22. | Rosenfeld AB. MOSFET dosimetry on modern radiation oncology modalities. Radiat Prot Dosimetry. 2002;101:393-398. |
| 23. | Cheung T, Yu PKN, Butson MJ. Low-dose measurement with a MOSFET in high-energy radiotherapy applications. Radiat Meas. 2005;39:91-94. |
| 24. | Ramani R, Russell S, O'Brien P. Clinical dosimetry using MOSFETs. Int J Radiat Oncol Biol Phys. 1997;37:959-964. |
| 25. | Butson MJ, Cheung T, Yu PK. Peripheral dose measurement with a MOSFET detector. Appl Radiat Isot. 2005;62:631-634. |
| 26. | Cherpak A, Studinski RC, Cygler JE. MOSFET detectors in quality assurance of tomotherapy treatments. Radiother Oncol. 2008;86:242-250. |
| 27. | Oldham TR, McLean FB. Total ionizing dose effects in MOS oxides and devices. IEEE Trans Nucl Sci. 2003;50:483-499. |
| 28. | Gladstone DJ, Lu XQ, Humm JL, Bowman HF, Chin LM. A miniature MOSFET radiation dosimeter probe. Med Phys. 1994;21:1721-1728. |
| 29. | Tanyi JA, Krafft SP, Hagio T, Fuss M, Salter BJ. MOSFET sensitivity dependence on integrated dose from high-energy photon beams. Med Phys. 2008;35:39-47. |
| 30. | Cygler JE, Saoudi A, Perry G, Morash C, E C. Feasibility study of using MOSFET detectors for in vivo dosimetry during permanent low-dose-rate prostate implants. Radiother Oncol. 2006;80:296-301. |
| 31. | Thomson N. Operator's manual for the mobile MOSFET dosimetry system. Ottawa: Best Medical Canada Ltd 2007; . |