INTRODUCTION
Placenta abnormalities happen when the chorionic villi infiltrate the uterine wall too much. This abnormality is currently classified as a spectrum disorder, with three different conditions of abnormal placentation representing different grades of pathological invasiveness of the placenta[1,2]. Abnormal placentation, including placenta accreta, increta, and percreta, is collectively termed placenta accreta spectrum (PAS) and represents a major risk factor for life-threatening postpartum hemorrhage (PPH). These conditions occur as a consequence of a deep penetration of the chorionic villi in the decidua and in the uterine wall. When the chorionic elements surpass the basal layer of the decidua, the condition is known as placenta accreta. When the villi extend into the myometrium, the condition is termed placenta increta. When the villi surpass even the myometrium and reach the peritoneal serosa up to other organs, the condition is termed placenta percreta[3,4].
In the case of a prenatal diagnosis of placenta increta or percreta, there is the risk of a dangerous extension to the bladder or to adjacent structures, and demolition surgery (cesarean section plus hysterectomy and bladder and other structure resection) is mandatory[5,6]. The invasion of extrauterine structures and the risk of severe abdominal hemorrhage lead to the possibility of bladder and/or ureteral injuries while performing surgery. Therefore, it is recommended to deal with these situations by means of a preemptive elective intervention rather than approaching them in an emergency setting.
Prior to demolition surgery, an intra-arterial balloon catheter is used to perform a prophylactic occlusion balloon (POB)[7]. This method allows target arteries to be quickly occluded for a small amount of time soon after the delivery of the newborn, leading to a reduction of complications, particularly blood loss (BL). This minireview focused on the indications, materials, technique, complications, and outcomes of POB of the abdominal aorta (POB-AA), POB of common iliac arteries (POB-CIA) and POB of internal iliac arteries (POB-IIA) for the prevention of peripartum hemorrhage in pregnant females with placenta abnormalities.
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Placental abnormalities occur in around 0.03%-0.07% of pregnancies[8]. Abnormal placentation can occur in a focal spot of the placenta or across the entire placental insertion area. In the latter case, it is referred to as total placenta accreta, increta, or percreta. Some conditions may act as predisposing factors for the PAS (i.e. hysterotomy scars following previous cesarean section or previous myomectomy, hysteroscopic resection of uterine septa or fibroids, placenta insertion in the lower uterine segment also known as placenta previa, previous manual removal of the placenta, uterine curettages and/or revisions, assisted reproductive technology, induced abortions, endometritis sequelae, advanced multiparity, or previous use of intrauterine devices)[2,9].
Even though pathogenesis of PAS is still unknown, the most prevailing theory on its pathogenesis is correlated to the abovementioned predisposing factors as prior uterine surgery and subsequent uterine scars lead to a defective decidualization, facilitating the chorionic villi to abnormally adhere to the myometrium[2,10]. On the other hand, the occurrence of placental abnormalities in primigravid females without previous uterine procedures could be related to fibroids, adenomyosis, or other uterine pathology resulting in endometrial defects that lead to abnormal adherence of the chorionic villi[10].
The increase of cesarean sections in the last decades has led to an increase in the frequency of placental abnormalities. Placenta accreta is seen in around 4.1% and 13.3% of females with 1 and ≥ 2 previous cesarean deliveries, respectively[11]. The incidence is higher in females with placenta previa and previous history of cesarean section, ranging from 11.1% in females with 1 cesarean delivery to 60.0% in females with ≥ 3 previous cesarean deliveries[12]. This confirms that the hysterotomy scar represents a significant risk factor for pathological adherence of the placenta and that the risk increases with the number of previous cesarean sections[3,13].
CLINICAL PRESENTATION
The only way to timely address the risks related to the PAS is to perform prenatal diagnosis[5,14]. In routine clinical practice the differentiation between placenta accreta, increta, and percreta is almost always based on presumptive criteria and diagnostic imaging evaluation because the effective diagnosis can only be based on a histological evaluation of the uterus and the placenta that only occurs in the rare occurrence of postpartum hysterectomy or myometrial resection.
Histologically, in areas of placenta accreta, increta, or percreta, there is hypoplasia or absence of the decidua, determining a direct contact between the chorionic villi and the myometrium[15]. Pathological placental adherences do not have specific and pathognomonic clinical presentation as most are asymptomatic or have vague symptoms, like vaginal bleeding and abdominal cramping[16]. Premonitory prenatal signs include the evidence of microhematuria/macrohematuria in the absence of other potential causes, raising the suspicion of bladder involvement, or an abnormally elevated serum concentration of alpha-fetoprotein, human chorionic gonadotropin, and pregnancy-associated plasma protein A, provided that other causes have been excluded[17].
Even though placental abnormalities usually occur during the second half of the pregnancy, they may also present as early pregnancy complications, leading to spontaneous and induced abortions. Other clinical presentations in advanced cases can be with intra-abdominal hemorrhage or metrorrhagia as reported by Roeters et al[18]. They described a case of massive intraperitoneal hemorrhage at 14 weeks of gestational age caused by placenta percreta.
DIAGNOSIS
Accurate prenatal diagnosis is vital in order to anticipate complications and optimize perinatal outcomes. Ultrasound remains the first-line imaging modality due to its accessibility and elevated diagnostic performance. Some remarkable B-mode ultrasonographic features include the loss or thinning of the retroplacental hypoechoic zone, the irregularity of the uterine-bladder interface, and the presence of multiple intraplacental lacunae, which present a “moth-eaten” appearance. Taipale et al[19] demonstrated that grayscale ultrasound integrated with color-Doppler provides valuable diagnostic features for PAS, including turbulent blood flow within the lacunae and hypervascularity at the placental-myometrial interface, suggesting deep placental invasion. Color-Doppler evaluation also improves diagnostic sensitivity by identifying abnormal vascular patterns, such as bridging vessels and subplacental hypervascularity[19].
Another useful feature is uterine artery (UA) color-Doppler spectrum analysis. It has been explored as a predictive tool for adverse pregnancy outcomes, including placental insufficiency and hypertensive disorders. Schwarze et al[20] identified that abnormal UA waveforms, particularly the persistence of diastolic notching, were significantly associated with the development of preeclampsia and intrauterine growth restriction, indicating their potential role in early screening strategies.
In cases where the operator may find himself faced with cases of diagnostic doubt or unfavorable anatomy, especially those involving posterior placentation or maternal obesity, magnetic resonance imaging (MRI) plays a fundamental role in diagnosis. MRI enables better discrimination of the depth and morphology of placental invasion due to its superior contrast resolution. MRI findings suggestive of PAS include uterine bulging, dark intraplacental bands on T2-weighted images, and thinning or disruption of the myometrium. Taipale et al[19] reported that MRI effectively contributed to the prenatal diagnosis of placenta percreta, especially in cases where ultrasound findings were inconclusive.
Warshak et al[21] conducted a comparative study to evaluate the diagnostic performance of ultrasound vs MRI for placenta accreta. The findings showed that ultrasound had a sensitivity of 77% and specificity of 96%, while MRI achieved a sensitivity of 88% and specificity of 100%, indicating that MRI might be superior in accurate characterization of the extent of the placental invasion[21]. Moreover, the authors emphasized the role of combined imaging strategies and multidisciplinary planning in reducing the risks associated with PAS. The integration of B-Mode ultrasound, color-Doppler imaging, and MRI provides the necessary diagnostic approach for PAS disorders. Early detection facilitates appropriate peripartum planning, potentially mitigating the risks of massive hemorrhage and improving maternal and neonatal outcomes.
INDICATION TO TREAT
Cesarean hysterectomy remains the conventional treatment for managing PAS disorders. However, this procedure can be emotionally challenging, particularly for younger females or those wishing to preserve their fertility. While there are alternative approaches designed to remove the portion of the placenta invading the uterine wall, these techniques may lead to an increased risk of delayed bleeding and may necessitate a later hysterectomy[22,23]. Significant intraoperative BL may require massive transfusions, which in turn can cause complications such as fluid overload, acute respiratory distress syndrome, disseminated intravascular coagulation, and infections. Furthermore, surgical risks, including damage to the intestines, bladder, or ureters, are a concern[24].
Endovascular preventive management has emerged as an effective minimally invasive technique for controlling pelvic hemorrhage, with particular significance in obstetric settings such as placenta disorders. Endovascular arterial bleeding control may be performed with the use of (POB-AA), POB-CIA, or hypogastric arteries (POB-IIA), or even with UA embolization. Traditionally, POB serves to reduce the BL during the cesarean section in patients with PAS. The aim of the procedure is to bring an occlusion balloon to the target site and inflate it during the surgical intervention[25,26]. The procedure of POB is mainly performed to control bleeding, preserve the uterus, and reduce morbidity during planned hysterectomy[27].
MATERIALS
Procedural equipment to perform a safe POB in patients with PAS should include vascular sheaths [usually between 4-French (Fr) and 7-Fr, both long and short], 0.035 J-tip hydrophilic guidewires (at least 150-cm long), 4-Fr or 5-Fr diagnostic catheters with various tip shapes (the most commonly used are C1-2, multipurpose, S-W, SIM1-2), compliant or non-compliant occlusion-balloon catheters for iliac arteries or aorta. To reach a more distal vascular district it is a good option to use a microcatheter (< 3 Fr) and microguidewire (0.018 and 0.014) to prevent or at least reduce arterial spasms; however, this procedure usually does not require microcatheter and microguidewire use[28,29].
There is still no consensus on the type of occlusion balloon, in particular for deciding between compliant vs non-compliant balloons. Both have advantages and disadvantages due to their intrinsic properties and behavior[30]. Specifically, compliant balloons are softer and adapt to the vessel shape, reducing the risk of vessel wall damage (e.g., dissection, rupture); however, they usually need a larger introducer sheath[31]. On the other hand, non-compliant balloons, which are mainly used in angioplasty procedures, can fit in a smaller introducer vascular sheath and are designed to maintain their size and shape even when inflated with high pressure. This characteristic makes them good for this procedure as the balloon inflation is usually performed “blindly” in the operating theater without the aid of a C-arm. However, these balloons are less flexible, leading to a worse “matching” with the vessel lumen, increasing the risks of vessel wall damage[32]. Balloon choice should be made on a case-by-case basis, taking into account the site of occlusion, the vessel size, and associated patient vascular pathologies (i.e. vasculitides) or coagulopathies. When using non-compliant balloons, it is advised to manually inflate the balloon rather than using an inflation device.
In addition to the classical equipment for POB, it is recommended to always have on the shelf everything that could be needed to treat every type of complication. In particular, it is mandatory to have embolic materials, such as microparticles (250 µm and larger), gelfoam, coils, plugs, liquid embolic agents (i.e. cyanoacrylate, ethylene-vinyl alcohol-based embolics) as well as bare metal stents and covered stents. Percutaneous arterial closure devices can be useful and are usually used when the vascular access is over 7-Fr, although there is no limit. Closure devices can be avoided with vascular sheath up to 7-Fr, preferring manual compression[33].
PROCEDURAL TECHNIQUE
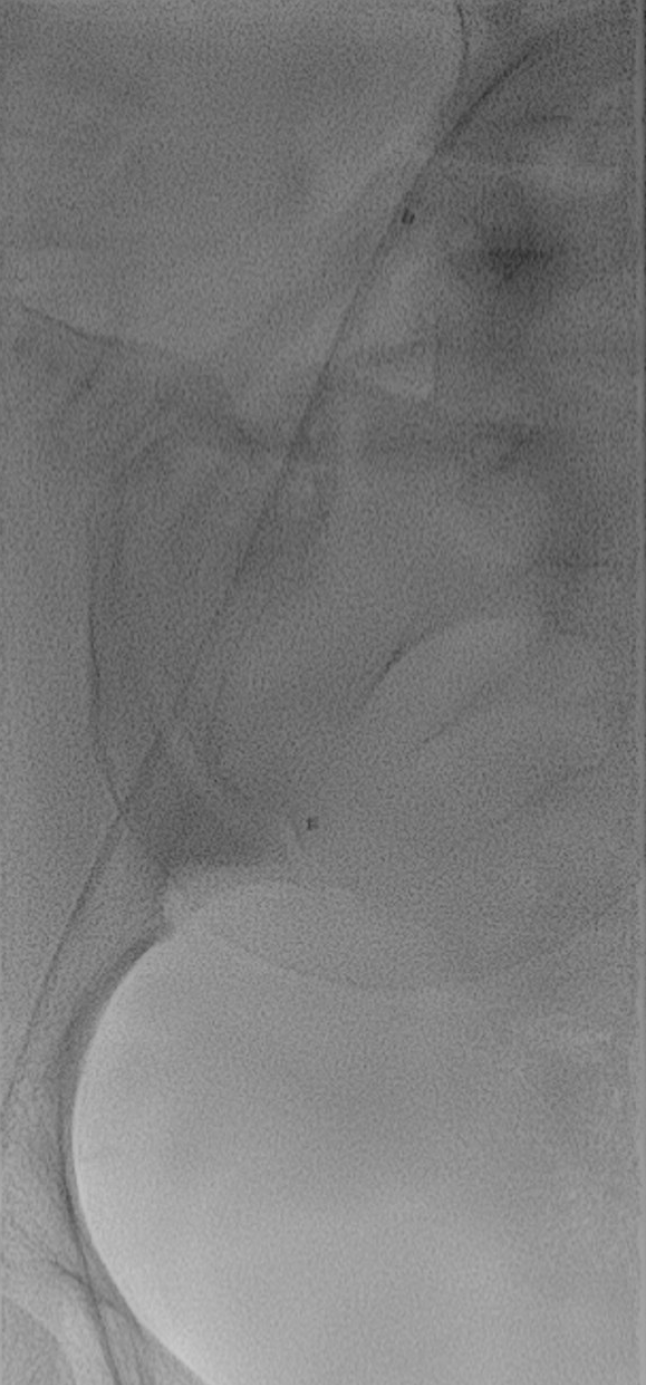
The aim of the POB is to bring the occlusion-balloon catheters to the target arteries (Figure 1). Procedural techniques of POB can vary between operators and different centers. The site of procedure (i.e. interventional radiology suite or surgery suite), fixed or mobile C-arm, operator (i.e. interventional radiologist, vascular surgeon, gynecologist), target artery (i.e. aorta, common iliac arteries, internal iliac arteries), starting moment of balloon inflation, and inflation and deflation time could be different based on site-specific needs. However, every gynecological arterial endovascular procedure should be performed in an angiographic (or hybrid) suite, with digital subtraction angiography and standard angiographic equipment[28,34].
Figure 1 Digital subtraction angiography.
Balloon positioning between the right internal iliac artery and the common iliac artery in a 24-years-old pregnant female.
For POB procedures, digital subtraction angiography should be avoided in order to reduce fetal X-ray exposure according to the “as low as reasonably achievable” principles[35-37]. The median radiation dose reported by Dai et al[38] was less than 100 mGy. The use of X-rays can cause fetal teratogenic risk, but this risk does not increase if the radiation dose is inferior to 100 mGy as highlighted by the International Commission on Radiological Protection. A total of 21 out of 22 studies included in the meta-analysis by Dai et al[38] found radiation doses were inferior to 100 mGy.
Another important consideration is that the time of the procedure is less for POB-AA with respect to POB-CIA or POB-IIA, leading to a decreased radiation dose. The POB-AA procedure requires one vascular sheath and one balloon and no need to cross the abdominal bifurcation, whereas two vascular sheaths and two balloons are needed to cross the abdominal bifurcation and reach contralateral common/internal iliac arteries for POB-CIA and POB-IIA[27]. Also, ipsilateral vascular access saves time and thus radiation with respect to contralateral vascular access.
A sterile field must be prepared at the access points and excellent hand disinfection must be performed by the operators before the procedure. Gynecological interventional radiology procedures generally require dedicated anesthesiological assistance and hemodynamic support. The choice between general anesthesia and conscious sedation or local anesthesia should be based on site-specific procedural settings (i.e. procedure performed in the operating room just before the cesarean section vs procedure performed in the angio-suite)[28,39]. Fetal safety should always be taken into account when choosing the correct type of anesthesia. Every POB procedure needs a unilateral or bilateral retrograde common femoral artery access, obtained with the Seldinger technique and under ultrasound guidance. Then the appropriately sized vascular sheath can be placed[28,29].
During POB-AA, a unilateral retrograde common femoral artery access could be done, while during other types of POB a bilateral common femoral artery access is almost always necessary. Bilateral access can be ipsilateral in cases of POB-CIA (right access for right CIA, left access for left CIA) or can be contralateral in case of POB-IIA (right access for left IIA, left access for right IIA) performing an aorto-iliac cross-over[40]. Some authors perform bilateral common femoral access for POB-AA, while others also suggest the brachial approach for POB-IIA[41,42]. The brachial artery approach for balloon placement, leaving the femoral arteries free from the introducer sheath and the balloon catheters, could grant better and easier patient positioning in the gynecological position during delivery[42]. However, the approach from the upper arm has the drawback of a greater endovascular distance from the target site; moreover, due to the risks of access site complications, this approach should be performed only by experienced operators[43].
Under fluoroscopic guidance, the balloon catheter is brought over a hydrophilic guidewire in the target site and is inflated to evaluate the correct cessation of the blood flow. The occlusion balloon catheters can be placed in infra renal AA, CIA, IIA, or in the UA although studies have shown that occlusion of the infrarenal AA and IIA are the most widely practiced methods[25-27].
Several studies have focused on POB-AA. However, there is still debate on whether the aorta should be occluded and where. The aortic zone in which the occlusion balloon should be inflated is not clearly determined. Liu et al[44] reported that a zone-II occlusion (between celiac trunk and the most distal renal artery) significantly reduced the BL compared with a zone-III occlusion (below the renal arteries), without increasing harm if the occlusion was performed for no more than 15 min. However, even though a higher site of occlusion should lead to reduced BL during surgery, the ischemic adverse events could increase and involve a larger extension of tissues and organs[45]. Aortic occlusion could be suggested in selected cases in which the bleeding is believed to not be safely managed with a lower occlusion (i.e. CIA or IIA) due to the presence of arteries arising from “above” (such as the ovaria arteries) that are expected to greatly increase the BL during surgery.
Liu et al[27] did not find significant differences in the length of hospital stay, PPH, and time of cesarean section between POB-AA and POB-IIA. When the correct position of the balloon was demonstrated, the operator completely deflated it, and the patient was directly transferred to the operating room (if the procedure and the cesarean section were not performed in the same suite)[39]. Inflation of the occlusion-balloons in both IIA could be performed simultaneously with extraction of the fetus by the operator[39].
Kyozuka et al[29] reported the inflation of the balloon being performed immediately before the cesarean section, usually by an emergency physician or an obstetrician with experience in this interventional radiology procedure, with removal of the balloon catheters within the operating room. The operator could deflate the balloons at the end of the surgical intervention[39]. The time of inflation should be as short as possible, in particular for POB-AA, to reduce the possible complications, such as ischemic and vascular injury[27]. To reduce all the possible complications, some authors suggest intermittently inflating the balloons for a maximum duration of 40 min for POB-AA and 4 h for POB-IIA, with 10-15 min of deflation intervals to temporarily restore the blood supply[27,38].
Abouda et al[39] reported balloon inflation in case of POB-IIA not exceeding 60 min, while Huo et al[41] reported a mean time of 39.7 min for POB-AA. As reported by Yin et al[26], during POB-AA the balloon was inflated after cord clamping and in case of active bleeding for no longer than 10 min with a 1-min deflation interval. The balloon was left deflated if the bleeding became inactive[26]. After the surgical demolition, if a C-arm is available in the operating theatre, then the anterior branch of both IIA can be embolized with the use of gelfoam before deflating and removing the balloon catheters to prevent PPH[46].
A multidisciplinary approach involving obstetricians, anesthesiologists, and interventional radiologists is essential for optimal patient selection and procedural planning[1,2]. Occlusion balloon catheters should always be removed under fluoroscopic guidance.
COMPLICATIONS
Major complications include artery thrombosis (the most common balloon-related vascular complication; incidence 1%-5%) due to hypercoagulability of maternal blood, vascular intimal injury during insertion of the balloon catheter and the blocking time of balloon occlusion, artery dissection, groin hematoma, pseudoaneurysm, balloon migration, ischemic events of the lower limbs, reperfusion injury of tissues and organs, acute renal failure, and rarely artery rupture (even with death)[26,27,38,47]. Other studies included uterine necrosis, intrauterine synechiae, endometrial atrophy, secondary amenorrhea, and hypomenorrhea. Another possible complication is postembolization syndrome, inclusive of transient low abdominal pain or buttocks pain/numbness, and low-grade fever with/without leukocytosis. No significant difference was found in terms of complications between POB-AA and POB-IIA by Liu et al[27].
OUTCOME
The most important factors to consider as objectives of POB are BL, PPH, and peripartum hysterectomy. The latter after cesarean section could be performed as a life-saving treatment in patients with placenta previa (incidence: 5.28%-10.83%) due to PPH[47]. The most important causes of emergent peripartum hysterectomy are abnormal placentation (25.0%-73.3%), uterine atony (20.6%-43.0%), and uterine rupture (11.4%-45.5%). Yang et al[47] reported BL ranging from 271.4 mL to 2080.0 mL [mean cumulative BL: 1280 mL; 95% confidence interval (CI): 986-1574), of which the mean volume during cesarean section was 1225 mL (95%CI: 908-1543) and during cesarean hysterectomy was 1526 mL (95%CI: 1002-2050)[48].
Other outcomes we consider are length of hospital stay and RBC transfusions (RBCT). The POB significantly improves the rate of all these consequences[38,42,48-50]. Many studies[51-55] found a significant difference between patients treated with POB and those who were not[56-60] and specifically observed a significant difference in BL[61-65]. The most recent studies demonstrated good outcomes for POB in terms of BL and reducing complications during cesarean sections.
However, some authors suggest that POB could not prevent massive bleeding because during pregnancy a rich system of blood vessels is developed. The majority of studies available in the literature are non-randomized prospective and retrospective trials. There is a paucity of randomized controlled trials necessary to demonstrate the safety and efficacy of this procedure and to identify the type of patient with PAS disorders who can benefit most from the procedure. Moreover, the majority of available studies include a small cohort of patients[25].
For these reasons, the Eastern Cooperative Oncology Group and International Federation of Gynecology and Obstetrics guidelines show caution regarding the effectiveness of POB, in particular for the presence of numerous conflicting studies. On the other hand, many centers perform this procedure anyway with the results reported in this minireview[25,66].
The indications for POB-AA as opposed to POB-CIA or POB-IIA are still not clear, and this procedure should be evaluated by a multidisciplinary team composed of gynecologists, obstetricians, diagnostic and interventional radiologists, urologists, and vascular surgeons[45]. The great majority of available literature studies are focused on POB-AA. Various attempts have been made to occlude more and more cephalad vessels, starting from the IIA to the CIA up to the aorta[67]. BL and blood transfusion were significantly lower between POB-AA, POB-IIA, and POB-UA compared with the non-POB intervention, but the POB-AA group was the best treatment for reducing BL[38,41].
The BL estimated by Abouda et al[39] was from 2888 mL ± 863 mL in the control group and 1828 mL ± 324 mL in the POB-IIA group (P < 0.01), whereas the need for RBCT reduction was 10 ± 5 in the control group vs 4 ± 3 in the POB-IIA group (P < 0.01). Kyozuka et al[29] reported BL of 2312 g (95%CI: 49-4577) during cesarean section. The complete meta-analysis conducted by Liu et al[27] showed a significant difference in BL [mean difference (MD): -410.61 mL, 95%CI: -779.74 to -41.47, P < 0.001] compared between POB-AA vs POB-IIA. However, no statistically significant differences were noticed regarding RBCT volume, although it was less in the patients with POB-AA than POB-IIA (MD: -344.50 mL, 95%CI: -735.74 to 46.74, P = 0.08).
Other studies showed a clear reduction in the need for RBCT between the POB group and the non-POB group. However, in the meta-analysis by Nankali et al[68], the authors concluded that it cannot be established if POB-IIA gives advantage in term of RBCT[55,57,59] due to conflicting data between different studies[62,65,69]. The hysterectomy rate was lower in some studies[49,52,55,58], with a significant difference between the POB and non-POB groups[61,62]. The meta-analysis by Nankali et al[68] reported a hysterectomy rate for the POB-IIA group and non-POB group of 8.9% and 31.2%, respectively, and a difference in BL of 3.21 mL ± 0.38 mL.
The hysterectomy rate reported by Dai et al[38] was reduced in the POB-AA and POB-IIA groups compared with the non-POB group. Between the POB-IIA, POB-CIA, and POB-AA groups there was no significant difference observed; POB-CIA and POB-AA were the best options to reduce the hysterectomy rate. Between the POB-CIA and non-POB groups the difference was not statistically significant. The POB-AA and POB-IIA treatment groups had a notably lower RBCT compared with the non-POB group. However, no significant difference among the POB-IIA, POB-CIA, POB-AA, and POB-UA subgroups was noted by Dai et al[38], and the probability of reduced RBCT was higher in POB-AA[47]. The Lowest hysterectomy rates during cesarean section were associated with POB-AA in comparison with the non-POB group. However, it was similar with the POB-IIA group[27].
The risk of death is avoided in 15000 pregnancies per year globally through the use of blood products, hence RBCT is crucial in the management of patients with abnormal placentation[70]. The better effect in reducing RBCT was with POB-AA procedure with a mean reduction of 600 mL due to occlusion of collateral circulation during POB-AA[38]. Huo et al[41] estimated BL of 3167.65 mL ± 3255.71 mL, hysterectomy rate of 17.6%, and intensive care unit admission rate of 41.2%. Liu et al[27] reported non-significant differences in hysterectomy rates (odds ratio = 0.99, 95%CI: 0.22-4.44, P = 0.99] and UA embolization (odds ratio = 0.65, 95%CI: 0.36-1.18, P = 0.15) between POB-AA and POB-IIA, and they reported reduced duration of balloon dilatation in POB-AA than POB-IIA (MD: –5.34 min, 95%CI: -9.91 to -0.77, P = 0.02).
A secondary but important outcome is the X-ray dose exposition. The National Committee on Radiological Protection recommended a fetal radiation exposure value less than 150 mGy as a standard dose. However, Yin and Hu[26] showed in their study a value less than 20 mGy. Liu et al[27] reported a higher fetal radiation dose in POB-IIA than POB-AA (MD: -20.81 mGy, 95%CI: -31.84 to -9.78, P < 0.001).
From our best practical knowledge, however, POB-AA shows better outcomes in terms of complication rates. The choice of the POB procedure should be made by the interventional radiologist/vascular surgeon within the surgeon team. When the operator works in an interventional radiology suite, and the patient is brought to the surgical theater after the POB procedure, it is better to choose POB-IIA, as ischemic complications are less frequent. If the operator and obstetric surgeon work together in a hybrid suite, POB-AA could be the best option. The complication rate is low during POB, and in some studies no procedure-related complications were reported, despite a large number of procedures performed[26,29].
CONCLUSION
POB performed before a cesarean section in patients with PAS is useful to reduce surgery complications, in particular BL. Although widely used in many centers worldwide, it is not yet standard in most important gynecological guidelines due to some discordant results in literature. The choice between different devices, target artery, and technical execution is not standardized. More information about POB-AA is available in the literature in comparison to the other POB procedural variants. The rate of procedural complications is low. Real effectiveness and safety of this procedure should be evaluated through randomized studies to compare POB of different arteries, to standardize and optimize the procedural protocol, and to better manage the patients with placental abnormalities.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade A, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade C
P-Reviewer: Nagamine T S-Editor: Luo ML L-Editor: Filipodia P-Editor: Lei YY