Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.489
Revised: August 27, 2024
Accepted: September 10, 2024
Published online: September 28, 2024
Processing time: 58 Days and 16.1 Hours
According to the population statistics in 2023, there were 110000 people aged over 100 years in China, and the experience of using Paxlovid (nirmatrelvir/ritonavir) for centenarians is particularly valuable. This article reports our experience of using Paxlovid in a centenarian with the novel coronavirus disease 2019 (COVID-19) infection.
A 103-year-old female with mild COVID-19 and renal insufficiency was given sufficient Paxlovid for 2 days and a half dose for 3 days. During treatment, the patient was complicated with lung infection and heart failure, and nucleic acid remained positive. After expert consultation, a full dose of Paxlovid was given again on the 9th day of admission for 2 days and a half dose for 3 days. Mean
This case suggests that Paxlovid can be used cautiously in centenarians with renal insufficiency and two courses of treatment can be considered in patients with persistent positive nucleic acid.
Core Tip: We report a 103-year-old female with renal insufficiency and a history of hypertension. The patient was treated with two courses of Paxlovid at a full dose for 2 days and a half dose for 3 days. Although she had lung infection and heart failure during the treatment, they all improved after treatment and she was discharged from hospital 40 days after admission. This case suggested that Paxlovid can be applied cautiously in centenarians with coronavirus disease 2019 patients with renal insufficiency, and but the liver and kidney function of the patient should be closely monitored.
- Citation: Zhang YX, Tang J, Zhu D, Wu CY, Liang ML, Huang YT. Prolonged course of Paxlovid administration in a centenarian with COVID-19: A case report. World J Radiol 2024; 16(9): 489-496
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/489.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.489
Coronavirus disease 2019 (COVID-19) is a pandemic virus that has swept the globe in recent years. According to the World Health Organization (WHO), as of February 21, 2023, the total number of confirmed cases of COVID-19 in the world had exceeded 750 million, and the death rate exceeded 6.85 million. Although the WHO declared in May 2023 that COVID-19 no longer constitutes a "public health emergency of international concern", COVID-19 still occur. Paxlovid is approved by the United States Food and Drug Administration on December 22, 2021 for mild to moderate COVID-19 patients over the age of 12 years and weighing at least 40 kg. Paxlovid is the first oral COVID-19 drug approved in the United States[1]. We here report a case of a 103-year-old COVID-19 patient who received two courses of Paxlovid, with good outcome.
A 103-year-old elderly female was admitted to hospital 4 days after being found to have poor mental performance and poor appetite.
On December 24, 2022, the patient developed poor mental state, fatigue, poor appetite, oliguria, occasional cough and sputum. Her temperature was not monitored and no special attention was given. On December 27, her family members developed symptoms of influenza, and a throat swab tested positive for nucleic acid, while the patient was also tested positive for a throat swab.
She had a history of right femoral neck injury, on a wheelchair for 8 years, hypertension for more than 1 year, and highest blood pressure of 180/100 mmHg, without any medication, and Alzheimer's disease, and has not been vaccinated against COVID-19. The patient had no history of smoking, alcohol, drug or food allergies.
There was no family history of genetic disorders.
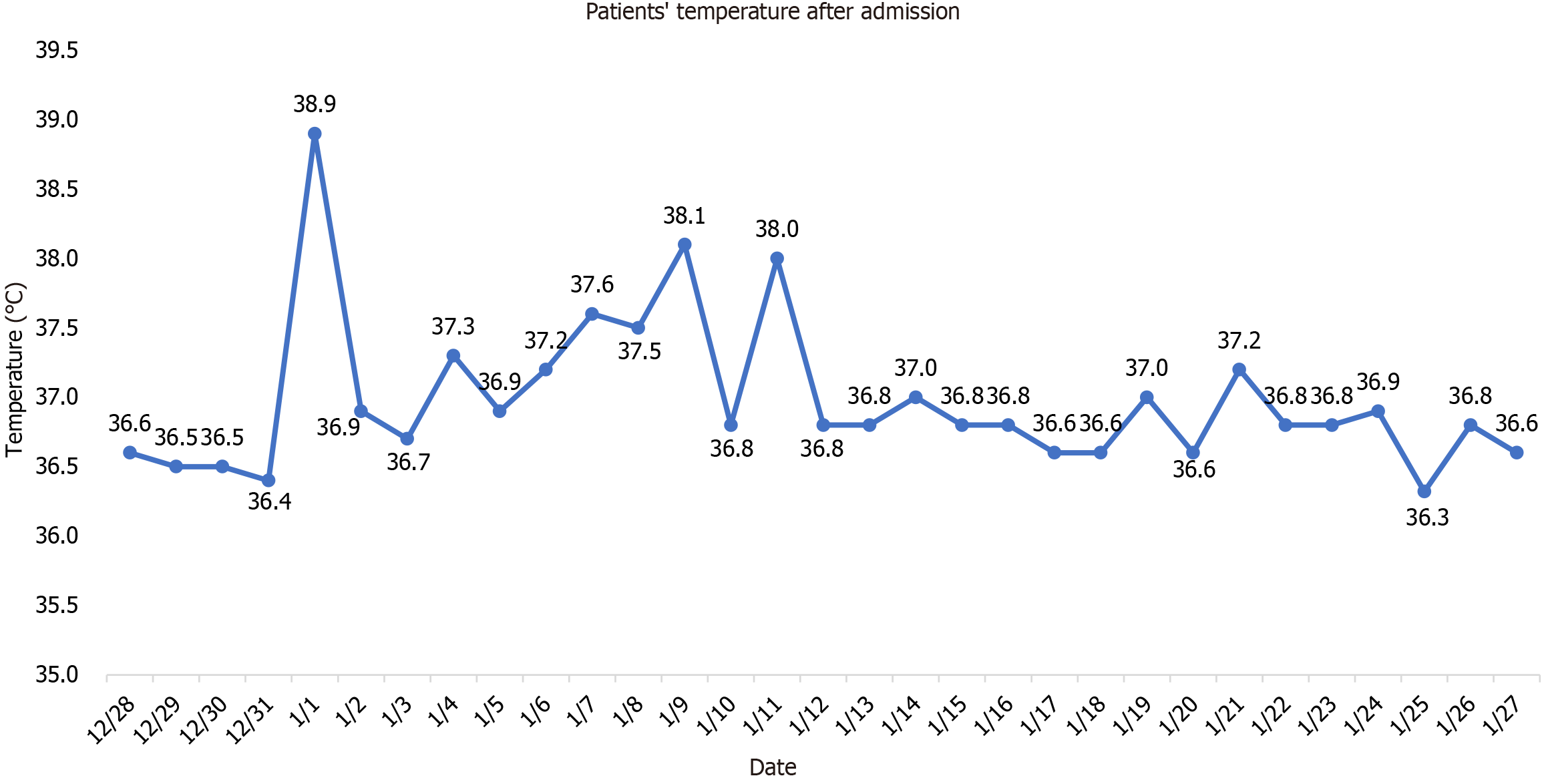
Body temperature: 36.6 °C, blood pressure 152/76 mmHg, breathing 24 times /min, blood oxygen saturation at rest state 94%, oxygen inhalation 3 L/min 97%, body weight 50 kg, mental fatigue, shallow coma, uncooperative physical examination, tingling response, a little dry and wet rale could be heard in the upper lung, low respiratory sound in the lower right lung. Heart rate 95 beats/min, in the aortic auscultation area 3/6 grade diastolic wind like murmurs could be heard. Abdominal tenderness, no tenderness, no rebound pain, no edema in lower extremities were observed.
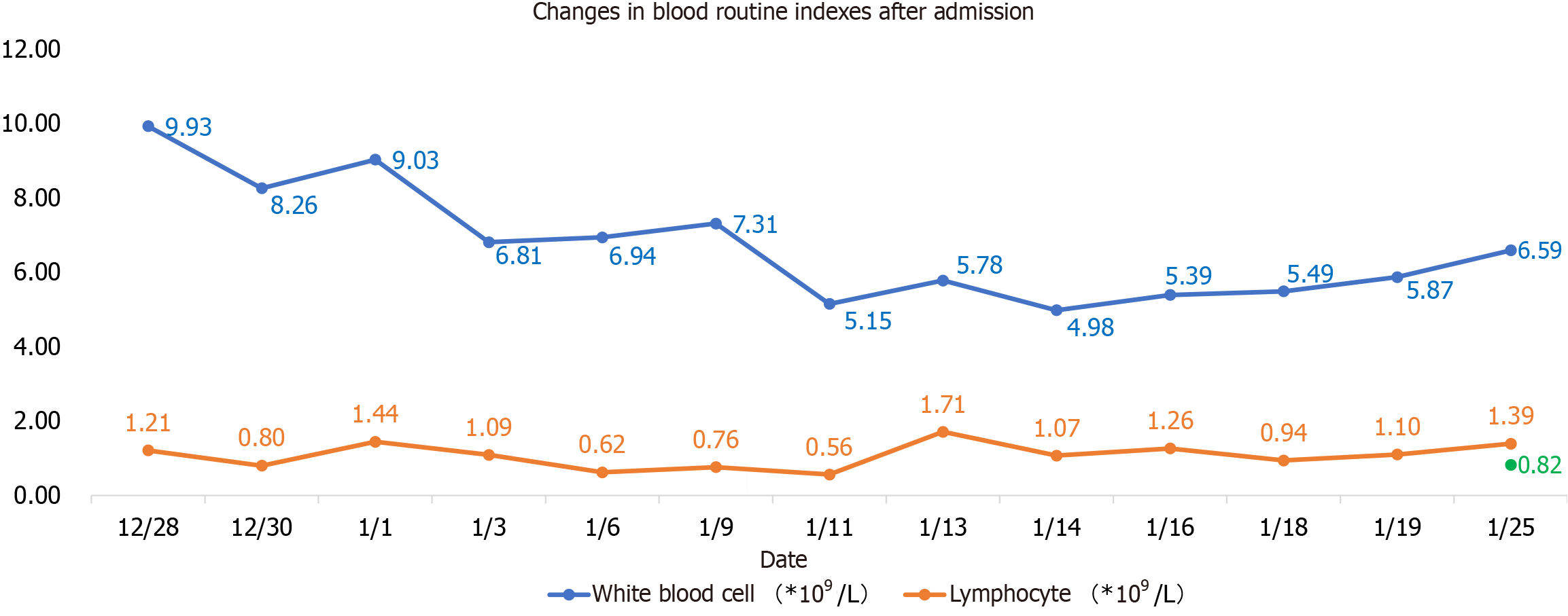
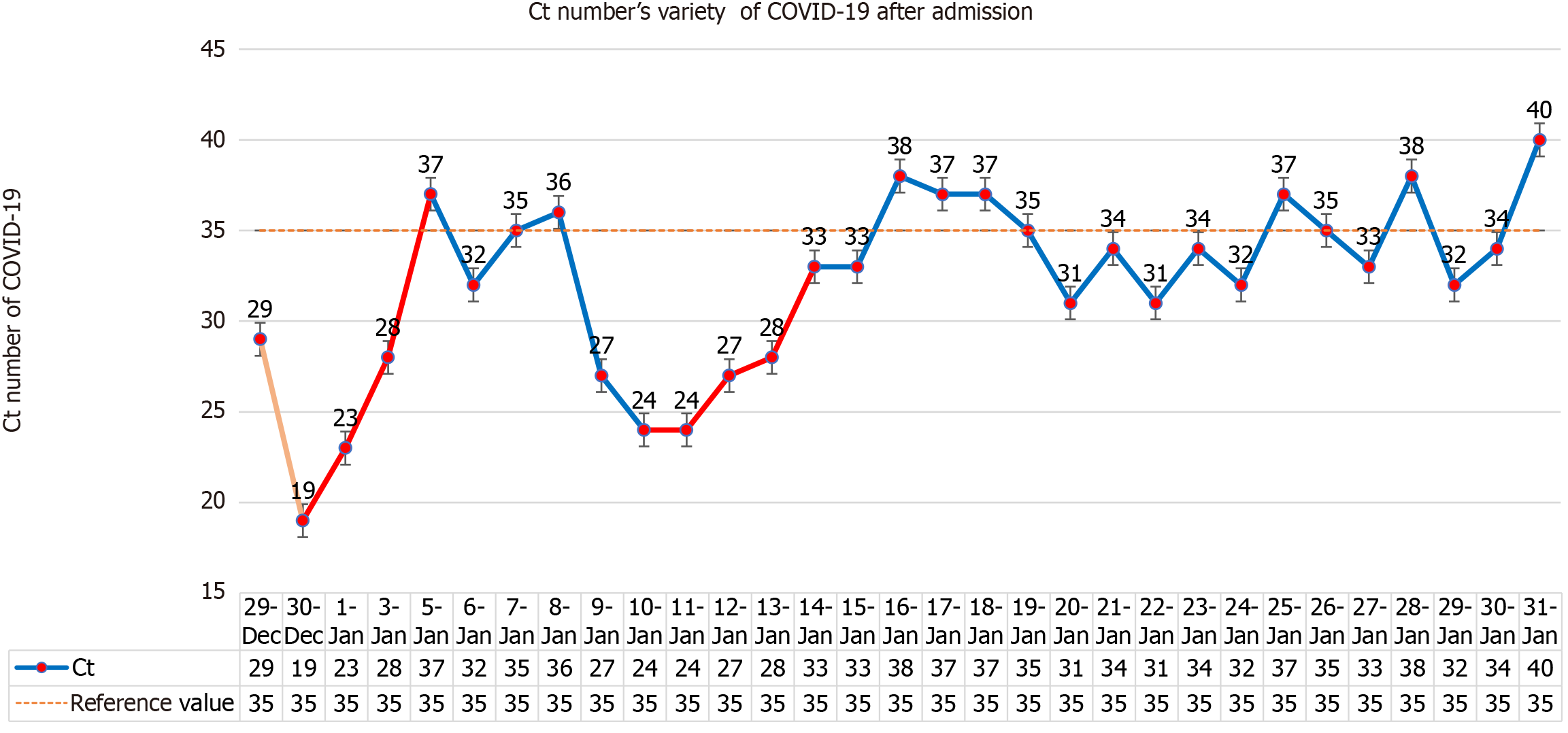
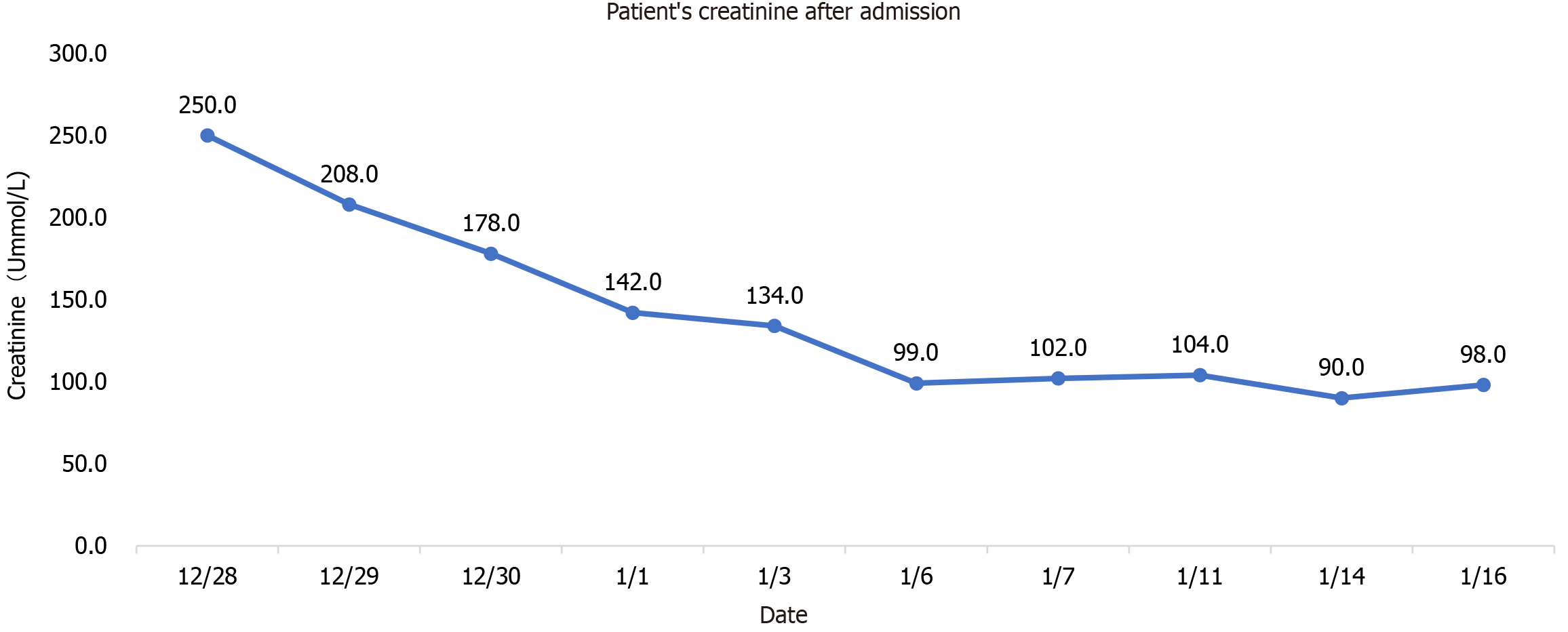
Blood routine examination showed white blood cells 9.93 × 109/L, neutrophil percentage 80.6%, lymphocyte count 1.21 × 109/L, sodium 170 mmol/L, chlorine 130.2 mmol/L, creatinine 250 um mol/L, nucleic acid positive, Ct value 29, D-2 polymer 5.0 mg/L, Procalcitonin 0.107 ng/mL, C-reactive protein 20.07 mg/L, N-terminal pro-brain natriuretic peptide (NT-proBNP) 1604 pg/mL, interleukin-6 29.19 pg/mL, ferritin 100.58 ng/mL, D2 polymer 5.0 mg/L, blood gas analysis (after oxygen absorption): PH 7.306, carbon dioxide partial pressure 44.5 mmHg, oxygen partial pressure 164 mmHg, oxygen saturation 99%, and lactate 2.3 mmol/L. Lactate dehydrogenase, myocardial enzyme, liver function and blood coagulation routine were all normal. On January 6, 2023, NT-proBNP reached the highest value of 6258 pg/mL, white blood cells and neutrophils were the highest on the first day of admission, 9.93 × 109/L and 80.6%, respectively, and lymphocyte count was the lowest on the 14th day of admission, 0.56 × 109/L, as shown in Figure 1. The highest value of procalcitonin was 0.107 pg/mL at the first check after admission, and the lowest value of epidermal growth factor receptor (eGFR) was 7.2% at admission and then gradually increased, as shown in Table 1.
| Date | White blood cells (× 109/L) | Ratio of white blood cells (%) | Lymphocyte count (× 109/L) | NT-proBNP (pg/mL) | Creatinine (umoL/L) | Creatinine clearance rate (%) | Procalcitonin (pg/mL) |
| December 28 | 9.93 | 80.60% | 1.21 | 250 | 7.7 | ||
| December 29 | 208 | 9.2 | |||||
| December 30 | 8.26 | 78.40% | 0.8 | 1604 | 178 | 10.8 | 0.107 |
| January 1 | 9.03 | 76.10% | 1.44 | 142 | 13.5 | 0.147 | |
| January 3 | 6.81 | 73.80% | 1.09 | 134 | 14.3 | ||
| January 6 | 6.94 | 78.8 | 0.62 | 6258 | 99 | 19.4 | 0.095 |
| January 7 | 102 | 18.8 | |||||
| January 9 | 7.31 | 75.50% | 0.76 | 5992 | |||
| January 11 | 5.15 | 78.40% | 0.56 | 5266 | 104 | 18.5 | 0.113 |
| January 13 | 5.78 | 54.40% | 1.71 | 0.091 | |||
| January 14 | 4.98 | 65.10% | 1.07 | 4890 | 90 | 21.4 | 0.1 |
| January 16 | 5.39 | 57.80% | 1.26 | 4201 | 98 | 19.6 | 0.087 |
| January 18 | 5.49 | 63.70% | 0.94 | ||||
| January 19 | 5.87 | 61% | 1.1 | 2890 | |||
| January 25 | 6.59 | 60.70% | 1.39 |
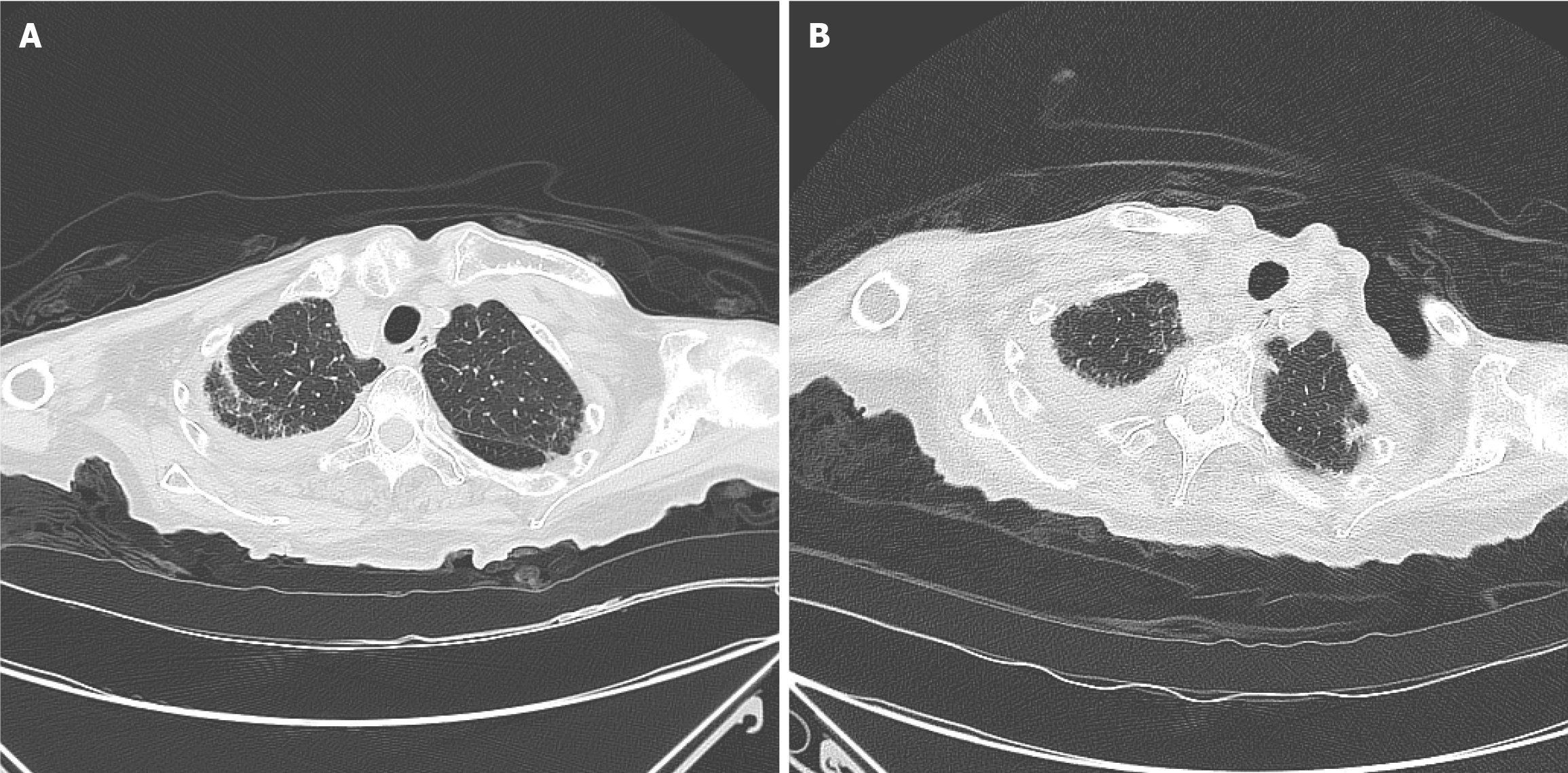
Upon admission, the patient's chest computed tomography (CT) revealed a mass in the upper lobe of the right lung, infectious lesions in both lungs, and a small amount of fluid on both sides, mainly on the right side. Upon discharge, chest CT indicated that inflammation of both lungs was slightly improved, as shown in Figure 2. Heart color ultrasonography showed ejection fraction 58%, and the left ventricular wall was slightly thickened (12 mm).
According to the therapeutics and COVID-19: Living guideline of WHO[2], the patient was diagnosed with mild-to-moderate COVID-19 at admission: Pulmonary infection, heart failure, and electrolyte disturbance during hospitalization.
The hospital organized consultations among experts inside and outside the hospital. On December 28, 2022, 150 mg of Nirmatrelvir and 100 mg of ritonavir were given for the first time, and on December 31, 300 mg of nirmatrelvir and 100 mg of ritonavir were given every 12 hours, with a full dose for 3 days and a half dose for 2 days. As the patient had dementia, she had poor cooperation with eating, and a gastric tube was inserted. All food and medication were administered via the gastric tube. The lowest Ct value of the patient was 19 on December 29, 2022. After Paxlovid and symptomatic treatment, the patient's mental state, cough, poor appetite and fatigue symptoms were improved sig
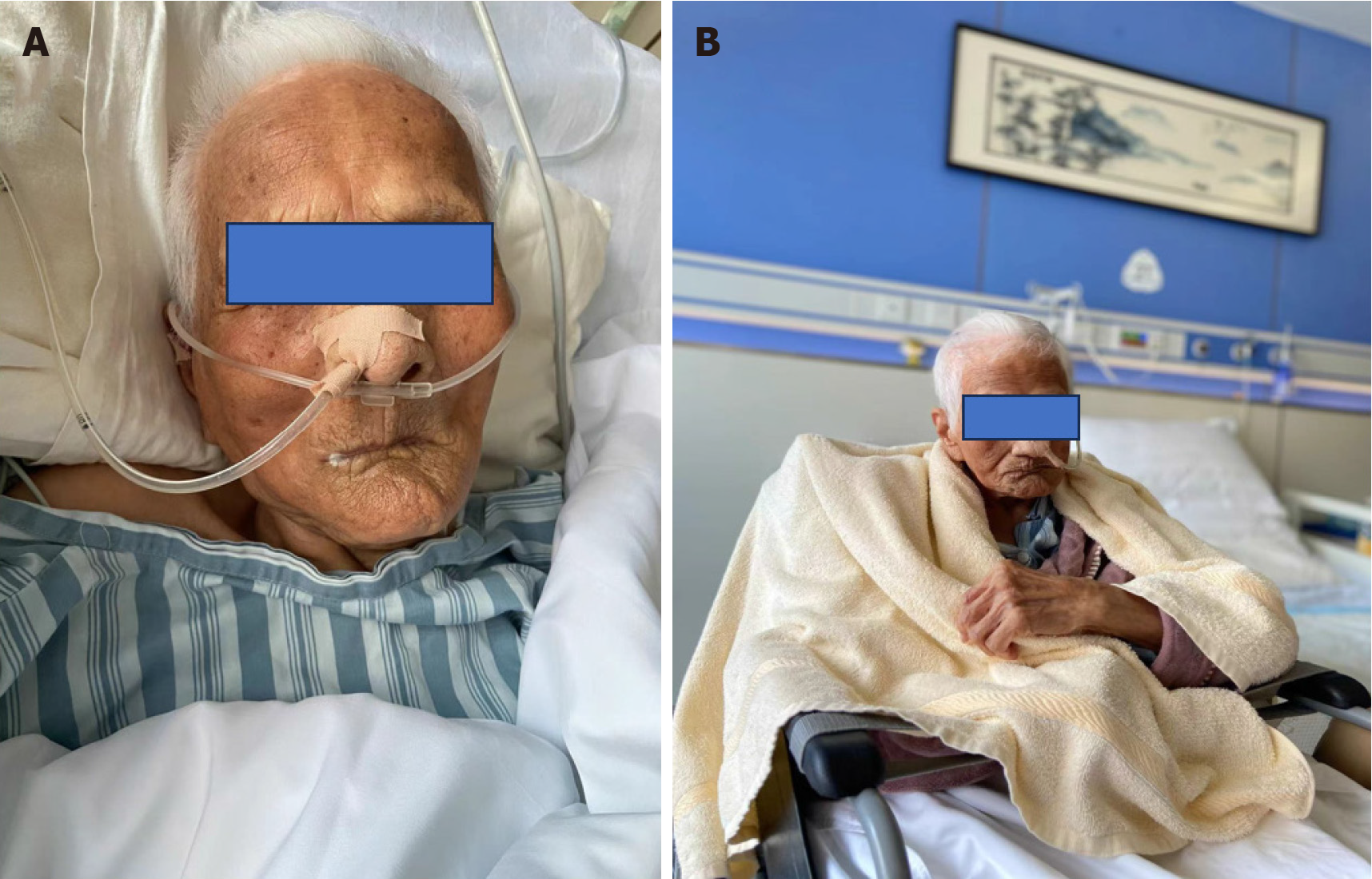
The patient was discharged from hospital on February 8, 2023 with no fever, no obvious cough and sputum, no shortness of breath, and with mental recovery. Figure 6 shows the comparison in the status of the patient at hospitalization and discharge. The patient's mental state was fine during a telephone follow-up 3 months after discharge, and she continued to be fed through a nasal feeding tube.
With the development of economy and the improvement of living standards, the number of long-lived people in China is increasing. According to the population statistics in 2023, there were 110000 people aged over 100 years in China. The experience of medication for centenarians is particularly valuable. We report a case of a 103-year-old female suffering from COVID-19. The experience of using Paxlovid for 2 courses in this patient is worth learning. From December 8, 2022 to January 12, 2023, a total of 59938 COVID-19 related deaths were reported[3] in medical institutions across China, according to the National Health Commission. According to the Xinhua net the majority of COVID-19 deaths were among the elderly, with an average age of 80.3 years, and 90.1% aged 65 or above, and 56.5% aged 80 or above. The elderly with cardiovascular diseases, obesity, diabetes and weakened immunity, are vulnerable groups[4-6]. The severe disease rate and death rate in elderly COVID-19 patients are significantly higher than those of the general population. The mechanism may be related[7] to inflammatory and immune factors in this aging group. The patient reported in this paper is 103 years old, and has a history of hypertension and belongs to a high-risk COVID-19 patient, which meets the indications for Paxlovid administration[8-9]. Wiersinga et al[10] reports among patients hospitalized in the intensive care unit, the case fatality is up to 40%. The "COVID-19 Diagnosis and Treatment Protocol (Trial Version 9)" issued in March 2022 Lists Paxlovid as the first choice for antiviral treatment. The United States Phase III clinical trial showed that Paxlovid can reduce the hospitalization rate and mortality rate of COVID-19 patients by 89%[1]. Paxlovid is indicated for the treatment of adults with mild-to-moderate COVID-19 who have a high risk of progression to severe disease, such as advanced age, chronic kidney disease, diabetes, cardiovascular disease, chronic lung disease and other high risk factors or severe disease[11-14]. Lu et al[15] studied 180 patients with an average age of 77 years and an average duration of treatment of 10 days, suggesting that the key factors leading to the prolonged treatment of elderly patients with COVID-19 in China are the lack of Paxlovid treatment and the absence of vaccination. All of the above proved that Paxlovid is effective and safe in the treatment of COVID-19.
There are four lessons worth learning from the use of Paxlovid for the long-lived elderly in this case. The first is the treatment plan, full dose for 3 days and half dose for 2 days. This dose is based on the antiviral effect of sufficient dose, and the patient is a long-lived elderly person with low eGFR (less than 30 mL/min), so as to minimize the side effects of the drug. The second is to use two courses of treatment, the second course of treatment is based on the fact that the patient's nucleic acid was positive and the Ct value had a downward trend. Considering the rise of viral load or the signs of the novel coronavirus recurrence, and the drug resistance of centenarians being weaker than that of ordinary people, the antiviral course may be longer. The obvious increase in Ct value after the second course of treatment also verifies our idea. The third is eGFR < 30 mL/min, which is prohibited according to the instructions. Since patient had no previous history of kidney disease, had low intake of food and fever after infection with COVID-19, and the creatinine increase was considered to be prerenal, nasal feeding is applied to supply fluid and food and the creatinine was significantly reduced. This suggests that the use of some drugs is limited by eGFR. However, we should distinguish whether the patient has underlying kidney disease or renal insufficiency secondary to this disease. If the latter is the case, we can use drugs more aggressively, but in either case, kidney function needs to be closely monitored. The fourth is the use of nasal feeding, the use in the drug instructions is oral, patients with Alzheimer's disease have basically lost their ability to eat autonomously, nasal feeding is the only route to ensure the normal intake of drugs. Although these four drugs are off-label, they are all appropriate treatments for the patient's condition. They are carried out under the condition of repeatedly informing the patient's family of the related risks and closely monitoring liver and kidney function, electrolytes and the patient's condition. The new effects and new use methods of many commonly used drugs in clinical practice are proved by continuous practice. Although the present patient is an individual case, it still provides us with some experience in the use of antiviral drugs for centenarians. The centenarians should use antiviral drugs as soon as possible after diagnosis of COVID-19, which can be given nasally if oral administration is not possible. The course of antiviral drugs may be longer than that of the general population, and the eGFR limit is not so strict, but the liver and kidney function should be closely monitored.
For centenarians infected with COVID-19, Paxlovid should be used as soon as possible to avoid the development of severe disease. If oral administration is not possible, nasal feeding can be used. Generally, one course of treatment is recommended. Due to immune senescence in the elderly, if the nucleic acid has not turned negative, two courses can be used, and the reference standard for eGFR for centenarians can be appropriately adopted. However, close monitoring of liver and kidney function is required.
| 1. | Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, Goldstein LH, Saliba W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin Infect Dis. 2023;76:e342-e349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 333] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 2. | Therapeutics and COVID-19: living guideline [Internet]. Geneva: World Health Organization, 2022 . [PubMed] |
| 3. | Mardomi A, Khosroshahi HT. Dialysis-Induced Immune Dysregulations and Their Possible Impacts on COVID-19. Iran J Kidney Dis. 2021;15:161-167. [PubMed] |
| 4. | Bailey A, Harris MA, Bogle D, Jama A, Muir SA, Miller S, Walters CA, Govia I. Coping With COVID-19: Health Risk Communication and Vulnerable Groups. Disaster Med Public Health Prep. 2021;17:e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zhu Q, Zhang Y, Kang J, Chen Z, Peng M, Chen M, Zhang G, Xiang D, Xiao S, Li H, Mei Y, Yang J, Qi X, Cai D, Ren H. Weakened humoral and cellular immune response to the inactivated COVID-19 vaccines in Chinese individuals with obesity/overweight. Genes Dis. 2023;10:608-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Plumb ID, Feldstein LR, Barkley E, Posner AB, Bregman HS, Hagen MB, Gerhart JL. Effectiveness of COVID-19 mRNA Vaccination in Preventing COVID-19-Associated Hospitalization Among Adults with Previous SARS-CoV-2 Infection - United States, June 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 7. | Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 8. | Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 9. | Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 979] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 10. | Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2691] [Cited by in RCA: 3180] [Article Influence: 636.0] [Reference Citation Analysis (0)] |
| 11. | Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, Pypstra R, Rusnak JM; EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1885] [Cited by in RCA: 1599] [Article Influence: 533.0] [Reference Citation Analysis (0)] |
| 12. | Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr. 2021;15:102329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Senderovich H, Vinoraj D, Stever M, Waicus S. Efficacy of COVID-19 treatments among geriatric patients: a systematic review. Ther Adv Infect Dis. 2022;9:20499361221095666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Catlin NR, Bowman CJ, Campion SN, Cheung JR, Nowland WS, Sathish JG, Stethem CM, Updyke L, Cappon GD. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 M(pro) inhibitor in animal models. Reprod Toxicol. 2022;108:56-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Lu G, Zhang Y, Zhang H, Ai J, He L, Yuan X, Bao S, Chen X, Wang H, Cai J, Wang S, Zhang W, Xu J. Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave. Emerg Microbes Infect. 2022;11:2045-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |