Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.105962
Revised: April 12, 2025
Accepted: May 21, 2025
Published online: June 28, 2025
Processing time: 134 Days and 21.5 Hours
Acute kidney injury (AKI) is a frequent complication after liver transplantation (LT). How to realize the early diagnosis of AKI, perform active intervention, and reduce the mortality of post-LT patients is an urgent problem to be solved.
To investigate the accuracy of hepatorenal index (HRI) and renal resistive index (RRI) in monitoring of early AKI after LT.
This observational study included adult deceased-donor LT recipients at our cen
Of 121 patients were included in the study (mean age: 50.18 ± 8.88years; female: 17.36%). AKI developed in 53 patients (43.80%). The AKI and non-AKI groups were similar in terms of their baseline characteristics. An HRI of ≤ 1.12 on POD 1 detected AKI with a sensitivity of 62.30% and a specificity of 87.80% [area under the receiver operating characteristic curve (AUC) = 0.801, P < 0.01]. An RRI of ≥ 0.65 on POD 1 detected AKI with a sensitivity of 87.80% and a specificity of 67.60% (AUC = 0.825, P < 0.01). The HRI combined with the RRI was more effective at detecting AKI than either the HRI or RRI alone (AUC = 0.890, P < 0.01). The HRI increased as AKI resolved while the RRI decreased as AKI re
The HRI and RRI are non-invasive bedside indices that can identify the occurrence and recovery of early AKI after LT.
Core Tip: In this observational study, we examined the monitoring value of the hepatorenal index and renal resistive index for acute kidney injury in deceased-donor liver transplantation recipients. We conclude that the hepatorenal index and renal resistive index are non-invasive bedside indices that can identify the occurrence and recovery of acute kidney injury, which aids in the intervention and adjustment of treatment plans, thereby improving the prognosis of patients with acute kidney injury after liver transplantation.
- Citation: Zhang D, Sun J, Xu CS, Yang ZZ, Wu XD, Zhao K, Cai JZ, Wang JH. Role of sonographic hepatorenal index and renal resistive index in monitoring of acute kidney injury after liver transplantation. World J Radiol 2025; 17(6): 105962
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/105962.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.105962
Acute kidney injury (AKI) is a frequent complication after liver transplantation (LT). AKI negatively impacts patient out
Post-LT AKI is multifactorial in origin and has been linked to liver disease severity, graft quality, pre-LT renal dys
Several tools are used to diagnose AKI, including urine output, serum creatinine, urinary protein estimation, and frac
Ultrasound is the method of choice to detect potential complications during the postoperative period of LT as it is fast and safe. Therefore, compared with other imaging methods, ultrasound plays an indispensable role in patient monitoring after LT[6]. Two-dimensional ultrasound can observe renal parenchymal echo. In some renal parenchyma diseases, changes in echogenicity are observed[7]. The hepatorenal index (HRI) is more objective and can avoid individual diffe
One of the cardinal features of AKI is intrarenal vasoconstriction. The ultrasound Doppler-derived renal resistive index (RRI) reflects renal blood flow. Several studies have confirmed the role of the RRI in predicting AKI after major surgeries, such as cardiac and liver surgery[8,9]. However, no studies have examined its diagnostic utility after LT.
In this study, we aimed to explore the role of the HRI and RRI in monitoring the occurrence and recovery of AKI after LT.
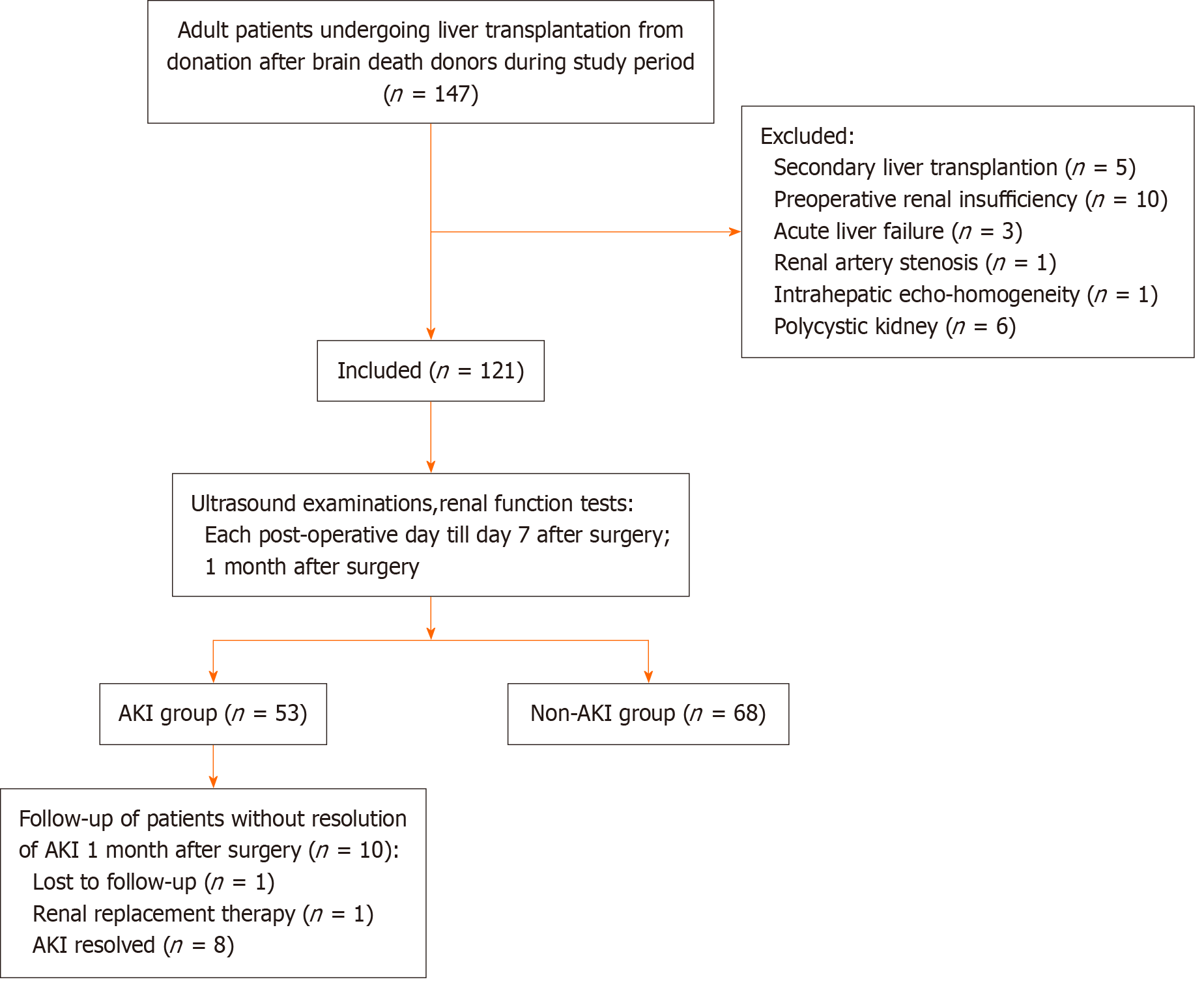
Consecutive adult patients (≥ 18 years) from whom consent was obtained, who satisfied the inclusion criteria, and who underwent deceased-donor LT for chronic liver disease or hepatocellular carcinoma between February 2022 and February 2023 were included (n = 121) (Figure 1).
Hemodynamics differ between patients undergoing living-donor and deceased-donor LT; therefore, we excluded patients who underwent living-donor LT to maintain data homogeneity and exclude bias. We also excluded: (1) Patients aged < 18 years; (2) Patients with anatomic malformations that may interfere with the determination of ultrasonic para
All LT procedures were performed by the same surgical team. Standard surgical steps were followed as per our insti
AKI was defined according to the KDIGO criteria[4], as follows: (1) An increase in serum creatinine by ≥ 0.3 mg/dL within 48 hours or an increase in serum creatinine to ≥ 1.5-times that of baseline within the last 7 days; or (2) A urine output of < 0.5 mL/kg/hour for 6 hours. According to the KDIGO criteria, AKI severity was further divided into stage 1, 2, or 3.
Renal recovery was defined as the disappearance of AKI criteria, as previously defined at the Acute Disease Quality Initiative conference in 2017[10].
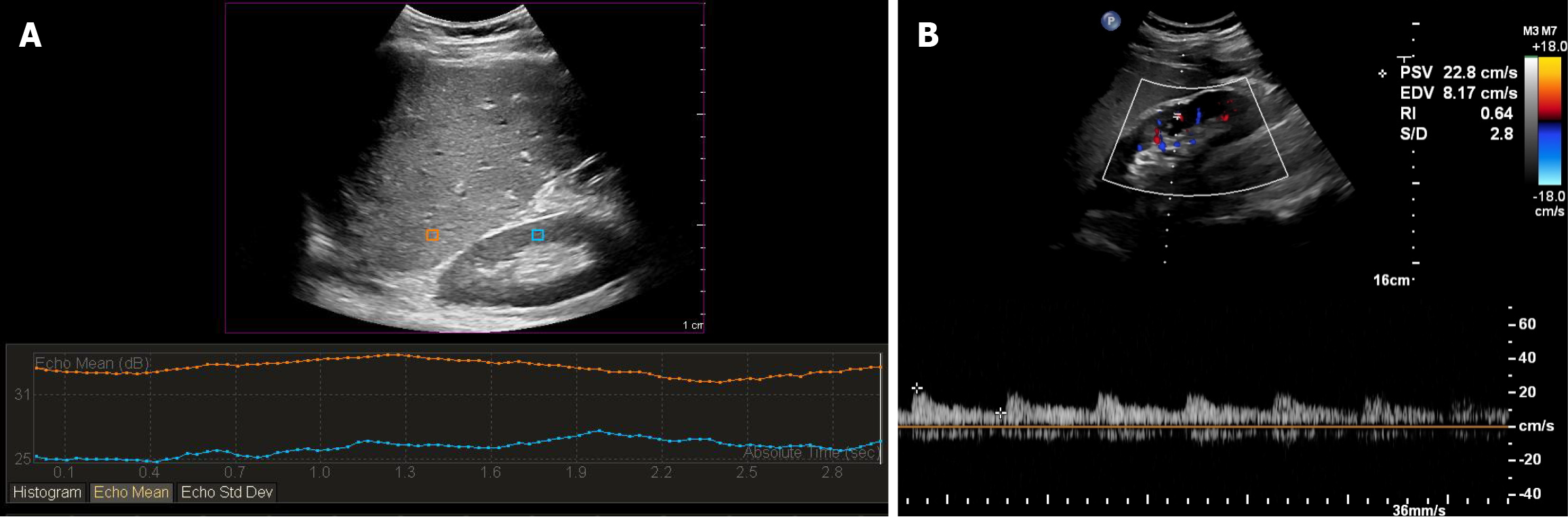
The patients were examined daily within the first 7 postoperative days (PODs). Ultrasound examinations were performed using PHILIPS EPIQ 7 ultrasound scanners with a C5-1-MHz convex probe. The patients were lying in the supine position with the right arm above the head. The probe was positioned in the right subcostal coronal position until stable images of the liver and renal parenchyma could be obtained. The angle of the probe was adjusted to avoid blocking the frame on the ultrasonic image. The liver–kidney junction was located in the center of the ultrasound image so as to avoid the influence of lateral attenuation on the echo intensity measurement. The images were analyzed using Q-LAB. Regions of interest (ROIs) were chosen to contain only hepatic and renal parenchyma, without any visible vessels, renal sinus, or medulla (Figure 2A). To avoid image distortion, the hepatic and renal ROIs were selected in the same plane and with the same depth. The HRI was calculated by dividing the mean echo intensity of the pixels within the selected hepatic ROI by the mean echo intensity of the pixels within the selected ROIs of the right renal cortex.
To prevent bias, standard protocols for RRI measurements were followed for all patients. First, three Doppler samples were obtained from each of three regions of the kidney, including the upper, middle, and lower poles. The RRI was measured at the level of the interlobar or arcuate arteries (Figure 2B). Following that, we calculated the mean RRI of each of the three regions of the kidney, as mentioned above.
Since the HRI before LT could not be used as the baseline value, we collected images at 1 month after LT surgery for patients whose kidney function had returned to normal.
All statistical analyses were performed using SPSS version 25.0. Continuous variables are reported as the mean with standard deviation or as median with range. Categorical variables are reported as numbers and proportions. To compare categorical datasets, the χ2 test or Fisher’s exact test was used. The paired t-test was used to compare the temporal change in the HRI and the RRI for the same subset of patients over the course of the postoperative period. A P value of < 0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve analysis was performed to deter
From February 2022 to February 2023, a total of 147 adult patients underwent deceased-donor LT at our center. Twenty-six patients were excluded due to secondary LT (n = 5), preoperative renal insufficiency (n = 10), acute liver failure (n = 3), renal artery stenosis (n = 1), intrahepatic echo-homogeneity (n = 1), and polycystic kidneys (n = 6). Finally, 121 patients were included in the study. The mean age of the patients was 50.18 years, and 17.36% of the patients were female. The average body mass index (BMI) was 24.30 kg/m2. The most common primary diagnosis was malignant neoplastic disease (42.96%), followed by hepatitis B cirrhosis (38.84%). The mean Model for End-Stage Liver Disease (MELD) score was 13.67 (range: 4.16-32.78). AKI developed in 53 patients (43.80%) and resolved within 7 days after surgery in 43 patients (81.13%) (Table 1). AKI did not resolve in 10 patients, and we followed up with these patients. One patient was not followed up at our center after surgery, and one patient required renal replacement therapy (RRT). AKI resolved in the remaining eight patients within 2 months after LT surgery.
| Postoperative characteristics | |
| AKI | 53 (43.80) |
| Stage 1 | 30 (56.60) |
| Stage 2 | 18 (33.96) |
| Stage 3 | 5 (9.43) |
| PODs of AKI diagnosis | |
| 1 | 49 (92.45) |
| 2 | 4 (7.55) |
| PODs when AKI resolved | |
| 2 | 4 (9.30) |
| 3 | 6 (13.95) |
| 4 | 19 (44.19) |
| 5 | 6 (13.95) |
| 6 | 8 (18.60) |
| AKI not resolved | 10 (18.87) |
| Stage 1 | 2 (20) |
| Stage 2 | 3 (30) |
| Stage 3 | 5 (50) |
There was no significant difference between the two groups in terms of co-morbid status, patient demographics, MELD score, or primary diagnosis. Patients in the AKI group had a higher BMI (26.02 kg/m2vs 22.96 kg/m2, P < 0.01), Cys C (1.32 vs 1.14 mg/L, P = 0.02) and a longer mean surgical duration (8.69 vs 8.03 hours, P = 0.03). Patients in the AKI group required more platelet transfusion during LT surgery (Table 2). Logistic regression analysis indicated that BMI [odds ratio (OR) = 1.267, confidence interval (CI): 1.121-1.433] and preoperative serum level of Cys C (OR = 4.038, CI: 1.219-13.375) are independent risk factors for early AKI after LT.
| AKI | Non-AKI | P value | |
| Age, years | 50.49 ± 8.51 | 44.94 ± 9.04 | 0.73 |
| Female sex | 8 (15.09) | 13 (19.12) | 0.56 |
| BMI, kg/m2 | 26.02 ± 4.17 | 22.96 ± 3.46 | < 0.01 |
| Comorbidities | |||
| Diabetes mellitus | 7 (13.21) | 4 (5.88) | 0.17 |
| Hypertension | 6 (8.82) | 5 (9.43) | 0.91 |
| Etiology of liver failure | 0.77 | ||
| Malignant neoplastic disease | 23 (43.40) | 29 (42.60) | |
| Virus (HBV) | 24 (45.30) | 23 (33.80) | |
| Ethanol | 4 (7.50) | 7 (10.30) | |
| Cholestatic liver disease | 1 (1.9) | 4 (5.90) | |
| Autoimmune disease | 1 (1.9) | 5 (7.40) | |
| MELD score | 14.61 ± 5.94 | 12.94 ± 6.45 | 0.15 |
| Creatinine, μmol/L | 78.47 ± 19.49 | 74.25 ± 19.26 | 0.24 |
| Urea nitrogen, mmol/L | 5.21± 2.00 | 5.14± 1.84 | 0.85 |
| Cys C, mg/L | 1.32 ± 0.42 | 1.14 ± 0.33 | 0.02 |
| eGFR, mL/minute/1.73 m2 | 81.84 ± 4.18 | 88.63 ± 30.58 | 0.19 |
| Albumin, g/L | 33.27 ± 5.10 | 33.09 ± 7.32 | 0.88 |
| IVC cross clamp | 0.86 | ||
| Partial (n = 2) | 1 (1.89) | 1 (1.47) | |
| Complete (n = 119) | 52 (98.11) | 67 (98.53) | |
| Mean intraoperative blood products transfused | |||
| Erythrocytes | 4.5 (2-10) | 6 (4-8.5) | 0.72 |
| Platelets | 0 (0-1) | 0 (0-0) | < 0.01 |
| Surgical duration, hours | 8.69 ± 1.66 | 8.03 ± 1.54 | 0.03 |
| Anhepatic duration, minutes | 41.26 ± 12.94 | 38.13 ± 10.23 | 0.14 |
| HRI on POD 1 | 1.08 ± 0.21 | 1.40 ± 0.35 | < 0.01 |
| RRI on POD 1 | 0.69 ± 0.04 | 0.63 ± 0.05 | < 0.01 |
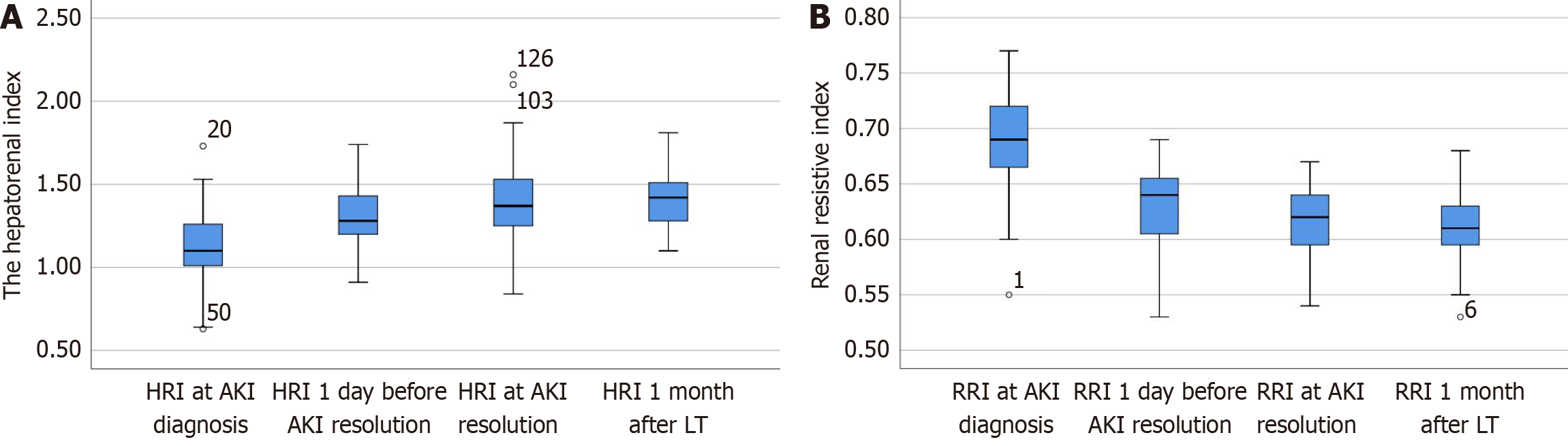
Paired comparisons were made between: (1) The HRI and RRI on the day of AKI diagnosis (HRId and RRId); (2) The HRI and RRI 1 day before AKI resolution (HRIr-1 and RRIr-1); (3) The HRI and RRI on the day of AKI resolution (HRIr and RRIr), and (4) The HRI and RRI at 1 month after LT surgery (HRIm and RRIm). These results are presented in Figure 3 and Table 3. The HRId was lower than the HRIm (1.13 vs 1.38, respectively; n = 43, P < 0.01), and generally remained this way until 1 day before resolution (1.28 vs 1.38; n = 43, P = 0.04). The HRIr was similar to the HRIm (1.37 vs 1.38, respec
| n | P value | ||
| HRId vs HRIm | 1.13 vs 1.38 | 43 | < 0.01 |
| HRIr-1 vs HRIm | 1.28 vs 1.38 | 43 | 0.04 |
| HRIr vs HRIm | 1.37 vs 1.38 | 43 | 0.92 |
| HRId vs HRIr-1 | 1.13 vs 1.28 | 43 | < 0.01 |
| HRId vs HRIr | 1.13 vs 1.37 | 43 | < 0.01 |
| HRIr-1 vs HRIr | 1.28 vs 1.37 | 43 | < 0.01 |
| RRId vs RRIm | 0.69 vs 0.61 | 43 | < 0.01 |
| RRIr-1 vs RRIm | 0.63 vs 0.61 | 43 | < 0.01 |
| RRIr vs RRIm | 0.61 vs 0.61 | 43 | 0.62 |
| RRId vs RRIr-1 | 0.69 vs 0.63 | 43 | < 0.01 |
| RRId vs RRIr | 0.69 vs 0.61 | 43 | < 0.01 |
| RRIr-1 vs RRIr | 0.63 vs 0.61 | 43 | 0.001 |
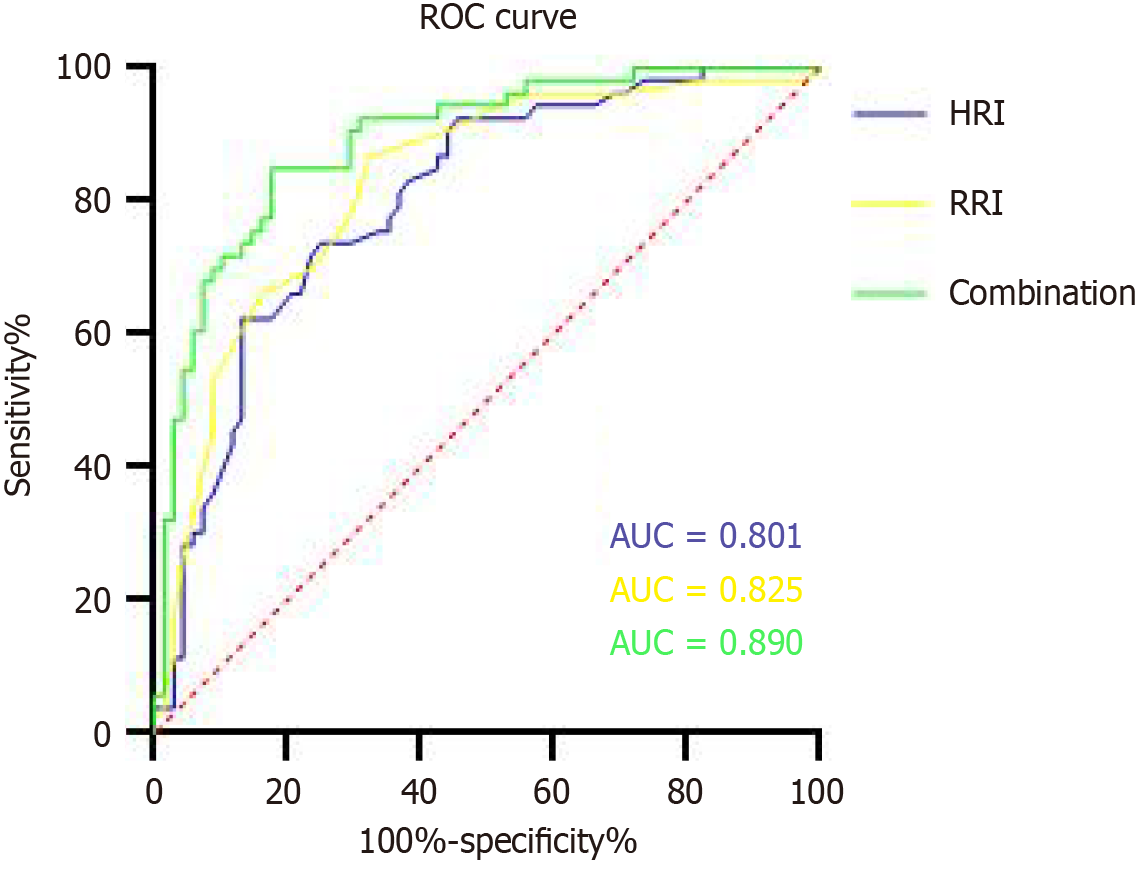
The ROC curve analysis showed that the HRI of ≤ 1.12 on POD 1 detected AKI with a sensitivity of 62.30% and a specificity of 87.80% [area under the ROC curve (AUC) = 0.801 (CI: 0.722-0.880), P < 0.01], while the RRI of ≥ 0.65 on POD 1 indicated AKI with a sensitivity of 87.80% and a specificity of 67.60% [AUC = 0.825 (CI: 0.750-0.901), P < 0.01]. The HRI combined with the RRI was more effective for detecting AKI than either the HRI or RRI alone [AUC = 0.890 (CI: 0.831-0.948), P < 0.01] (Figure 4).
AKI represents a frequent and clinically significant complication following LT, with substantial implications for both short- and long-term patient outcomes[11]. The reported incidence of post-LT AKI varies widely from 5% to 94%, with 10%-20% of patients exhibiting long-term renal dysfunction[12,13]. Post-LT AKI is associated with an increased length of hospital stay and higher morbidity, including CKD development and greater mortality[11]. A previous study emphasized the link between moderate AKI and lower graft and patient survival rates[2]. In the present study, the incidence of post-LT AKI within the first 7 PODs was 43.80%, which is similar to previous reports[1,9]. We observed that all patients who developed AKI experienced this injury within the first 2 PODs, with the most common day of diagnosis being POD 1 in more than 90% of patients. The majority of AKI (90.57%) episodes were mild-to-moderate in severity and resolved spontaneously without requiring RRT. Patients with early recovery of AKI did not develop delayed AKI after surgery. A previous study also reported that most mild and moderate cases of post-LT AKI are reversible[14]. Among the patients who did not recover early, one was lost to follow-up and one required RRT. The remaining eight patients recovered within 2 months after surgery. There were no fatalities in either group within the first 7 PODs after LT.
The development of post-LT AKI is multifactorial, involving an interplay of pre-LT comorbidities, donor and recipient features, intraoperative factors, and postoperative variables. Pre-transplant renal dysfunction, which is a common problem in patients with cirrhosis, is a significant comorbidity. Higher creatinine, higher blood urea nitrogen, and the presence of renal dysfunction or AKI prior to transplantation were identified as independent risk factors for post-LT AKI in a previous meta-analysis[11]. Patients with hepatorenal syndrome and baseline CKD were not included in the present study. In this study, while the AKI and non-AKI groups did not differ in terms of liver disease severity, patient demographics, and co-morbid status, patients in the AKI group had higher BMI values. A previous study showed that obesity significantly impacts post-LT renal function. A BMI of > 27.5 kg/m2 was independently associated with post-LT AKI with an OR of 2.5[15]. According to the literature, the majority of patients (> 60%) who undergo orthotopic LT experience AKI, leading to acute renal failure in the early postoperative period, which may be linked to early mortality as high as 40%, compared with only 5% in other LT recipients[16,17]. In the present study, 98.35% of patients underwent orthotopic LT, which suggested a high risk of AKI. We observed that more platelets were required during surgery in the AKI group. According to earlier research, intraoperative platelet transfusion is an independent risk factor for 1- and 5-year survival after LT because it leads to acute lung injury[18]. We also found that a long surgical duration was a risk factor for AKI following LT (8.69 vs 8.03, P = 0.03). In a recent report, it was noted that patients with a long surgical duration (> 480 minutes) were 6.567-times more likely to develop AKI than their counterparts with a shorter surgical duration[19].
Interstitial edema is present in many acute kidney diseases, including AKI. Furosemide blocks chloride ion transport in the ascending branch of the loop of Henle, reducing reabsorption from the tubule into the blood, resulting in increased interstitial water. Tuma et al[20] studied changes in renal echo during interstitial edema by injection of furosemide and showed that interstitial edema resulted in enhanced renal cortical echo. However, the routine sonographic interpretation of enhanced renal cortical echo is based on a subjective impression. As a result, the interpretation is observer-driven, with interobserver and intraobserver variability and limited reproducibility and comparability. The HRI is an objective computer-calculated index, which overcomes the limitation of interobserver/intraobserver variability. Several studies have used the HRI to evaluate steatosis, showing that it is a sensitive, non-invasive method for steatosis quantification[21]. The HRI can diagnose small amounts of liver fat that would be missed by conventional sonography[22]. Currently, few studies have reported the application of the HRI in the context of AKI. The present study demonstrated that the HRI decreased when AKI occurred, and as AKI resolved, the HRI increased. An HRI of ≤ 1.12 on POD 1 indicated AKI with a sensitivity of 62.30% and a specificity of 87.80%. The HRI therefore provides a more objective and accurate basis for sonographers to identify AKI in the early stage and assists clinicians to prevent and control injury in a timely manner, in turn improving the prognosis of patients. Current evidence strongly associates AKI with systemic hemodynamic insta
This study is the first to use the HRI to monitor post-LT AKI. The advantages of this study include the robust metho
In conclusion, both the HRI and RRI have a clear role in monitoring the occurrence and recovery of AKI in LT recipients. The HRI combined with the RRI is more effective for detecting AKI than either the HRI or RRI alone.
| 1. | Dong V, Nadim MK, Karvellas CJ. Post-Liver Transplant Acute Kidney Injury. Liver Transpl. 2021;27:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | de Haan JE, Hoorn EJ, de Geus HRH. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract Res Clin Gastroenterol. 2017;31:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Caragata R, Emerson S, Santema ML, Selzner N, Sapisochin G, Wang S, Huszti E, Van Klei W, McCluskey SA. Intraoperative hypotension and the risk of acute kidney injury following liver transplantation. Clin Transplant. 2023;37:e15053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3348] [Article Influence: 257.5] [Reference Citation Analysis (0)] |
| 5. | Rossiter A, La A, Koyner JL, Forni LG. New biomarkers in acute kidney injury. Crit Rev Clin Lab Sci. 2024;61:23-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Banegas-Deras EJ, Mazón-Ruiz J, Romero-González G, Ruiz-Cobo JC, Sanz-García C, Serrano-Soto M, Sánchez E, Argaiz ER. Acute kidney injury and point-of-care ultrasound in liver cirrhosis: redefining hepatorenal syndrome. Clin Kidney J. 2024;17:sfae112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Tuma J. [CME Sonography 99/Answers: Kidney Echo Changes]. Praxis (Bern 1994). 2021;110:510-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Wu H, Liu K, Darko IN, Xu X, Li L, Xing C, Mao H. Predictive value of renal resistive index for the onset of acute kidney injury and its non-recovery: A systematic review and meta-analysis . Clin Nephrol. 2020;93:172-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Shankar V, Raj A, Singhal S, Sahni R, Goyal N, Venuthurimilli A, Olson MT, Chatterji C. Doppler-derived renal resistive index helps predict acute kidney injury in patients undergoing living-related liver transplantation. Clin Transplant. 2021;35:e14263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1058] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 11. | Thongprayoon C, Kaewput W, Thamcharoen N, Bathini T, Watthanasuntorn K, Lertjitbanjong P, Sharma K, Salim SA, Ungprasert P, Wijarnpreecha K, Kröner PT, Aeddula NR, Mao MA, Cheungpasitporn W. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Hizomi Arani R, Abbasi MR, Mansournia MA, Nassiri Toosi M, Jafarian A, Moosaie F, Karimi E, Moazzeni SS, Abbasi Z, Shojamoradi MH. Acute Kidney Injury After Liver Transplant: Incidence, Risk Factors, and Impact on Patient Outcomes. Exp Clin Transplant. 2021;19:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | MacDonald AJ, Karvellas CJ. Acute kidney injury: A critical care perspective for orthotopic liver transplantation. Best Pract Res Clin Anaesthesiol. 2020;34:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, Wang H; ISN AKF 0by25 China Consortiums. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 333] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 15. | Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, Kim CS, Gwak MS, Kim GS. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS One. 2015;10:e0136230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | O'Riordan A, Wong V, McQuillan R, McCormick PA, Hegarty JE, Watson AJ. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant. 2007;7:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Zhu M, Li Y, Xia Q, Wang S, Qiu Y, Che M, Dai H, Qian J, Ni Z, Axelsson J, Yan Y. Strong impact of acute kidney injury on survival after liver transplantation. Transplant Proc. 2010;42:3634-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Zhou ZQ, Fan LC, Zhao X, Xia W, Luo AL, Tian YK, Wang XR. Risk factors for acute kidney injury after orthotopic liver transplantation: A single-center data analysis. J Huazhong Univ Sci Technolog Med Sci. 2017;37:861-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Tuma J, Schwarzenbach HR, Nováková B, Jungius KP, Kuchta M. [The quantitative measurement of the echogenicity of the renal parenchyma]. Praxis (Bern 1994). 2008;97:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Tanpowpong N, Panichyawat S. Comparison of sonographic hepatorenal ratio and the degree of hepatic steatosis in magnetic resonance imaging-proton density fat fraction. J Ultrason. 2020;20:e169-e175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Kjaergaard M, Lindvig KP, Hansen CD, Detlefsen S, Krag A, Thiele M. Hepatorenal Index by B-Mode Ratio Versus Imaging and Fatty Liver Index to Diagnose Steatosis in Alcohol-Related and Nonalcoholic Fatty Liver Disease. J Ultrasound Med. 2023;42:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | De Souza FM, De Carvalho AV, Ferraz IS, Damiano AP, Brandão MB, Nogueira RJN, De Souza TH. Acute kidney injury in children undergoing cardiac surgery: predictive value of kidney arterial Doppler-based variables. Pediatr Nephrol. 2024;39:2235-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Gupta K, Bhurwal A, Law C, Ventre S, Minacapelli CD, Kabaria S, Li Y, Tait C, Catalano C, Rustgi VK. Acute kidney injury and hepatorenal syndrome in cirrhosis. World J Gastroenterol. 2021;27:3984-4003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (7)] |