Published online Nov 28, 2024. doi: 10.4329/wjr.v16.i11.668
Revised: October 10, 2024
Accepted: November 12, 2024
Published online: November 28, 2024
Processing time: 160 Days and 19.5 Hours
Incidental pulmonary nodules are an increasingly common finding on computed tomography (CT) scans of the thorax due to the exponential rise in CT examinations in everyday practice. The majority of incidental pulmonary nodules are benign and correctly identifying the small number of malignant nodules is cha
To prospectively compare ULDCT chest combined with MBIR with SDCT chest in the analysis of solid pulmonary nodules.
A prospective cohort study was conducted on adult patients (n = 30) attending a respiratory medicine outpatient clinic in a tertiary referral university hospital for surveillance of previously detected indeterminate pulmonary nodules on SDCT chest. This study involved the acquisition of a reference SDCT chest followed immediately by an ULDCT chest. Nodule identification, nodule characterisation, nodule measurement, objective and subjective image quality and radiation dose were compared between ULDCT with MBIR and SDCT chest.
One hundred solid nodules were detected on ULDCT chest and 98 on SDCT chest. There was no significant difference in the characteristics of correctly identified nodules when comparing SDCT chest to ULDCT chest protocols. Signal-to-noise ratio was significantly increased in the ULDCT chest in all areas except in the paraspinal muscle at the maximum cardiac diameter level (P < 0.001). The mean subjective image quality score for overall diagnostic acceptability was 8.9/10. The mean dose length product, computed tomography volume dose index and effective dose for the ULDCT chest protocol were 5.592 mGy.cm, 0.16 mGy and 0.08 mSv respectively. These were significantly less than the SDCT chest protocol (P < 0.001) and represent a radiation dose reduction of 97.6%.
ULDCT chest combined with MBIR is non-inferior to SDCT chest in the analysis of previously identified solid pulmonary nodules and facilitates a large reduction in radiation dose.
Core Tip: Recent advancements in computed tomography (CT) hardware and software have facilitated the development of ultra-low-dose imaging protocols that have the potential to significantly reduce radiation dose while, crucially, maintaining image quality and diagnostic integrity. Previously identified indeterminate solid pulmonary nodules may be effectively monitored with ultra-low-dose CT chest with the added benefit of a large reduction in radiation dose.
- Citation: O'Regan PW, Harold-Barry A, O'Mahony AT, Crowley C, Joyce S, Moore N, O'Connor OJ, Henry MT, Ryan DJ, Maher MM. Ultra-low-dose chest computed tomography with model-based iterative reconstruction in the analysis of solid pulmonary nodules: A prospective study. World J Radiol 2024; 16(11): 668-677
- URL: https://www.wjgnet.com/1949-8470/full/v16/i11/668.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i11.668
Incidental pulmonary nodules are an increasingly common finding in routine patient care secondary to the exponential rise in utilisation of chest computed tomography (CT)[1,2]. The majority of incidental pulmonary nodules are benign and correctly identifying malignant nodules poses a diagnostic challenge[3]. Based on various morphological nodule metrics, indeterminate solid pulmonary nodules are frequently followed with serial chest CT to monitor for changes that may represent malignancy. Typical solid pulmonary nodule features that correlate with likelihood of malignancy include size, internal features (e.g. cavitation), border characteristics (e.g. smooth, spiculated) and perinodular surround characteristics (e.g. pleural tethering)[4-6]. Hendrix et al[7] report that in a cohort of almost 75000 patients between 2008 and 2019 the percentage of patients with pulmonary nodules increased from 38% to 50% and the proportion of stage 1 lung cancers doubled. These findings highlight the paramount importance of correctly identifying and characterising pulmonary nodules.
The concept of low-dose CT (LDCT) chest imaging was proposed by Naidich et al[8] in 1990. The National Lung Screening Trial, a randomised control trial in 2011, demonstrated a 20% reduction in mortality when LDCT chest was utilised in favour of traditional chest radiography in screening an asymptomatic high risk population[9]. LDCT has been utilised for over a decade in screening programmes of high risk patients for lung malignancy and its ability to characterise solid pulmonary nodules is well established[10]. However, the use of serial ionising radiation examinations in an asymptomatic population raises concern regarding cumulative effective dose (ED) and subsequent carcinogenesis[11]. Ultra-LDCT (ULDCT) chest imaging protocols have been developed in an effort to further reduce this ionising radiation burden. Inadequate signal to noise ratio (SNR) has typically been the most common limitation of ULDCT techniques and recent advances in hardware and software in a range of clinical settings, have shown potential in surmounting this limitation. Model-based iterative reconstruction (MBIR) takes advantage of statistical algorithm techniques to model x-ray production, tissue attenuation and sources of noise in a CT examination which allows large reductions in noise in the reconstructed images[12].
ULDCT chest imaging protocols with MBIR have been shown to facilitate both a large reduction in radiation dose and produce CT images capable of providing comparable diagnostic accuracy of respiratory pathology in comparison with the more traditional filtered back projection reconstruction technique[13-15]. Maintaining diagnostic integrity is essential when adjusting imaging protocols. The ability of ULDCT chest protocols to detect and characterise pulmonary nodules has been demonstrated in numerous in vitro studies[16-19]. Currently, the clinical utility of ULDCT chest in the asse
In this study, we aimed to prospectively compare ULDCT chest combined with MBIR with standard dose CT (SDCT) chest in the detection, measurement and characterisation of solid pulmonary nodules. A secondary aim was to compare the radiation dose between the two protocols.
Following institutional ethical board approval (Clinical Research Ethics Committee reference number: ECM4(g)1/3/16 & ECM3(nnnn)9/3/21) a prospective cohort study was conducted. The study population consisted of adult patients (n = 30) attending respiratory outpatient clinic for surveillance of previously detected indeterminate solid pulmonary nodules on SDCT chest.
Inclusion criteria for the study were as follows: > 35 years of age, ability to provide informed written consent, and current or former smoker. Exclusion criteria were as follows: Unable to give informed consent, active malignancy, pregnancy or any condition, or ailment precluding the ability to lie flat for the duration of the scan.
Each potential participant was given information in simple language about the objective, methods, and risks of study participation. The study procedure which involved the acquisition of reference SDCT chest followed by an ULDCT chest was explained to all subjects. If aggregable to participation, written informed consent was obtained.
A SDCT and an ULDCT chest without intravenous contrast were acquired with a 64-row multi-detector CT system (Discovery CT750 HD; GE Healthcare, Waukesha, WI, United States). Our previously published MBIR ULDCT chest protocol was utilised in all ULDCT acquisitions[14]. Briefly, this involved a tube voltage: 80 Kv; tube current: 20 mA; gantry rotation time: 0.4 seconds; pitch factor: 1.375; and FOV of 32 cm. Scanning was performed at end-inspiration from lung apices to bases. No additional expiratory phase imaging was performed. Images were acquired at slice thickness of 0.625 mm.
Nodules were detected, measured and characterised, according to the Fleischner Society Guidelines, independently on review of the PACS system on a dedicated workstation (Advantage Workstation VolumeShare 2, Version 4.4, GE Medical Systems, Milwaukee, WI, United States) by two consultant radiologists with a subspeciality interest in chest radiology[4].
Objective image quality analysis was performed independently on a dedicated workstation (Advantage Workstation VolumeShare 2, Version 4.4, GE Medical Systems, Milwaukee, WI, United States) by two readers in line with our previously published work[20]. The readers were blinded to the scanning protocol used and the order of the datasets was randomized. Briefly, attenuation values were measured in Hounsfield units (HU) at three levels: Aortic arch, carina, and the maximum cardiac diameter. Measurements were recorded by placing circle histograms of equal size (diameter, 10 mm) in the descending aorta and paraspinal muscles of the posterior chest wall at each level. These regions of interest (ROIs) were placed in a homogenous area, taking care to avoid fat planes and blood vessels. The standard deviation of the mean attenuation in the ROI served as an objective measure of image noise. The SNR of each ROI was calculated by dividing the mean HU by its standard deviation. Measurements were taken three times by each operator to reduce error and the mean recorded. The mean of both readers’ measurements was used for analysis.
The ability to identify and characterise the pulmonary nodules on the ULDCT chest protocol was subjectively scored by two readers on a scale of 1-10, with 1 being unacceptable, 5 acceptable and 10 excellent. The ULDCT chest was also subjectively rated for overall diagnostic acceptability on the same scale. The mean of both readers’ measurements was used for analysis. The subjective image quality of the ULDCT chest with MBIR was assessed independently and not directly compared with the corresponding SDCT chest for each patient. SDCT chest is our institution’s ‘gold standard’ for the assessment of lung nodules and is considered 10/10 on subjective image quality assessment.
Radiation dose data were taken from individual institutional reports. The CT dose index volume (CTDIvol) in mGy and dose length product (DLP) in mGy.cm were recorded. The ED was calculated using a conversion factor of 0.014 as per validated literature[21].
Statistical analysis was carried out using SPSS version 28 (IBM SPSS Inc., Chicago, Il). Data were exported into SPSS from Microsoft Office Excel (Microsoft Corporation, CA, United States) for statistical analysis. Descriptive statistics were utilised for patient demographics. Following Shapiro-Wilk normality testing, a paired t-test was utilised to compare the ULDCT and SDCT protocols. Intraclass correlation coefficient (ICC) was used to evaluate interrater reliability. A P value of < 0.05 was considered statistically significant. Data are presented as median and standard deviation unless otherwise specified in the text.
Thirty patients (13 female; 17 male) (mean age 64 ± 12.3 years) were included. Each patient underwent both SDCT chest and ULDCT chest with MBIR examinations and these findings were compared with previously performed index imaging to establish a baseline nodule burden. The mean duration between index and follow up examinations was 217 days.
One hundred and twenty-two nodules (100 solid nodules) were detected on ULDCT chest (mean of 4.1 nodules), and 116 nodules (98 solid nodules) were detected on SDCT chest (mean of 3.9 nodules; Table 1).
| Identified nodules | Index SDCT | Study SDCT | ULDCT |
| Solid nodules | 94 | 98 | 100 |
| Ground glass opacities | 5 | 4 | 5 |
| Calcified granulomas | 10 | 11 | 14 |
| Part solid nodules | 4 | 3 | 3 |
| Total | 113 | 116 | 122 |
The number of solid pulmonary nodules on SDCT chest in comparison with index CT were similar with the expected interval resolution and subsequent development of a small number of pulmonary nodules.
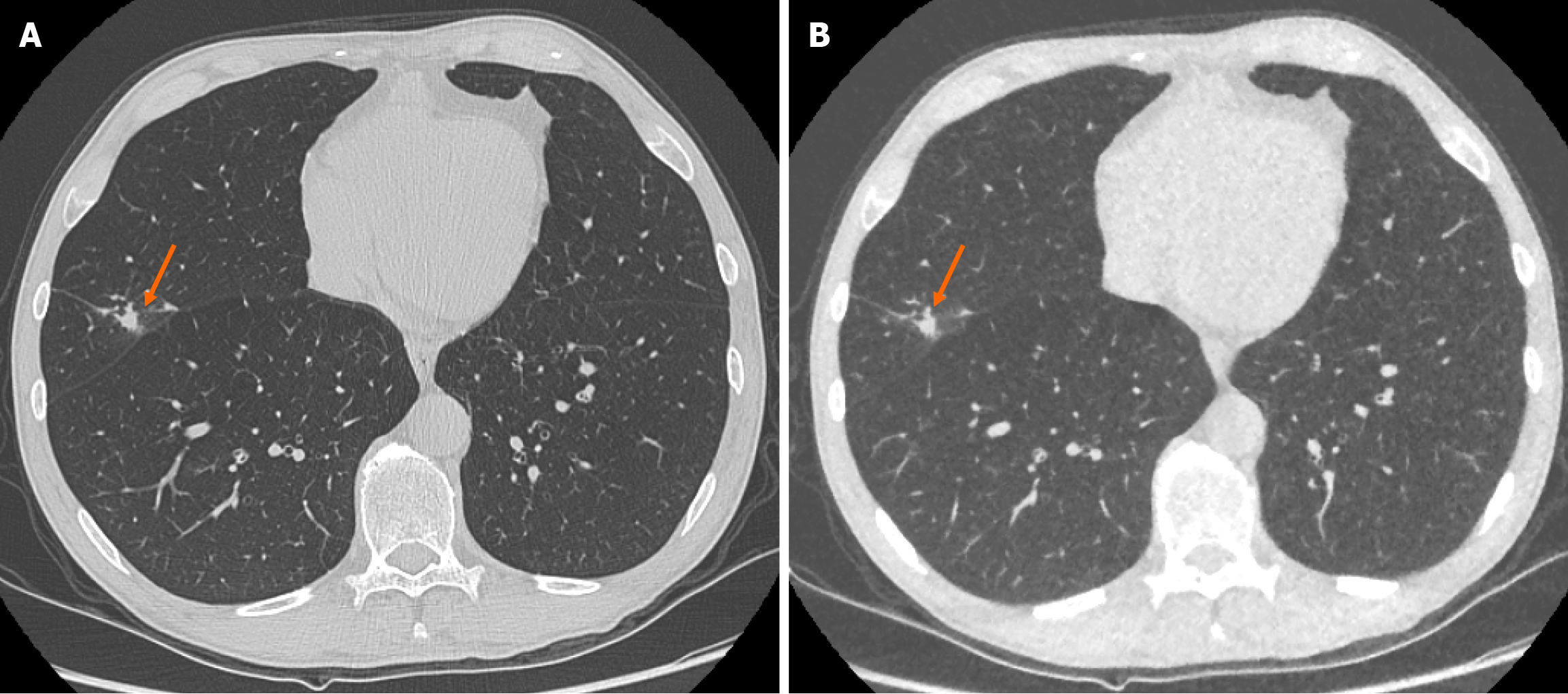
There was no significant change in size of the solid pulmonary nodules detected between ULDCT chest with MBIR and SDCT chest protocols. The mean pulmonary nodule size for ULDCT chest was 4.51 ± 2.47 mm and for SDCT chest was 4.47 ± 2.53 mm (P = 0.328; Figure 1). ICC as an indication of interrater agreement for nodule size on ULDCT was r = 0.961 and on SDCT, r = 0.933 (excellent reliability).
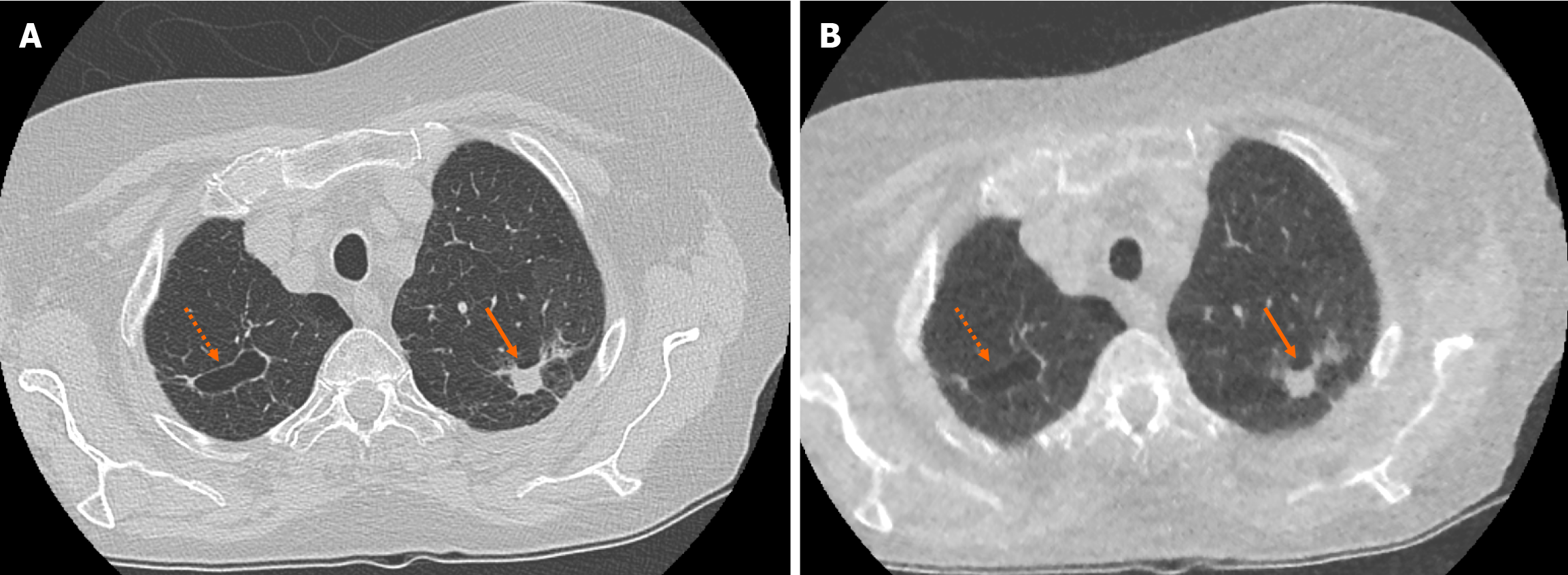
There was no significant difference in the characteristics of correctly identified nodules when comparing SDCT chest to ULDCT chest with MBIR protocols (P = 0.09). For example, there was no significant difference in the ability to characterise lesions as cavitating or spiculated (Figure 2).
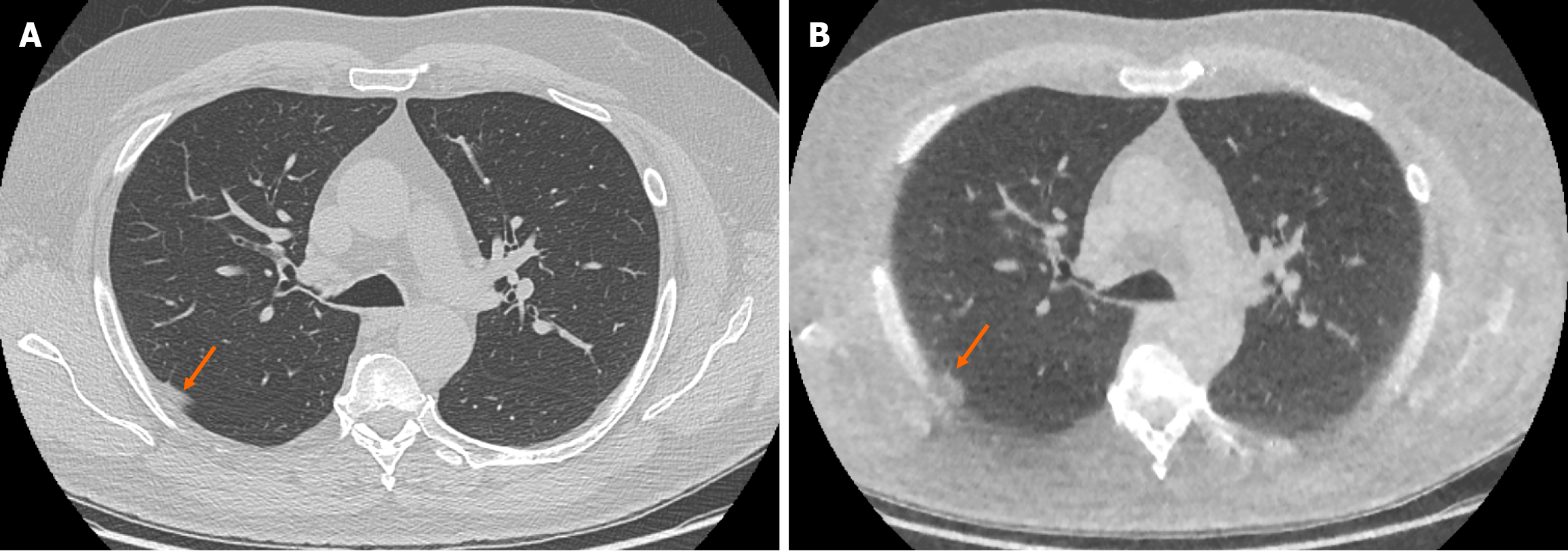
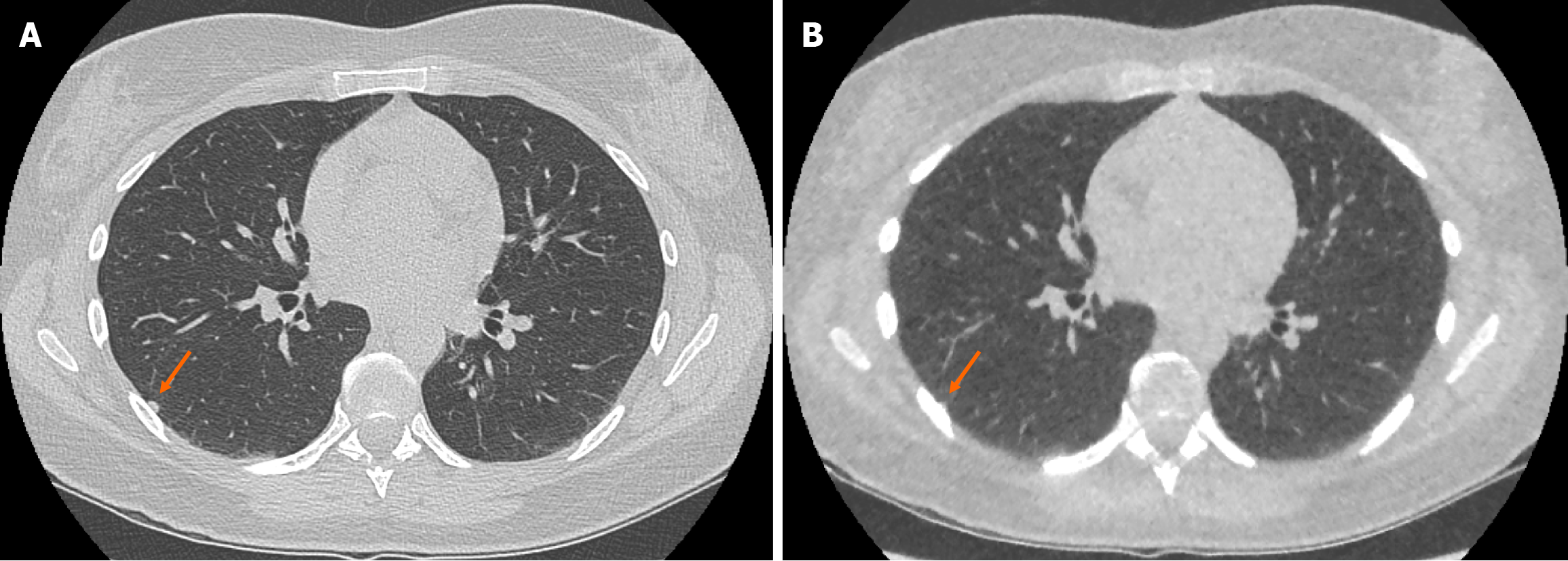
The ULDCT chest protocol demonstrated a small number of false positive and false negative pulmonary nodules when compared to the traditional SDCT chest protocol (Figure 3 and Figure 4 respectively). These minor discrepancies did not reach statistical significance.
Objective noise and SNR were measured in the aortic lumen and paraspinal muscles at the level of the aortic arch, carina and largest cardiac diameter (Table 2). Noise was significantly reduced in the paraspinal muscles at the level of the aortic arch and carina in the ULDCT chest protocol (reconstructed with MBIR) in comparison with the SDCT chest protocol (P < 0.001). SNR was significantly increased in the ULDCT chest with MBIR in comparison to SDCT chest in all areas except in the paraspinal muscle at the maximum cardiac diameter level (P < 0.001). The remainder of the results did not reach statistical significance.
| Level | 10 mm ROI | Noise (HU) | SNR | ||||
| ULDCT | SDCT | P value | ULDCT | SDCT | P value | ||
| Aortic arch | Aortic lumen | 22.28 ± 3.6 | 21.06 ± 2.9 | 0.149 | 2.86 ± 0.8 | 1.53 ± 0.3 | < 0.001 |
| Paraspinal muscles | 20.86 ± 3.6 | 27.29 ± 4 | < 0.001 | 2.84 ± 0.7 | 1.89 ± 0.4 | < 0.001 | |
| Carina | Aortic lumen | 23.8 ± 12.4 | 22.98 ± 4.2 | 0.359 | 2.63 ± 0.9 | 1.57 ± 0.6 | < 0.001 |
| Paraspinal muscles | 22.33 ± 4.4 | 28.28 ± 4.9 | < 0.001 | 2.35 ± 1.1 | 1.72 ± 0.5 | < 0.001 | |
| Max. cardiac diameter | Aortic lumen | 23.14 ± 7.9 | 27.7 ± 12.9 | 0.061 | 2.14 ± 0.9 | 1.17 ± 0.6 | < 0.001 |
| Paraspinal muscles | 23.44 ± 3.2 | 23.37 ± 4 | 0.472 | 1.66 ± 0.6 | 1.61 ± 0.6 | 0.318 | |
The mean subjective image quality score, from a maximum score of 10, for the ability to identify and characterise the pulmonary nodules on ULDCT with MBIR was 7.8 ± 1.48 and overall diagnostic acceptability was 8.9 ± 0.93. The ICC for diagnostic satisfaction had an r value of 0.548 and quality of nodule visualisation r value was 0.511 (moderate reliability).
The mean DLP, CTDIvol and ED for the ULDCT chest with MBIR protocol were 5.592 mGy.cm, 0.16 mGy and 0.08 mSv respectively. These were significantly less than the SDCT chest protocol with mean DLP, CTDIvol and ED of 237.1 mGy.cm, 7.2 mGy and 3.21 mSv respectively (P < 0.001). This represents an overall radiation dose reduction of 97.6%.
This prospective cohort study demonstrates that ULDCT chest combined with MBIR is adequate when compared to SDCT chest in the identification, measurement and characterisation of previously identified solid pulmonary nodules while facilitating a 97.6% associated reduction in radiation dose (mean ED of 0.08 mSv vs 3.21 mSv).
Excessively long computer processing times is a typical limitation of advanced reconstruction algorithms and MBIR represents the latest attempt to overcome this and facilitate its routine inclusion in clinical practice[22]. A clear benefit of CT radiation dose reduction techniques are the potential benefits in paediatric patient cohorts in particular. MBIR has been shown to be superior to other reconstruction techniques in imaging children[23,24]. Carcinogenesis risk due to lifetime cumulative ED from medical imaging is a challenging topic without a definitive consensus. A large systematic review and dose-response meta-analysis assessing over 110 million adults over 3 continents found an inordinate increase in cancer risk in adults that positively correlated with CT radiation dose exposure[25]. Given the current data, a prudent approach is one that endeavours to reduce the radiation dose delivered to the patient without compromising diagnostic integrity. In concordance with other published work, our MBIR protocol facilitated a large reduction in radiation dose while maintaining diagnostic integrity[26].
With such a large reduction in radiation dose there are inevitable concerns regarding the potential consequences of misdiagnosis. In our prospective study of 30 patients, the ULDCT chest with MBIR protocol identified slightly more solid pulmonary nodules than the SDCT chest protocol (100 vs 98 solid pulmonary nodules respectively). When considering inherent false positive and false negative results in any diagnostic test and interobserver variability, this slight dis
Gheysens et al[28] report that in a prospective study of 63 patients, a scoutless fixed-dose ULDCT chest was com
In a study of 99 patients, Miller et al[30] assessed the reliability of ULDCT chest in identifying pulmonary nodules in comparison to a reference LDCT chest and, in line with our study, found excellent sensitivity and specificity with a significant radiation dose reduction. Paks et al[31] have shown ULDCT chest to be comparable to SDCT chest for solid pulmonary nodules > 2 mm in a prospective 57 patient cohort and propose its utilisation in serial imaging of known pulmonary nodules. Multiple groups have prospectively compared ULDCT chest with LDCT or SDCT chest and concluded adequate image quality and sensitivity in nodule detection[32-35].
These findings highlight the value of continued in vitro and in vivo research in ultra-low dose CT imaging and represent encouraging progress in the field.
Objective image quality was improved in our ULDCT chest with MBIR protocol with increased SNR in almost all assessed areas, substantiating recent advances in hardware and software and in particular the power of MBIR. MBIR is particularly useful in thoracic imaging given the high inherent contrast between background normal lung parenchyma and solid pulmonary nodules[36]. Iterative reconstruction methods produce less image noise than traditional filtered back projection methods[37]. Subjective image quality was more than acceptable in our study in terms of nodule identification (mean score 7.8/10) and diagnostic acceptability (mean score 8.9/10) for both readers. The ability for diagnostic radiologists to trust the objective and subjective image quality of ULDCT is essential for ongoing advancements in the field.
Reduction or elimination of common CT related image artifacts becomes increasingly relevant in ULDCT with the inevitable reduction in signal as a consequence of the reduced radiation dose. Recent phantom work by Watanabe et al[38] has shown the addition of a dedicated tin filter photon shield reduced pacemaker related artefact in ULDCT and subsequently improved pulmonary nodule detectability. The ability of clinical imaging to diagnose patients at ever reducing radiation doses with adequate image quality is heavily supported by phantom and other non-clinical research which facilitates ongoing meaningful advances in imaging technique development.
The exciting new field of advanced image analysis through radiomics and deep learning algorithms has the potential to improve nodule characterisation and subsequent patient prognostication. Automatic nodule detection software in various forms have been utilised in ULDCT nodule detection and characterisation to good effect[26,39]. The limitations of heterogeneity in image acquisition, accurate data segmentation, limited reproducibility of results across different radiomics platforms and the novelty of the field have prevented advanced imaging analysis being utilised routinely in current daily practice[40].
This study had several limitations including its limited sample size of data from a single centre. Volumetric analysis is not routinely performed in our institution and therefore did not form a part of this study. However, the ability of MBIR to facilitate accurate volumetric analysis has been shown in a phantom study by Chen et al[41]. Our study did not assess the upfront identification of pulmonary nodules with ULDCT in patients without documented prior nodules or any change in pulmonary nodules over time.
ULDCT chest combined with MBIR is non-inferior to SDCT chest in the identification, measurement and characterisation of previously identified solid pulmonary nodules and facilitates a reduction in radiation dose of up to 97.6%. We propose the use of ULDCT chest in the routine follow-up of previously identified indeterminate solid pulmonary nodules.
| 1. | Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015;192:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 497] [Article Influence: 55.2] [Reference Citation Analysis (1)] |
| 2. | Hammerschlag G, Cao J, Gumm K, Irving L, Steinfort D. Prevalence of incidental pulmonary nodules on computed tomography of the thorax in trauma patients. Intern Med J. 2015;45:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, Yousaf-Khan U, Horeweg N, van 't Westeinde S, Prokop M, Mali WP, Mohamed Hoesein FAA, van Ooijen PMA, Aerts JGJV, den Bakker MA, Thunnissen E, Verschakelen J, Vliegenthart R, Walter JE, Ten Haaf K, Groen HJM, Oudkerk M. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 2101] [Article Influence: 420.2] [Reference Citation Analysis (0)] |
| 4. | MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, Rubin GD, Schaefer-Prokop CM, Travis WD, Van Schil PE, Bankier AA. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284:228-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 1553] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 5. | Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, Franks K, Gleeson F, Graham R, Malhotra P, Prokop M, Rodger K, Subesinghe M, Waller D, Woolhouse I; British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70 Suppl 2:ii1-ii54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 660] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 6. | Christensen J, Prosper AE, Wu CC, Chung J, Lee E, Elicker B, Hunsaker AR, Petranovic M, Sandler KL, Stiles B, Mazzone P, Yankelevitz D, Aberle D, Chiles C, Kazerooni E. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Chest. 2024;165:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Hendrix W, Rutten M, Hendrix N, van Ginneken B, Schaefer-Prokop C, Scholten ET, Prokop M, Jacobs C. Trends in the incidence of pulmonary nodules in chest computed tomography: 10-year results from two Dutch hospitals. Eur Radiol. 2023;33:8279-8288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Naidich DP, Marshall CH, Gribbin C, Arams RS, McCauley DI. Low-dose CT of the lungs: preliminary observations. Radiology. 1990;175:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 200] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8099] [Cited by in RCA: 7692] [Article Influence: 549.4] [Reference Citation Analysis (0)] |
| 10. | Marshall HM, Bowman RV, Yang IA, Fong KM, Berg CD. Screening for lung cancer with low-dose computed tomography: a review of current status. J Thorac Dis. 2013;5 Suppl 5:S524-S539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 11. | Mayo-Smith WW, Hara AK, Mahesh M, Sahani DV, Pavlicek W. How I do it: managing radiation dose in CT. Radiology. 2014;273:657-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 992] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 13. | Ichikawa Y, Kitagawa K, Nagasawa N, Murashima S, Sakuma H. CT of the chest with model-based, fully iterative reconstruction: comparison with adaptive statistical iterative reconstruction. BMC Med Imaging. 2013;13:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Moloney F, Kavanagh RG, Ronan NJ, Grey TM, Joyce S, Ryan DJ, Moore N, O'Connor OJ, Plant BJ, Maher MM. Ultra-low-dose thoracic CT with model-based iterative reconstruction (MBIR) in cystic fibrosis patients undergoing treatment with cystic fibrosis transmembrane conductance regulators (CFTR). Clin Radiol. 2021;76:393.e9-393.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Lin S, Lin M, Lau KK. Efficacy of model-based iterative reconstruction in cystic fibrosis assessment using CT. Clin Radiol. 2019;74:569.e19-569.e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Doo KW, Kang EY, Yong HS, Woo OH, Lee KY, Oh YW. Accuracy of lung nodule volumetry in low-dose CT with iterative reconstruction: an anthropomorphic thoracic phantom study. Br J Radiol. 2014;87:20130644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Xie X, Willemink MJ, de Jong PA, van Ooijen PM, Oudkerk M, Vliegenthart R, Greuter MJ. Small irregular pulmonary nodules in low-dose CT: observer detection sensitivity and volumetry accuracy. AJR Am J Roentgenol. 2014;202:W202-W209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Zhou X, Zhang H, Jin X, Zhang X, Lu X, Han Q, Xiong X, Liu T, Feng Y, Tu W, Zhou T, Ge Y, Dong P, Liu S, Fan L. Ultra-low-dose spectral-detector computed tomography for the accurate quantification of pulmonary nodules: an anthropomorphic chest phantom study. Diagn Interv Radiol. 2023;29:691-703. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Milanese G, Silva M, Frauenfelder T, Eberhard M, Sabia F, Martini C, Marchianò A, Prokop M, Sverzellati N, Pastorino U. Comparison of ultra-low dose chest CT scanning protocols for the detection of pulmonary nodules: a phantom study. Tumori. 2019;105:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | O'Connor OJ, Vandeleur M, McGarrigle AM, Moore N, McWilliams SR, McSweeney SE, O'Neill M, Ni Chroinin M, Maher MM. Development of low-dose protocols for thin-section CT assessment of cystic fibrosis in pediatric patients. Radiology. 2010;257:820-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Harrison JD, Balonov M, Bochud F, Martin CJ, Menzel HG, Smith-Bindman R, Ortiz-López P, Simmonds JR, Wakeford R. The use of dose quantities in radiological protection: ICRP publication 147 Ann ICRP 50(1) 2021. J Radiol Prot. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Yamada Y, Jinzaki M, Hosokawa T, Tanami Y, Sugiura H, Abe T, Kuribayashi S. Dose reduction in chest CT: comparison of the adaptive iterative dose reduction 3D, adaptive iterative dose reduction, and filtered back projection reconstruction techniques. Eur J Radiol. 2012;81:4185-4195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Smith EA, Dillman JR, Goodsitt MM, Christodoulou EG, Keshavarzi N, Strouse PJ. Model-based iterative reconstruction: effect on patient radiation dose and image quality in pediatric body CT. Radiology. 2014;270:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Ernst CW, Hulstaert TL, Belsack D, Buls N, Van Gompel G, Nieboer KH, Buyl R, Verhelle F, De Maeseneer M, de Mey J. Dedicated sub 0.1 mSv 3DCT using MBIR in children with suspected craniosynostosis: quality assessment. Eur Radiol. 2016;26:892-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Cao CF, Ma KL, Shan H, Liu TF, Zhao SQ, Wan Y, Jun-Zhang, Wang HQ. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer. 2022;22:1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 26. | Yang L, Liu H, Han J, Xu S, Zhang G, Wang Q, Du Y, Yang F, Zhao X, Shi G. Ultra-low-dose CT lung screening with artificial intelligence iterative reconstruction: evaluation via automatic nodule-detection software. Clin Radiol. 2023;78:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Wataya T, Yanagawa M, Tsubamoto M, Sato T, Nishigaki D, Kita K, Yamagata K, Suzuki Y, Hata A, Kido S, Tomiyama N; Osaka University Reading Team. Radiologists with and without deep learning-based computer-aided diagnosis: comparison of performance and interobserver agreement for characterizing and diagnosing pulmonary nodules/masses. Eur Radiol. 2023;33:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 28. | Gheysens G, De Wever W, Cockmartin L, Bosmans H, Coudyzer W, De Vuysere S, Lefere M. Detection of pulmonary nodules with scoutless fixed-dose ultra-low-dose CT: a prospective study. Eur Radiol. 2022;32:4437-4445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Dunning CAS, Marsh JF Jr, Winfree T, Rajendran K, Leng S, Levin DL, Johnson TF, Fletcher JG, McCollough CH, Yu L. Accuracy of Nodule Volume and Airway Wall Thickness Measurement Using Low-Dose Chest CT on a Photon-Counting Detector CT Scanner. Invest Radiol. 2023;58:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Miller AR, Jackson D, Hui C, Deshpande S, Kuo E, Hamilton GS, Lau KK. Lung nodules are reliably detectable on ultra-low-dose CT utilising model-based iterative reconstruction with radiation equivalent to plain radiography. Clin Radiol. 2019;74:409.e17-409.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Paks M, Leong P, Einsiedel P, Irving LB, Steinfort DP, Pascoe DM. Ultralow dose CT for follow-up of solid pulmonary nodules: A pilot single-center study using Bland-Altman analysis. Medicine (Baltimore). 2018;97:e12019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Messerli M, Kluckert T, Knitel M, Wälti S, Desbiolles L, Rengier F, Warschkow R, Bauer RW, Alkadhi H, Leschka S, Wildermuth S. Ultralow dose CT for pulmonary nodule detection with chest x-ray equivalent dose - a prospective intra-individual comparative study. Eur Radiol. 2017;27:3290-3299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Kim Y, Kim YK, Lee BE, Lee SJ, Ryu YJ, Lee JH, Chang JH. Ultra-Low-Dose CT of the Thorax Using Iterative Reconstruction: Evaluation of Image Quality and Radiation Dose Reduction. AJR Am J Roentgenol. 2015;204:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Sui X, Meinel FG, Song W, Xu X, Wang Z, Wang Y, Jin Z, Chen J, Vliegenthart R, Schoepf UJ. Detection and size measurements of pulmonary nodules in ultra-low-dose CT with iterative reconstruction compared to low dose CT. Eur J Radiol. 2016;85:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Jin S, Zhang B, Zhang L, Li S, Li S, Li P. Lung nodules assessment in ultra-low-dose CT with iterative reconstruction compared to conventional dose CT. Quant Imaging Med Surg. 2018;8:480-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Neroladaki A, Botsikas D, Boudabbous S, Becker CD, Montet X. Computed tomography of the chest with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination: preliminary observations. Eur Radiol. 2013;23:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 37. | Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, Pfammatter T, Frauenfelder T, Alkadhi H. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 38. | Watanabe S, Urikura A, Ohashi K, Kitera N, Tsuchiya T, Kasai H, Kawai T, Hiwatashi A. Artifact reduction in low and ultra-low dose chest computed tomography for patients with pacemaker: A phantom study. Radiography (Lond). 2024;30:770-775. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Ma G, Dou Y, Dang S, Yu N, Guo Y, Han D, Fan Q. Improving Image Quality and Nodule Characterization in Ultra-low-dose Lung CT with Deep Learning Image Reconstruction. Acad Radiol. 2024;31:2944-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Prosper AE, Kammer MN, Maldonado F, Aberle DR, Hsu W. Expanding Role of Advanced Image Analysis in CT-detected Indeterminate Pulmonary Nodules and Early Lung Cancer Characterization. Radiology. 2023;309:e222904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Chen B, Barnhart H, Richard S, Robins M, Colsher J, Samei E. Volumetric quantification of lung nodules in CT with iterative reconstruction (ASiR and MBIR). Med Phys. 2013;40:111902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |