Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.446
Revised: August 23, 2024
Accepted: September 10, 2024
Published online: September 28, 2024
Processing time: 177 Days and 15.2 Hours
Cases of myelin oligodendrocyte glycoprotein (MOG) antibody-related disease have a history of coronavirus disease 2019 infection or its vaccination before disease onset. Severe acute respiratory syndrome virus 2 (SARS-CoV-2) infection has been considered to be a trigger of central nervous system autoimmune diseases.
Here we report a 20-year male with MOG-associated transverse myelitis after a SARS-CoV-2 infection. The patient received a near-complete recovery after stan
Attention should be paid to the evaluation of typical or atypical neurological symptoms that may be triggered by SARS-CoV-2 infection.
Core Tip: Here we present a case of myelin oligodendrocyte glycoprotein-associated disease after severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection. The patient received a near-complete recovery after standard immunological treatments. We suggest that attention should be paid to the evaluation of typical or atypical neurological symptoms that may be triggered by SARS-CoV-2 infection, and focus on the exclusion of coexisting autoimmune reactions or diseases.
- Citation: Zheng JR, Chang JL, Hu J, Lin ZJ, Lin KH, Lu BH, Chen XH, Liu ZG. Myelin oligodendrocyte glycoprotein-associated transverse myelitis after SARS-CoV-2 infection: A case report. World J Radiol 2024; 16(9): 446-452
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/446.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.446
Many cases of coronavirus disease 2019 (COVID-19) have been reported with co-existing neurological symptoms. Several co-exsiting autoimmune neurological diseases, including central (limbic and brainstem encephalitis, acute disseminated encephalomyelitis, and myelitis) and peripheral neurological diseases (Guillain-Barré and Miller fisher syndromes), have been reported[1]. The correlation between COVID-19 and neurological symptoms is yet not clear, with the possible mechanisms of autoimmunity and inflammation. Currently, the diagnosis standards and treatment options for COVID-related neurological diseases are ambiguous, leading to a possibly underestimated number of total cases and posing new challenges for clinical work in the neurological discipline.
Myelin oligodendrocyte glycoprotein (MOG) is a member of the immunoglobulin superfamily[2]. It is specifically expressed on the membrane surface of oligodendrocytes and the outermost layer of the myelin sheath, and its expression level usually reflects the degree of myelination. MOG has been proven to be one of the most common antigens in demyelinating diseases. MOG antibody-related disease (MOG-AD) accounts for 1.2% to 6.5% of all adult demyelinating syndromes, with acute disseminated encephalomyelitis, optic neuritis and myelitis as the common clinical symptoms[3].
In this case repot, we report a case of rapidly progressing acute myelitis presented with motor sensory disorders of both lower extremities and urinary retention after severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection. The patient was further diagnosed with MOG-associated transverse myelitis (TM) after being tested positive for anti-MOG-IgG in the cerebrospinal fluid (CSF). We also reviewed published cases of MOG-AD associated with SARS-CoV-2 infection, intending to improve clinicians’ understanding of MOG-AD after SARS-CoV-2.
A 20-year-old young male of Asian ethnicity was admitted to our hospital for lower limb weakness and sensory disturbance and urinary retention for 3 days.
Eight days prior to admission, the patient developed weakness and a fever with chills, and was diagnosed with COVID-19 after conformation of SARS-CoV-2 infection by a pharyngeal swab reverse transcription-polymerase chain reaction test. No other symptoms such as cough, sputum, chest tightness, or shortness of breath were present at that time. After 5 days of consistent fever, the patient began to develop symmetrical weakness in lower extremities, accompanied by symmetrical sensory impairment below the waist, inability to walk by himself, and difficulty in urination.
The patient was previously healthy.
He had previously received the SARS-CoV-2 adenovirus vaccine before infection and had no symptoms or discomfort at the time of vaccination.
At administration, the patient had a fever of 38.5 °C. Neurological examination revealed pyramidal tract impairment and sensory abnormalities, including decreased muscle strength in both legs (3/5 in medical research council scale of muscle strength rating system) and diminished tendon reflex, accompanied with hypesthesia below the T9 dermatome level [modified Rankin scale (mRS) 4 and expanded disability status scale (EDSS) 7.0]. His abdominal wall reflexes were elicited only at the left T7-T8 plane. And urinary retention resulted in his need for urinary catheterization.
The initial laboratory tests showed an elevated level of white blood cell count (15.69 × 109/L), C-reactive protein (84.590 mg/L), D-dimer (1.17 mg/L, fibrinogen equivalent units), and erythrocyte sedimentation rate (52 mm/hour), with no other general examination remarkable. A lumbar puncture was therefore performed. The pressure of the cerebral spinal fluid was remarkably over 330 mmH2O, with a raised white blood cell count (66 × 106/L), mononuclear cell percentage (91%), and elevated protein level (0.26 g/L). Oligoclonal bands were seen in both serum and CSF with the same pattern. A CSF test was positive for anti-MOG antibody (titration 1:32), while was negative for other antibodies, especially anti-aquaporin 4 antibodies.
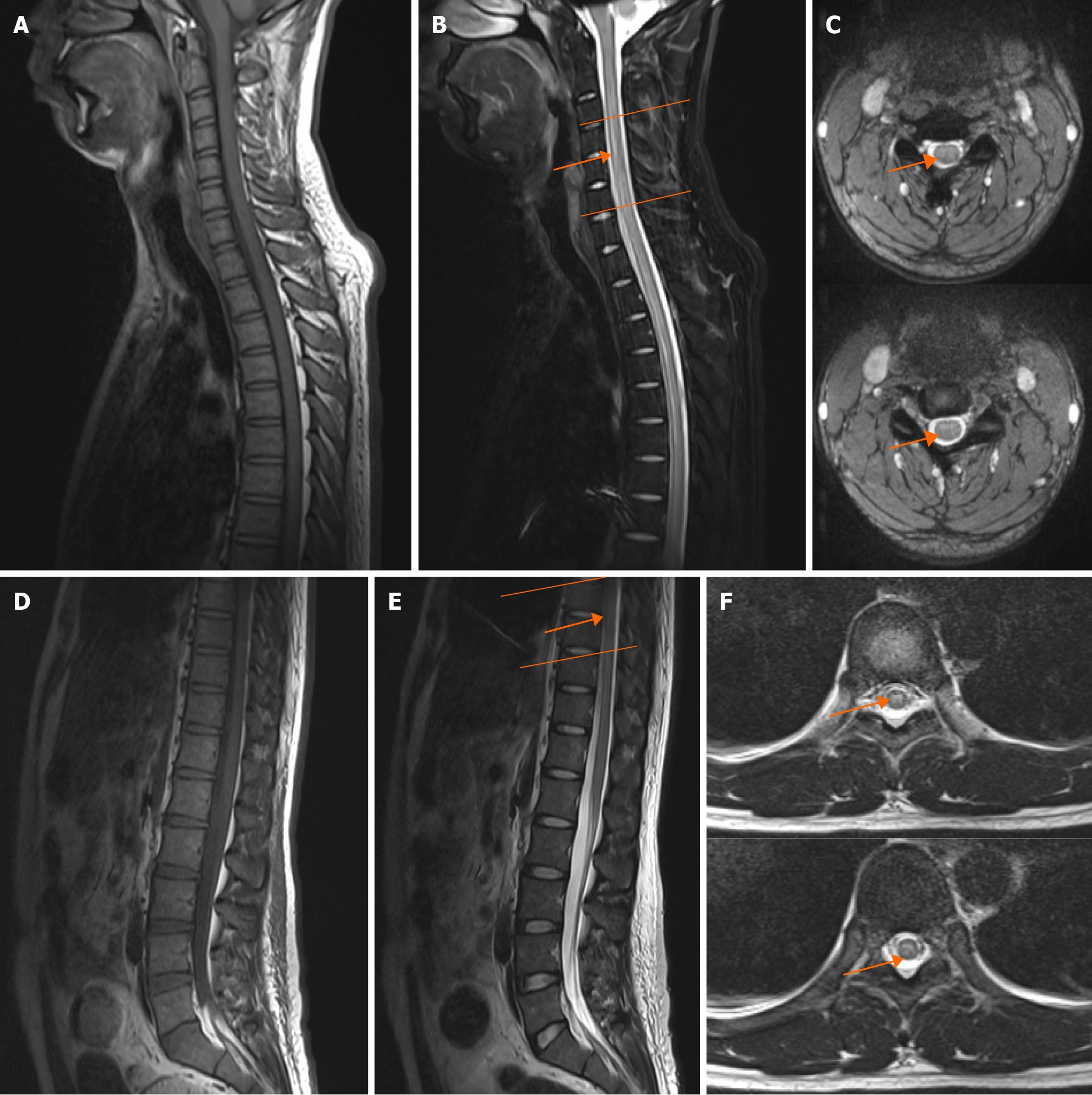
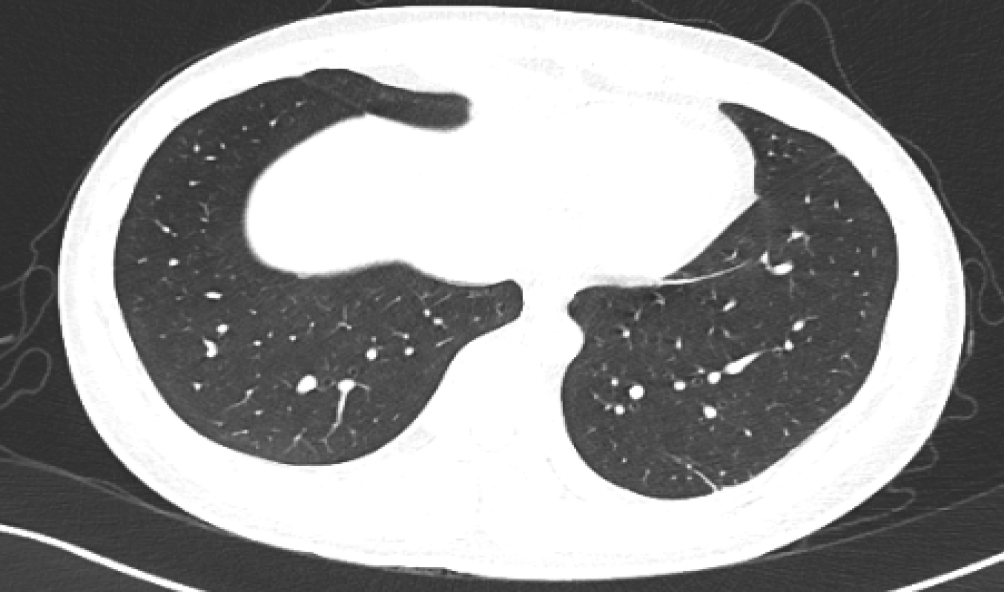
A magnetic resonance imaging (MRI) scan of the spinal cord revealed an abnormal signal in the C3-C6, T7-T8 segment resembling demyelinating lesion (Figure 1), presuming a diagnosis of TM. Neither typical glassy nor solid shadows were observed in his lung from an immediate chest computerized tomography scan (Figure 2).
Based on this patient’s recent history of SARS-CoV-2 infection, clinical presentation, laboratory findings, MRI, and CSF analysis, the diagnosis of MOG-TM was supported.
Pulsed methylprednisolone therapy [1000 mg × 3 days; 500 mg × 3 days; 250 mg × 3 days; 120 mg × 3 days followed by 65 mg (1 mg/kg/day) orally] was administered by our medical team.
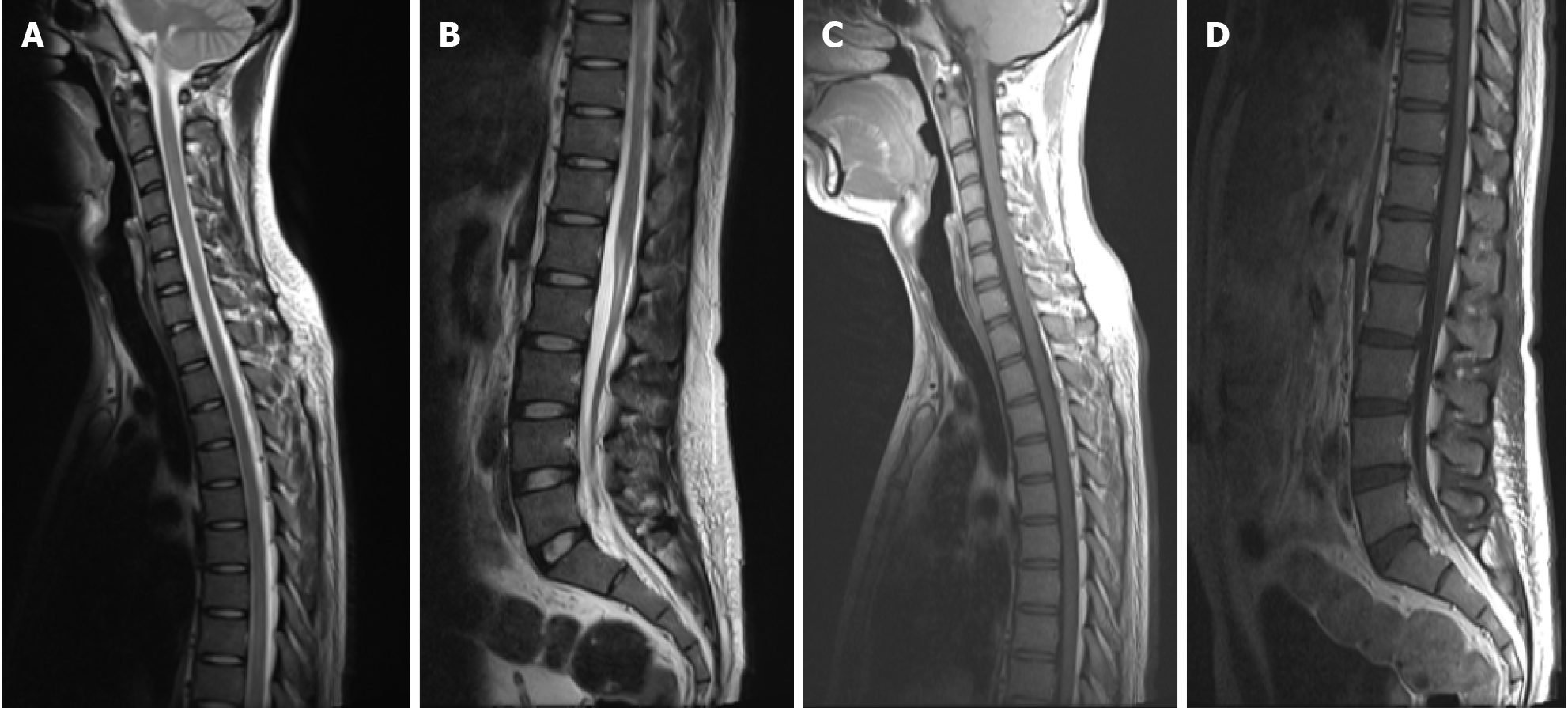
The patient responded well to steroid treatment with gradual improvements in symptoms. After a one-week of treatment, the patient’s muscle strength of the lower limbs was restored (4 +/5 in medical research council scale of muscle strength rating system score). He could walk independently and was mostly self-care, despite slight sensory abnormalities and mild urinary retention, for which a catheter was still needed. At re-exam in the second week, an MRI scan showed reduced volume of the abnormal signals compared with the previous scan (Figure 3). At discharge, the mRS score was at 2, and the EDSS score was at 3.5. A prescription of a long-term oral prednisolone until further reevaluation was given, with a slow tapering plan.
With the accumulation of cases affected by the COVID-19 pandemic, studies have found that a range of neurological complications such as cerebrovascular disease, encephalitis, and myelitis could occur in addition to the respiratory and cardiovascular symptoms[4-7], which is gaining more attention in clinical practice. COVID-19 related myelitis was first reported by Zhao et al[8], despite a lack in CSF and spinal MRI findings.
The number of reported MOG-AD cases seems slightly higher than that in the pre-pandemic period of COVID-19, and its coincidental occurrence should not be neglected[9,10]. COVID-19-associated MOG-AD can occur in patients of all ages, but is more commonly seen in younger patients. In these patients, myelitis or TM is usually accompanied by encephalitis (acute disseminated encephalomyelitis) and optic neuritis[10,11]. Our patient presented with typical neurological symptoms including lower extremity weakness, sensory impairment and urination retention, which is consistent with the reported cases. Similar to other previously reported cases, in the cross-sectional MRI scans of this reported patient, the spinal cord lesion showed a typical “cloudy” and “H-shaped” pattern[11-14]. A recent multimodal meta-analysis exhibited that both structural and functional alterations of right superior temporal gyrus, left insula and right orbitofrontal cortex have been found in COVID-19 patients compared with healthy persons. This study prompted that the cortex is more susceptible than the white matter in brain after SARS-CoV-2 infection, which may accordingly make an explanation why patients with SARS-CoV-2 associated MOG-TM mainly manifest the dysfunction of the grey matter in spinal cord[15]. However, direct evidence especially the multimodal imaging and autopsy examination of the spinal cord demonstrating the pathophysiological link, is still lacking to date, and further clinical and preclinical studies are still needed to reveal the underlying mechanisms of MOG-TM associated with COVID-19.
It is difficult to confirm the causality of SARS-CoV-2 infection and TM. Considering the characteristics of the cases available so far and the temporal relationship between SARS-CoV-2 infection and MOG-AD, it is very likely that this was an inflammatory para-infection or post-infection phenomenon, supporting the hypothesis of autoimmune process rather than viral damage directly. The homologous trimeric spike protein S in SARS-CoV-2 membrane has a receptor binding domain that recognizes angiotensin-converting enzyme 2 (ACE2) on target cells and the virus can subsequently fuse with the target cell membrane to complete viral replication and infection[16,17]. Since ACE2 receptors are present on brain glial cells and neurons, it is suggested that the nervous system is also highly susceptible to SARS-CoV-2[18]. The possible route for virus infection in the central nervous system may be through the central protrusion of the olfactory cells to the olfactory bulb or through the disruption of the blood-brain barrier by infecting vascular epithelial cells, which is further evidenced by the detection of SARS-CoV-2 genetic material in patients’ CSF[19,20]. However, SARS-CoV-2 polymerase chain reaction test was only positive in the CSF of a very small number of patients presenting with encephalitis or encephalomyelitis, and is usually negative in patients presenting with simple myelitis for the time being, contradicting the idea of direct SARS-CoV-2 infection on neuron[1,9]. MOG-AD can also occur secondarily to the infection of other viruses such as herpes simplex virus and Epstein-Barr virus, suggesting that viral infections may be triggers for the development of immune-mediated responses, but is not specific to a defined pathogen[3]. Previous study identified inflammatory markers [such as C-reactive protein, interleukin (IL)-2R, IL-6, IL-10, tumor necrosis factor-α] are significantly elevated in patients with severe COVID-19 infection, which underlie the cytokine inflammatory storm which leads to hyperpyrexia and respiratory failure[21,22]. However, in this reported case, neurological damage is much more evident than respiratory symptoms, suggesting that anti-MOG antibody activation, rather than inflammation itself, was playing a pivotal role. In addition, MOG-AD was found to be closely associated with SARS-CoV-2 vaccination. Indeed, MOG-AD after vaccination may induce more severe symptoms compared to non-vaccine related MOG-AD, represented by longer focal involvement of segments and even require multiple immunotherapy in some cases[23], while routine lab tests of CSF could be normal[23,24]. Therefore, according to the currently proposed criteria for a “probable” causal relationship between SARS-CoV-2 vaccination and neurological complications, SARS-CoV-2 infection or vaccination may lead to the process of molecular mimicry and epitope spread, which may contribute to the inflammation-associated MOG-Ig as a non-specific trigger releasing and finally result in MOG-AD[25].
As for disease regression, in this group of patients, immunotherapy with adequate steroid hormones is most commonly adopted in clinical practice, and most patients have good response to steroid treatments and are able to achieve clinical remission[1]. Immunoglobulin and plasma replacement are alternative clinical strategies. As with typical MOG-AD, patient with MOG-AD after SARS-CoV-2 infection could receive complete or near-complete recovery after treatments, and relapse cases are rare[1,11]. However, given the specificity of MOG antibodies, the current epidemiological setting of SARS-CoV-2, and the limited follow-up time, and it is recommended that such patients should be followed up for a longer period of time for assessment or to prevent relapse.
Here we present a case of MOG-AD after SARS-CoV-2 infection and a literature review of available reports. We suggest that attention should be paid to the evaluation of typical or atypical neurological symptoms that may be triggered by SARS-CoV-2 infection, and focus on the exclusion of coexisting autoimmune reactions or diseases. We suggest that SARS-CoV-2 infection may play a potential role in triggering and driving inflammation in the pathogenesis of MOG-AD, but direct pathophysiological evidence is still lacking. More prospective studies are needed to elucidate this possible association.
| 1. | Ariño H, Heartshorne R, Michael BD, Nicholson TR, Vincent A, Pollak TA, Vogrig A. Neuroimmune disorders in COVID-19. J Neurol. 2022;269:2827-2839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Pham-Dinh D, Mattei MG, Nussbaum JL, Roussel G, Pontarotti P, Roeckel N, Mather IH, Artzt K, Lindahl KF, Dautigny A. Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. Proc Natl Acad Sci U S A. 1993;90:7990-7994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 152] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, Amato MP, Asgari N, Banwell B, Bennett J, Brilot F, Capobianco M, Chitnis T, Ciccarelli O, Deiva K, De Sèze J, Fujihara K, Jacob A, Kim HJ, Kleiter I, Lassmann H, Leite MI, Linington C, Meinl E, Palace J, Paul F, Petzold A, Pittock S, Reindl M, Sato DK, Selmaj K, Siva A, Stankoff B, Tintore M, Traboulsee A, Waters P, Waubant E, Weinshenker B, Derfuss T, Vukusic S, Hemmer B. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 4. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30085] [Article Influence: 6017.0] [Reference Citation Analysis (3)] |
| 5. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9310] [Article Influence: 1862.0] [Reference Citation Analysis (0)] |
| 6. | Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020;382:2268-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1741] [Cited by in RCA: 1894] [Article Influence: 378.8] [Reference Citation Analysis (0)] |
| 7. | Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agrò FE. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 732] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 8. | Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. Preprint from: medRxiv 2020. [DOI] [Full Text] |
| 9. | Mariotto S, Carta S, Dinoto A, Lippi G, Salvagno GL, Masin L, Alberti D, Marignier R, Ferrari S. Is there a correlation between MOG-associated disorder and SARS-CoV-2 infection? Eur J Neurol. 2022;29:1855-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Johnsson M, Asztely F, Hejnebo S, Axelsson M, Malmeström C, Olausson T, Lycke J. SARS-COV-2 a trigger of myelin oligodendrocyte glycoprotein-associated disorder. Ann Clin Transl Neurol. 2022;9:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Lambe J, McGinley MP, Moss BP, Mao-Draayer Y, Kassa R, Ciotti JR, Mariotto S, Kunchok A. Myelin oligodendrocyte glycoprotein-IgG associated disorders (MOGAD) following SARS-CoV-2 infection: A case series. J Neuroimmunol. 2022;370:577933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Sarma D, Bilello LA. A Case Report of Acute Transverse Myelitis Following Novel Coronavirus Infection. Clin Pract Cases Emerg Med. 2020;4:321-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Sotoca J, Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4572] [Article Influence: 914.4] [Reference Citation Analysis (0)] |
| 15. | Guo Z, Sun S, Xiao S, Chen G, Chen P, Yang Z, Tang X, Huang L, Wang Y. COVID-19 is associated with changes in brain function and structure: A multimodal meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2024;164:105792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3499] [Cited by in RCA: 4379] [Article Influence: 875.8] [Reference Citation Analysis (0)] |
| 17. | Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3274] [Cited by in RCA: 3669] [Article Influence: 733.8] [Reference Citation Analysis (0)] |
| 18. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4761] [Cited by in RCA: 4696] [Article Influence: 939.2] [Reference Citation Analysis (0)] |
| 19. | Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264-7275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 961] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 20. | Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020;36:101642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 21. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6743] [Article Influence: 1348.6] [Reference Citation Analysis (0)] |
| 22. | McCray PB Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 851] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 23. | Jarius S, Bieber N, Haas J, Wildemann B. MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature. J Neurol. 2022;269:5198-5212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Sechi E, Buciuc M, Flanagan EP, Pittock SJ, Banks SA, Lopez-Chiriboga AS, Bhatti MT, Chen JJ. Variability of cerebrospinal fluid findings by attack phenotype in myelin oligodendrocyte glycoprotein-IgG-associated disorder. Mult Scler Relat Disord. 2021;47:102638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Butler M, Tamborska A, Wood GK, Ellul M, Thomas RH, Galea I, Pett S, Singh B, Solomon T, Pollak TA, Michael BD, Nicholson TR. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |