Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.107315
Revised: April 27, 2025
Accepted: June 7, 2025
Published online: June 28, 2025
Processing time: 79 Days and 2.8 Hours
Thyroid dysfunction during pregnancy is an important disease affecting the health of mothers and children. Two-dimensional (2D) shear wave elastography (SWE) is the newest ultrasonic elastography technology and its value in differen
To explore prospectively the value of 2D SWE quantitative analysis for the eva
We included outpatients of reproductive age in the Department of Gynecology in Shanghai Changning Maternity and Infant Health Hospital between March 2023 and March 2024 who had conventional ultrasound examination and 2D SWE of the thyroid. They also underwent transvaginal ultrasound examination to confirm early intrauterine pregnancy and serum thyroid stimulating hormone (TSH) level was measured. The patients were divided into pregnant with normal TSH, pre
A total of 108 patients were included in the study; 57 in the pregnant with normal TSH group, 18 in the pregnant with abnormal TSH group and 33 were in the nonpregnant with normal TSH group. Thyroid size, thyroid echotexture, 2D SWE quantitative parameters including mean elasticity in the region of interest and maximal elasticity in the region of interest showed no significant differences among the three groups (P > 0.05).
Conventional ultrasound and 2D SWE features could not reflect the level of serum TSH.
Core Tip: Two-dimensional shear wave elastography (2D SWE) is very useful in differentiating benign and malignant thyroid nodules. However, its value in evaluating and predicting thyroid function is unclear. We prospectively compared conventional ultrasound and 2D SWE results of thyroid in pregnant women with normal thyroid stimulating hormone (TSH), pregnant with abnormal TSH, and nonpregnant with normal TSH. Our results showed that thyroid size, thyroid echotexture, 2D SWE quantitative parameters including mean elasticity and maximal elasticity showed no significant differences among the three groups. 2D SWE features could not reflect serum TSH level and be unsuitable for monitoring thyroid function.
- Citation: Zhang HP, Chen ML, Zou J, Zhou YQ. Value of two-dimensional shear wave elastography quantitative analysis for evaluation of thyroid function in first trimester pregnancy. World J Radiol 2025; 17(6): 107315
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/107315.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.107315
Thyroid dysfunction during pregnancy is an important disease affecting the health of mothers and children, increasing the risk of gestational hypertension, abortion, premature delivery, low birth weight infants, stillbirth, etc., and endan
Conventional ultrasound is the first imaging choice and is important in the diagnosis of thyroid diseases. Two-dimensional (2D) shear wave elastography (SWE) is the newest ultrasonic elastography technique, which provides quantitative information about tissue stiffness. Through emitting the acoustic radiation force by the probe to pressurize the tissue, causing the tissue deformation and generating shear wave, tissue hardness can be indirectly measured by shear wave velocity, or be displayed in real time by color-coding shear wave velocity. The value of 2D SWE in differentiating benign and malignant thyroid nodules has been widely recognized for its high diagnostic efficacy[3-5].
The value of 2D SWE in evaluating and predicting thyroid function is unclear. Yucel et al[6] showed that SWE quan
Department of China’s Health Management suggests that all pregnant women should be screened for thyroid disease, and the preferred screening indicator is serum TSH[9]. If TSH is abnormal, further examinations including serum free triiodothyronine, free thyroxine, TG-Ab, TPO-Ab and thyroid ultrasound are necessary. If SWE examination of the thyroid were useful for evaluating thyroid function and reflecting serum TSH level, it would provide a new noninvasive imaging method for thyroid function assessment during pregnancy.
In this study, we prospectively performed thyroid conventional ultrasound and SWE examinations, evaluated the correlation between thyroid SWE examination results and serum TSH level, and compared those results with those in normal nonpregnant women. Our aim was to explore the value of 2D SWE quantitative analysis for evaluation of thyroid function in early pregnancy.
This was a prospective study and was approved by the Medical Ethics Committee of Shanghai Changning Maternity and Infant Health Hospital, approval No. CNFBLLKT-2020011. Written informed consent was obtained from each patient before ultrasound examination.
Outpatients in the Department of Gynecology in Shanghai Changning Maternity and Infant Health Hospital between March 2023 and March 2024 volunteered to join the study and met the inclusion criteria: (1) Age 18-45 years; (2) Without thyroid surgery or medication history; (3) Conventional ultrasound examination showed no thyroid space-occupying lesions; and (4) Transvaginal ultrasound examination confirmed an early intrauterine pregnancy with fetal heartbeat (gestational age 6-10 weeks), or transvaginal ultrasound examination together with HCG test excluded pregnancy. The exclusion criteria were: (1) Without serum TSH level acquired through laboratory examination within 2 weeks before and after ultrasound examination; (2) Abnormal serum TSH level for no-pregnant women; and (3) 2D SWE examination could not meet the criteria of quality control.
The participants were divided into the pregnant and non
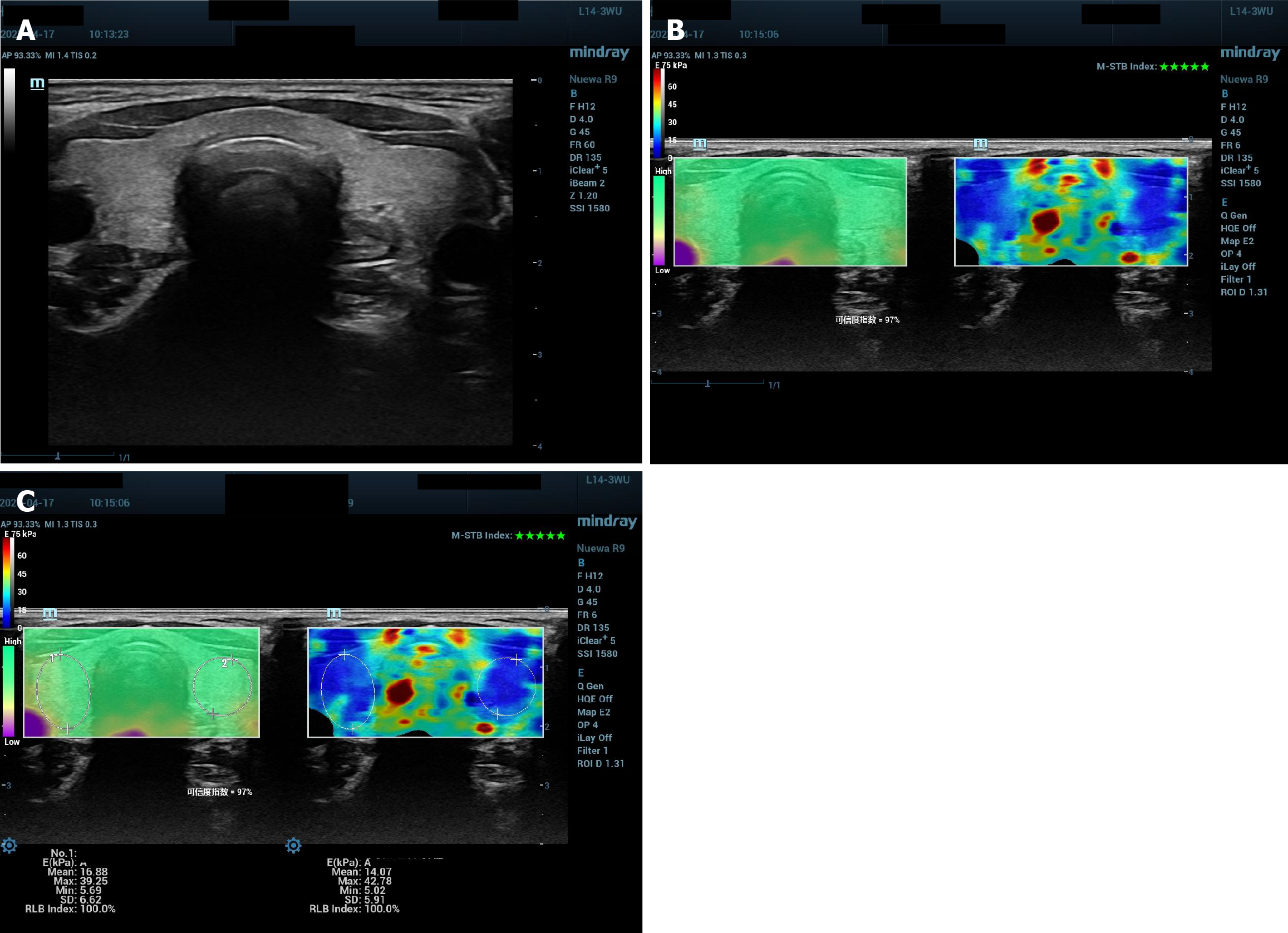
All the conventional ultrasound examinations were performed by a radiologist with 10 years’ experience. A Resona R9 diagnostic ultrasound system (Mindray Medical International, Shenzhen, China) with a linear array L14-3WU probe was used. The patient was instructed to lie on the examination bed, expose their neck adequately and breathe gently. A thorough scan of the thyroid was used first to exclude thyroid space-occupying lesions. The transverse diameters and anteroposterior diameters of the right lobe and left lobe were measured and the product of the transverse diameter and anteroposterior diameter of each lobe was calculated and recorded as the size of each lobe. Thyroid echotexture was observed and recorded as homogeneous or inhomogeneous (Figure 1A).
Before the study, the radiologist was specially trained in thyroid 2D SWE examination. For thyroid 2D SWE examination, the transducer frequency was set as 12 MHz, the depth of the image as 4 cm and dynamic range as 135 dB. A transverse section of thyroid including left lobe, right lobe and isthnus was chosen first for 2D SWE examination. A region of interest (ROI) was set to include the whole thyroid. The elasticity bar was set to a scale of 0-75 kpa with blue color indicate soft tissue and red color indicate hard. The elastographic map was held stably for at least 3 seconds and when the image met the quality control criteria (reliability index > 95% and motion-stability index to be five green stars, Figure 1B), it was frozen and saved. An oval ROI was drawn to include the right lobe; another oval ROI was drawn to include the left lobe. Quantitative parameters as mean elasticity (Emean) and maximal elasticity (Emax) were shown on the screen and recorded for further analysis (Figure 1C). A longitudinal section of the right lobe and a longitudinal section of the left lobe were chosen respectively for 2D SWE examination. An oval ROI was drawn to include the whole lobe and quantitative parameters as Emean and Emax were recorded for further analysis.
SPSS version 24.0 software (IBM Corporation, Chicago, IL, United States) was used for statistical analysis. P < 0.05 was considered statistically significant. Numerical variables are presented as mean ± SE, median (5th-95th percentile). The independent sample t-test and analysis of variance were used for comparisons if they showed normal distribution (using the Kolmogorov-Smirnov test); otherwise, nonparametric test including Mann–Whitney U test and Kruskal-Wallis H test were used instead. Numerical data are presented as a number (percentile) and compared in groups using the χ2 test.
One hundred and twelve patients (aged: 22-45 years; mean age 31.79 ± 4.52 years) were included. We excluded three patients without serum level of TSH acquired within 2 weeks before and after ultrasound examination and one nonpregnant women with abnormal TSH level. A total of 108 patients were included in the study; 57 were pregnant with normal TSH level (aged: 24-40 years; mean age 31.16 ± 3.59 years), 18 were pregnant with abnormal TSH level (aged: 26-45 years; mean age 31.50 ± 4.50 years); and 33 were nonpregnant with normal TSH level (aged: 22-45 years; mean age 33.03 ± 5.70 years). Patient age did not differentiate significantly among the three groups (F = 1.870, P = 0.159).
Conventional ultrasound results are shown in Table 1. Thyroid size and echotexture had no significant differences among the three groups.
| Characteristics | Size of thyroid right lobe1 (mm2) | Size of thyroid left lobe1 (mm2) | Thyroid echotexture | |
| Inhomogeneous | Homogeneous | |||
| Pregnant with normal TSH level (n = 57) | 180.44 ± 52.90, 180 (156-192) | 162.04 ± 44.85, 150 (140-168) | 15 (26.32) | 42 (73.68) |
| Pregnant with abnormal TSH level (n = 18) | 162.44 ± 44.90, 159 (129-182) | 159.72 ± 46.70, 157 (130-182) | 6 (33.33) | 12 (66.67) |
| Non-pregnant with normal TSH level (n = 33) | 160.21 ± 52.80, 144 (136.5-168) | 146.21 ± 41.97, 135 (130-150) | 11 (33.33) | 22 (66.67) |
| χ2 | 4.986 | 3.303 | 0.636 | |
| P value | 0.083 | 0.192 | 0.728 | |
2D SWE quantitative comparison among the three groups is shown in Table 2. 2D SWE quantitative parameters, as Emean and Emax, of transverse section or longitudinal section of the right or left lobe had no significant differences among the three groups. In the pregnant group, there were no correlations between serum TSH levels and 2D SWE quantitative parameters. Comparison of 2D SWE quantitative parameters between patients with abnormal and normal thyroid antibodies in the pregnant group is shown in Table 3. The above-mentioned 2D SWE quantitative parameters had no significant differences between the two groups.
| Characteristics | Emean of transverse thyroid right lobe | Emax of transverse thyroid right lobe | Emean of transverse thyroid left lobe | Emax1 of transverse thyroid left lobe | Emean1 of longitudinal thyroid right lobe | Emax of longitudinal thyroid right lobe | Emean1 of longitudinal thyroid left lobe | Emax of longitudinal thyroid left lobe |
| Pregnant with normal TSH level (n = 57) | 22.90 ± 5.23, 22.88 (21.53-24.12) | 41.92 ± 6.93, 41.04 (39.58-43.05) | 21.78 ± 5.54, 22.07 (19.76-24.37) | 39.56 ± 8.10, 40.36 (38.33-42.24) | 22.37 ± 5.59, 20.64 | 40.67 ± 7.52, 41.20 (37.45-43.25) | 23.81 ± 5.23, 23.23 (21.29-25.48) | 41.39 ± 7.05, 41.85 (37.82-43.27) |
| Pregnant with abnormal TSH level (n = 18) | 20.90 ± 4.87, 21.62 (17.59-23.72) | 38.68 ± 7.40, 41.05 (33.27-44.00) | 19.98 ± 4.67, 19.98 (18.13-22.35) | 40.06 ± 8.83, 42.01 (38.22-43.74) | 22.77 ± 5.42, 22.18 | 41.29 ± 5.47, 42.07 (38.94-44.63) | 24.28 ± 5.56, 24.77 (19.29-27.66) | 42.26 ± 7.07, 42.46 (39.00-47.30) |
| Non-pregnant with normal TSH level (n = 33) | 24.10 ± 5.79, 23.41 (21.44-26.32) | 39.61 ± 8.71, 41.47 (36.87-44.65) | 22.23 ± 4.75, 21.37 (20.08-24.91) | 40.47 ± 6.00, 40.75 (37.48-43.80) | 22.89 ± 6.36, 21.23 | 38.34 ± 6.50, 37.71 (34.92-41.22) | 23.25 ± 5.77, 22.30 (20.00-24.24) | 39.76 ± 7.80, 40.25 (34.34-42.65) |
| F/χ2 | 2.092 | 1.708 | 1.887 | 0.240 | 0.259 | 1.523 | 0.805 | 0.827 |
| P value | 0.129 | 0.186 | 0.157 | 0.887 | 0.878 | 0.223 | 0.668 | 0.440 |
| Characteristics | Emean of transverse thyroid right lobe | Emax of transverse thyroid right lobe | Emean of transverse thyroid left lobe | Emax1 of transverse thyroid left lobe | Emean1 of longitudinal thyroid right lobe | Emax of longitudinal thyroid right lobe | Emean1 of longitudinal thyroid left lobe | Emax of longitudinal thyroid left lobe |
| Pregnant with normal thyroid antibodies (n = 59) | 22.31 ± 4.95, 22.23 (21.13-24.00) | 41.15 ± 7.04, 40.75 (39.59-43.05) | 21.12 ± 5.39, 21.15 (18.60-23.37) | 39.45 ± 8.48, 40.36 (38.28-42.56) | 22.60 ± 5.68, 21.48 | 40.87 ± 7.12, 41.69 (37.68-43.25) | 23.96 ± 5.15, 23.41 (21.37-25.52) | 41.67 ± 7.25, 42.11 (38.74-43.27) |
| Pregnant with abnormal thyroid antibodies (n = 11) | 23.69 ± 7.04, 22.44 (18.14-30.02) | 40.98 ± 8.67, 38.01 (32.86-50.75) | 22.59 ± 4.68, 22.85 (19.78-26.62) | 40.19 ± 5.32, 41.32 (38.33-42.48) | 22.16 ± 5.03, 21.54 | 39.15 ± 6.16, 41.20 (33.36-44.13) | 23.95 ± 6.56, 20.45 (18.75-32.74) | 39.89 ± 6.99, 38.25 (33.08-48.71) |
| t/Z | 0.788 | 0.071 | 0.847 | 0.281 | 0.242 | 0.748 | 0.002 | 0.753 |
| P value | 0.434 | 0.944 | 0.365 | 0.779 | 0.809 | 0.457 | 0.999 | 0.454 |
Our results showed that thyroid size and echotexture shown on conventional ultrasound examinations, and 2D SWE quantitative parameters including Emean and Emax had no significant differences among the pregnant with normal TSH group, pregnant with abnormal TSH group, and nonpregnant with normal TSH group. Conventional ultrasound and 2D SWE quantitative parameters did not reflect serum TSH level and thyroid function.
Thyroid size and echotexture were usually considered to be relevant to diffuse thyroid disease (DTD) and thyroid dysfunction. DTD usually appears as diffuse hyperechogenic or hypoechogenic inhomogeneity with enlarged thyroid volume on conventional ultrasound[12,13]. Some DTDs, such as Hashimoto’s thyroiditis and Graves’ disease, have typical ultrasound features and usually can be diagnosed by conventional ultrasound[14,15]. However, as different types of DTD have multifaceted pathogenesis and different pathological changes over time, sometimes it is difficult to diagnose and differentiate DTDs by conventional ultrasound[16]. Our results showed that thyroid size and echotexture shown on conventional ultrasound had no significant differences between pregnant and nonpregnant women, or between pregnant women with normal serum TSH and abnormal serum TSH. Xuan showed similar results, and concluded that ultrasound features may not be consistent with serological results during pregnancy[17].
Normal thyroid glands are composed of follicles covered with cuboidal epithelium, with colloid in follicular lumen and connective tissue, blood vessels and lymphatic vessels among follicles. When lesions occur, the probable histological changes include follicular cell hyperplasia or hypertrophy, colloid accumulation or depletion, inflammatory cell infiltration and fibrous tissue proliferation. Those changes lead to the change in the stiffness of thyroid tissue, which is the theoretical foundation for thyroid SWE examination. The preliminary study of Sporea et al[18] concluded that thyroid SWE examination was a useful method to evaluate DTD and was able to predict the presence of DTD with sufficient accuracy. They also found that SWE quantitative parameters in patients with abnormal TSH were significantly higher than those with normal TSH, especially between patients with low levels of TSH and those with normal TSH[19]. Our study had different results and showed that 2D SWE quantitative parameters, as Emax and Emean, had no significant differences between pregnant women with normal and abnormal serum TSH levels. The possible reason for that might be the participant discrepancy between the two studies. Our study included pregnant women and outpatients of repro
We also found that 2D SWE quantitative parameters had no significant differences between pregnant women with abnormal and normal thyroid antibodies. TPO-Ab and TG-Ab could usually be positive in DTD patients before clinical symptoms appear and thyroid stiffness changes. So, 2D SWE quantitative parameters could not reflect the change of TPO-Ab and TG-Ab.
Although conventional ultrasound and 2D SWE features could not reflect thyroid function correctly, it is still important to perform thyroid ultrasound during pregnancy to detect thyroid nodules, monitor nodule size, diagnose malignancy and guide clinical management[20,21]. Our study had some limitations. First, participant numbers were insufficient, especially for abnormal serum TSH pregnant women. Second, patients in pregnant with abnormal TSH group were all with abnormally high serum TSH level, with no patients with abnormally low serum TSH level. Further prospective larger studies are needed to verify our results.
Conventional ultrasound and 2D SWE features did not reflect the level of serum TSH. Further prospective larger studies are needed to verify our results.
| 1. | Tsakiridis I, Giouleka S, Kourtis A, Mamopoulos A, Athanasiadis A, Dagklis T. Thyroid Disease in Pregnancy: A Descriptive Review of Guidelines. Obstet Gynecol Surv. 2022;77:45-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Bogović Crnčić T, Ćurko-Cofek B, Batičić L, Girotto N, Tomaš MI, Kršek A, Krištofić I, Štimac T, Perić I, Sotošek V, Klobučar S. Autoimmune Thyroid Disease and Pregnancy: The Interaction Between Genetics, Epigenetics and Environmental Factors. J Clin Med. 2024;14:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Zhao CK, Ren TT, Yin YF, Shi H, Wang HX, Zhou BY, Wang XR, Li X, Zhang YF, Liu C, Xu HX. A Comparative Analysis of Two Machine Learning-Based Diagnostic Patterns with Thyroid Imaging Reporting and Data System for Thyroid Nodules: Diagnostic Performance and Unnecessary Biopsy Rate. Thyroid. 2021;31:470-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Petersen M, Klemenz B, Schenke SA. [Elastography in thyroid nodules]. Laryngorhinootologie. 2023;102:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Borysewicz-Sańczyk H, Bossowski F, Anikiej K, Sawicka B, Michalak J, Dzięcioł J, Bossowski A. Application of shear wave elastography in the management of thyroid nodules in children and adolescents: our experience and a review of the literature. Front Endocrinol (Lausanne). 2024;15:1486285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Yucel S, Ceyhan Bilgici M, Kara C, Can Yilmaz G, Aydin HM, Elmali M, Tomak L, Saglam D. Acoustic Radiation Force Impulse Quantification in the Evaluation of Thyroid Elasticity in Pediatric Patients With Hashimoto Thyroiditis. J Ultrasound Med. 2018;37:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Liu J, Zhang Y, Ji Y, Wan Q, Dun G. The value of shear wave elastography in diffuse thyroid disease. Clin Imaging. 2018;49:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Li L, Zhang A, Chen D, Taragin BH, Luo X. Preliminary study of sound touch elastography in diffuse thyroid disease in children. Front Pediatr. 2022;10:964413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Compilation Committee of “Guidelines for the Prevention; Treatment and Management of Thyroid Diseases during Pregnancy and Childbirth”; Chinese Medical Association; Endocrinology Branch; Women's Health Branch of Chinese Preventive Medicine Association. Guidelines for prevention and management of thyroid diseases during pregnancy and perinatal period. Zhonghua Neifengmi Daixie Zazhi. 2022;38:539-551. [DOI] [Full Text] |
| 10. | Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1485] [Article Influence: 185.6] [Reference Citation Analysis (0)] |
| 11. | Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Słowińska-Klencka D, Wojtaszek-Nowicka M, Klencki M, Wysocka-Konieczna K, Popowicz B. The Presence of Hypoechoic Micronodules in Patients with Hashimoto's Thyroiditis Increases the Risk of an Alarming Cytological Outcome. J Clin Med. 2021;10:638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Huang J, Zhao J. Quantitative Diagnosis Progress of Ultrasound Imaging Technology in Thyroid Diffuse Diseases. Diagnostics (Basel). 2023;13:700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Vita R, Di Bari F, Perelli S, Capodicasa G, Benvenga S. Thyroid vascularization is an important ultrasonographic parameter in untreated Graves' disease patients. J Clin Transl Endocrinol. 2019;15:65-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Kosiak W, Piskunowicz M, Świętoń D, Batko T, Kaszubowski M. An additional ultrasonographic sign of Hashimoto's lymphocytic thyroiditis in children. J Ultrason. 2015;15:349-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Huang Q, Jiang W, Li J, Wen J, He J, Song W. Hashimoto's thyroiditis recognition from multi-modal data via global cross-attention and distance-aware training. Med Image Anal. 2025;102:103515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Xuan YH, Yue S, Jiang YX, Wu QQ. Ultrasonographic manifestations of thyroid with subclinical thyroid serological anomalies during pregnancy. Chin J Med Ultrasound (Electron Ed). 2018;15:213-217. |
| 18. | Sporea I, Vlad M, Bota S, Sirli RL, Popescu A, Danila M, Sendroiu M, Zosin I. Thyroid stiffness assessment by acoustic radiation force impulse elastography (ARFI). Ultraschall Med. 2011;32:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Sporea I, Sirli R, Bota S, Vlad M, Popescu A, Zosin I. ARFI elastography for the evaluation of diffuse thyroid gland pathology: Preliminary results. World J Radiol. 2012;4:174-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Panagiotakopoulos T, Chorti A, Pliakos I, Ioannidis A, Boudina M, Papavramidis T. Thyroid cancer and pregnancy: a systematic ten-year-review. Gland Surg. 2024;13:1097-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Langdon J, Gupta A, Sharbidre K, Czeyda-Pommersheim F, Revzin M. Thyroid cancer in pregnancy: diagnosis, management, and treatment. Abdom Radiol (NY). 2023;48:1724-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |