Published online Jul 28, 2022. doi: 10.4329/wjr.v14.i7.180
Peer-review started: January 5, 2022
First decision: June 16, 2022
Revised: June 26, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: July 28, 2022
Processing time: 202 Days and 20.9 Hours
There is increasing evidence on the utility of cardiac computed tomography (CCT) in infective endocarditis (IE) to investigate the valvular pathology, the extra-cardiac manifestations of IE and pre-operative planning. CCT can assist in the diagnosis of perivalvular complications, such as pseudoaneurysms and abscesses, and can help identify embolic events to the lungs or systemic vasculature. CCT has also been shown to be beneficial in the pre-operative planning of patients by delineating the coronary artery anatomy and the major cardiovascular structures in relation to the sternum. Finally, hybrid nuclear/computed tomography techniques have been shown to increase the diagnostic accuracy in prosthetic valve endocarditis. This manuscript aims to provide a contemporary update of the existing evidence base for the use of CCT in IE.
Core Tip: Cardiac computed tomography (CCT) has an expanding role in the management of infective endocarditis (IE). It has been shown to be superior to echocardiography for diagnosing perivalvular complications such as pseudoaneurysms and abscesses. CCT can also diagnose extra-cardiac manifestations of IE such as septic emboli to the lungs. It can assist in pre-operative planning by delineating the coronary anatomy and assessing vascular structures. Herein, we review the role of CCT in IE including the evidence base comparing CCT to echocardiography in diagnosing the valvular complications of IE and the use of CT in IE beyond valvular assessment.
- Citation: Hughes D, Linchangco R, Reyaldeen R, Xu B. Expanding utility of cardiac computed tomography in infective endocarditis: A contemporary review . World J Radiol 2022; 14(7): 180-193
- URL: https://www.wjgnet.com/1949-8470/full/v14/i7/180.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i7.180
Infective endocarditis (IE) is an infection of the endocardium, heart valves or intra-cardiac devices. It remains a challenging disease to diagnose and manage with high rates of morbidity and mortality[1,2]. Echocardiography remains the main imaging modality used in IE; more recently, however, there is an increasing evidence base for a multimodality imaging approach for IE. Complementary imaging modalities including cardiac computed tomography (CCT) now play increasingly important roles in diagnosis, risk stratification and management of IE. CCT has certain advantages compared to echocardiography in being able to investigate for perivalvular extension, extra-cardiac complications of IE, including metastatic spread and planning for surgery including assessing for coronary artery disease. Advancements in CT technologies, including the use of dedicated cardiac gated four-dimensional CCT, have expanded the applications of CT in IE, demonstrating good sensitivity and specificity for diagnosing the complications of IE. This article aims to review the available evidence for the use of CCT in IE.
The incidence of IE in the United States is estimated to be approximately 15 per 100000 persons annually[3,4], with Staphylococcus aureus (SA) being the most common pathogen followed by Viridans group Streptococci[5]. A number of risk factors have been identified for acquiring IE, including the presence of a prosthetic valve, a previous episode of IE, patients with untreated cyanotic congenital heart disease, injection drug use, poor dentition and pre-existing valvular heart disease[6]. The clinical presentation of IE can vary significantly from an acute life threatening illness to a more indolent chronic disease[7]. The most common presenting symptoms are: fever, cardiac murmur, heart failure or complications from septic emboli[8].
The Duke criteria were developed in 1994 to assist in the risk stratification of patients with suspected IE into definite, possible and rejected cases of IE[9]. These criteria have been since validated by a number of retrospective analyses, and underwent further modification in 2000 to reflect changing clinical practice and the emergence of SA as the most common pathogen encountered[10-12]. Despite the updated clinical criteria for diagnosis of IE, there often remains a delay in diagnosis for many patients, commonly due to a lack of microbiological criteria from impropriate antibiotic use, with worse outcomes seen in these patients[13,14]. Advances in CT technologies including improvements in both temporal and spatial resolution have enabled greater use of CCT for the diagnosis of IE. The European Society of Cardiology guidelines for the management of infective endocarditis reflect these advances in imaging techniques and include paravalvular lesions detected by CCT to be a major imaging criterion[15]. The 2020 American College of Cardiology/American Heart Association Guideline for the management of patients with valvular heart disease also recommend the use of CCT as an adjunctive imaging modality for IE[16].
Echocardiography, including transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) where appropriate, remain the first line imaging modality for the diagnosis and monitoring of IE[17,18]. There are three main echocardiographic findings that are considered major criteria for the diagnosis of IE: vegetations, abscesses/pseudoaneurysms and new dehiscence of a prosthetic valve[15]. In native valve endocarditis, the sensitivities for the diagnosis of a vegetation are approximately 70% for TTE and 96% for TEE, respectively[17]. For prosthetic valve endocarditis (PVE), the sensitivities for diagnosing a vegetation are approximately 50% for TTE and 92% for TEE, respectively[17]. There are many challenges in the diagnosis of IE with echocardiography, including small vegetations or embolization of the vegetation prior to imaging, difficulty visualizing the lesion in the setting of pre-existing valvular disease or with prosthetic valves. Small abscesses may also be challenging to diagnose, especially by TTE[19]. There are also many mimics of IE that could result in a false positive diagnosis, such as Lambl’s excrescence, fibroelastomas, thrombus, degenerative lesions, prosthetic material/sutures or marantic lesions[20]. CCT can therefore be helpful to assist in the diagnosis of IE, when there are equivocal findings by echocardiography or in challenging cases involving prosthetic valves[21].
For evaluation of cardiac valves, multiphase imaging using a retrospectively ECG-gated acquisition is required to obtain an isotropic data set. Images are acquired in spiral mode utilizing a low pitch of 0.16 to 0.5 during 5-10 R-R intervals with a section thickness of 0.60 mm. Thin collimation is used for optimal visualization of the valve leaflets, typically 64 mm × 0.6 mm. The tube voltage used is adapted to the patient’s weight, and can vary between 100 to 120 kV. Gantry rotation time of 0.28 s to 0.35 s is used[22-27]. The scan is typically performed in a limited field of view from the level of the carina to the cardiac apex.
Timing of the iodinated contrast bolus is important to optimize visualization of the involved cardiac valves. A monophasic contrast injection is most commonly used, with timing of the contrast bolus chosen for optimal visualization of the expected involved valve and cardiac chambers. Alternatively, biphasic contrast injection may be performed, which allows evaluation of all cardiac chambers and valves[28]. Premedication with beta blockers may be used to regulate heart rate to less than 65-70 bpm if not contraindicated. This improves image quality by reducing artefacts related to cardiac motion and valvular motion.
The isotropic data set acquired from the retrospectively gated acquisition allows for reconstruction in any desired plane. In addition to static images, imaging at multiple points during the cardiac cycle also allows for creation of 4D cine images, allowing for evaluation of valve leaflet motion and planimetry.
Because images are acquired throughout the entire cardiac cycle, this results in a significantly higher radiation dose penalty compared to prospectively gated CT as used typically in CT angiography of the coronary arteries. Radiation dose may be lowered utilizing methods such as iterative reconstruction and ECG-triggered radiation dose modulation. However, ECG-triggered dose modulation may result in suboptimal evaluation during the phase of reduced tube current, typically the systolic phase[29]. There are specific protocols used for visualizing the various cardiac and extra manifestations of IE and for pre-operative planning. Herein, we group all of these into an umbrella term of CCT, referring to ECG-gated CT of the chest with contrast. There are some situations, such as during investigation for septic emboli to the visceral organs, when abdominal imaging may also be needed.
CCT has the ability to assess for valvular lesions, perivalvular extension,
| CCT | TEE | |
| Vegetation | An irregular mass or thickening associated with the endocardium, native valve or prosthetic valve with low to intermediate attenuation | Mobile or non-mobile intracardiac mass on valve or other endocardial structures, including on implanted intracardiac material |
| Pseudoaneurysm | Perivalvular collection of contrast enhanced material usually adjacent to a valve with a visible direct communication | Abnormal perivalvular echo-free space with color-Doppler flow showing connection with the cardiovascular lumen |
| Abscess | Usually perivalvular collection of low attenuation material. Often has a thick layer of tissue in the wall of the collection that enhances with contrast | Usually perivalvular collection that can have an echodense or echolucent appearance without a communication to a lumen |
| Dehiscence of a prosthetic valve | Prosthetic valve misalignment with a tissue defect between the annulus and prosthesis | Evidence of excessive motion of a prosthetic valve. Occasionally, it is possible to see a defect between annulus and prosthesis and/or evidence of paravalvular leak on Doppler assessment |
| Perforation | Leaflet tissue defect that can be observed in two different views | Defect in a valve leaflet that may be seen visually as an interruption of tissue or by color flow across the defect |
| Fistula | An abnormal communication between two cardiac chambers that is contrast filled | An abnormal connection two neighboring lumen detected by color Doppler flow |
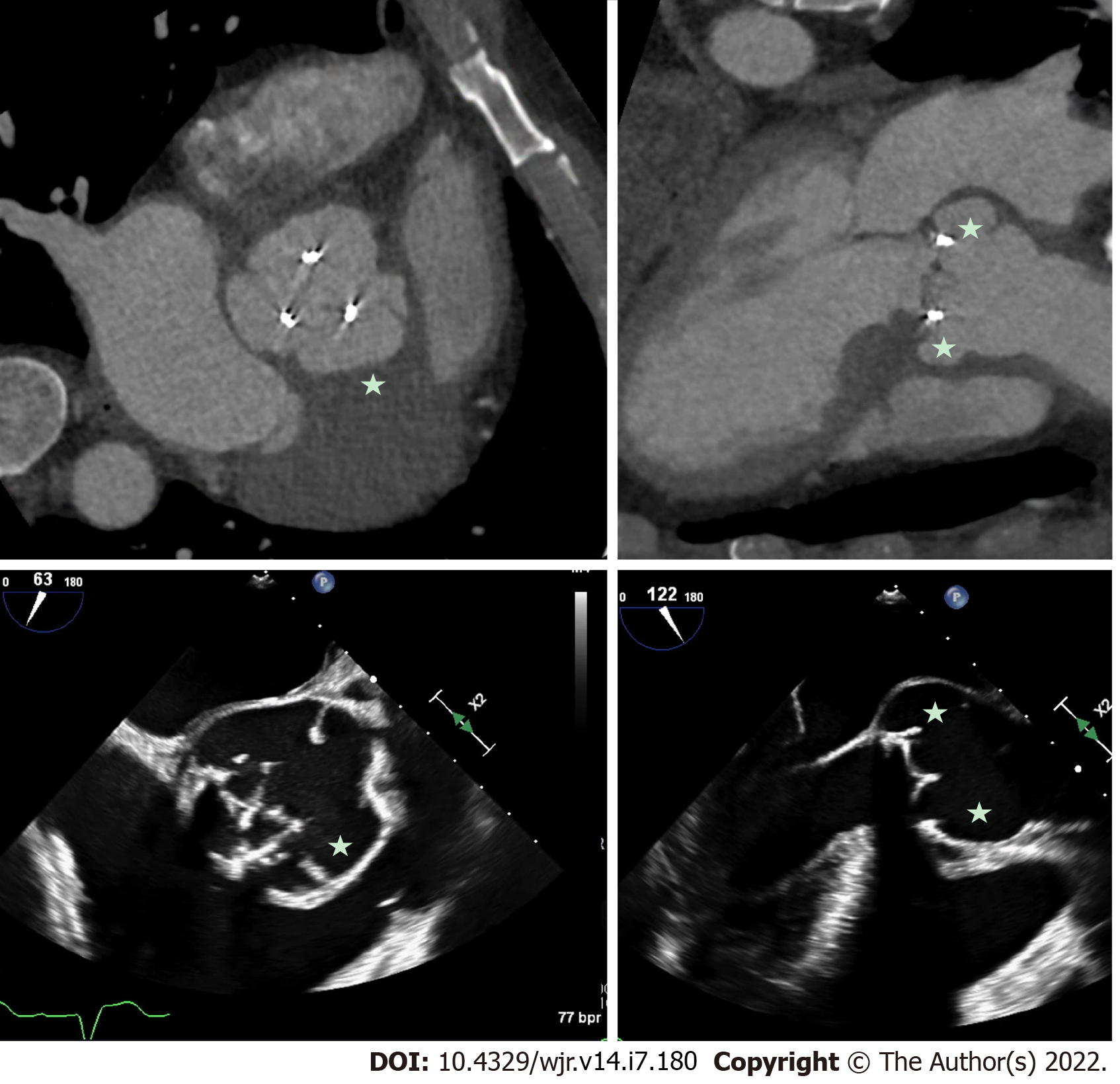
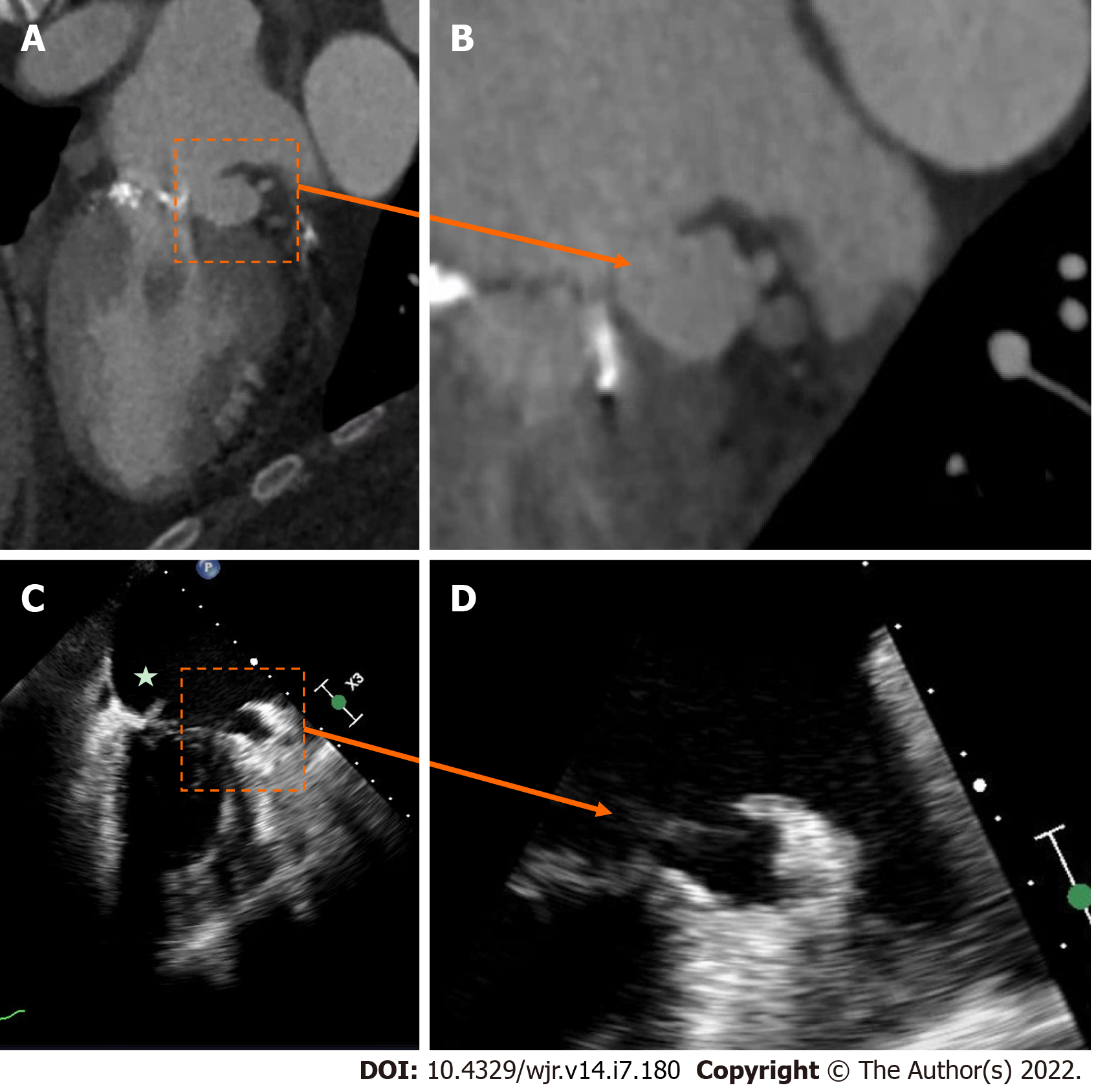
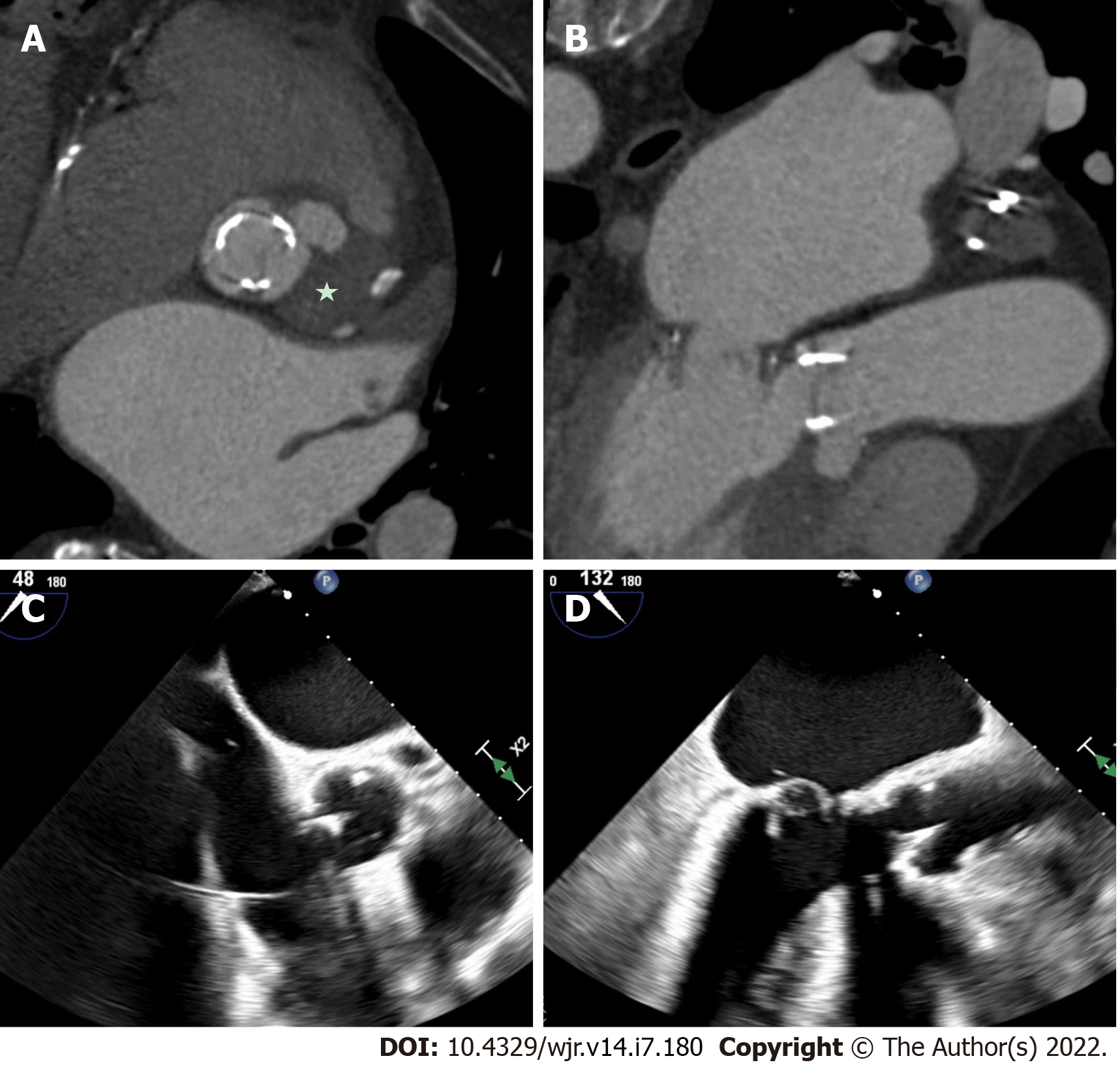
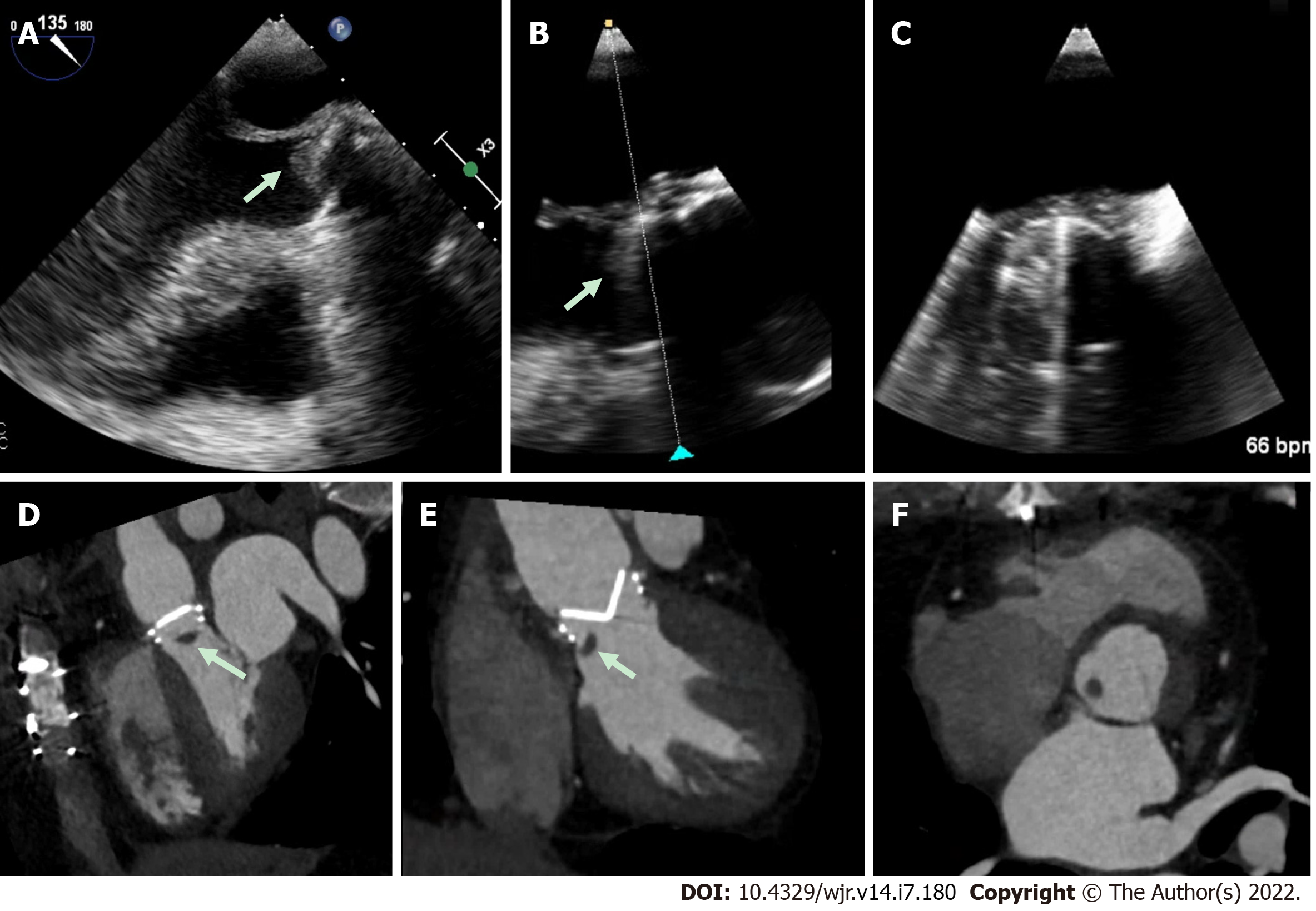
Perivalvular extension of IE, which includes pseudoaneurysms, abscesses and fistulae are associated with a higher rate of operative management and mortality[32-34]. A pseudoaneurysm is a perivalvular cavity that is in communication with the cardiovascular lumen which results from an abscess rupturing into a cavity[17]. On echocardiography, this appears as a pulsatile echo-free space with detectable Doppler color flow, while on CCT, it appears as an abnormal cavity close to the valve with direct communication with the heart chambers or major blood vessel[30]. An abscess is a closed cavity with necrosis and purulent material not in communication with a cardiovascular cavity[17] . On echocardiography, this appears as a thickened perivalvular area with a homogenous echo-dense or echo-lucent appearance. On CCT, abscesses appear as perivalvular collections of fluid encased in a thick layer of inflammatory tissue enhanced by the injection of contrast medium. See Figures 1-4 for examples of comparisons of TEE and CCT images in patients with perivalvular complications of IE (Table 2).
| Modality | CCT | TTE | TEE | PET/CT |
| Strengths | Ability to image the entire thorax; Improved detection of perivalvular complications; CAD Assessment; Pre-Operative planning; Detection of extra-cardiac emboli | Good Spatial resolution; Availability and portable; Low cost; Lack of radiation; Lack of contrast; Chamber quantification; Assess hemodynamics | Improved spatial and temporal resolution over TTE; Availability and low cost; Lack of radiation; Lack of contrast; Better sensitivity than TTE in PVE; Assess Hemodynamics | Improved detection of perivalvular complications; Improved diagnostic accuarcy in PVE detection of embolic events |
| Weaknesses | Higher cost; Radiation exposure; Nephrotoxicity; Lower sensitivity for small vegetations and leaflet perforation; Availability may be limited | Limited value in PVE; No tissue characterization; Low sensitivity for peri-valvular complications | No tissue characterization; May miss some peri-valvular complications; Invasive procedure requiring sedation (cannot be performed in some patients with esophageal issues) | Limited availability; Higher cost; Radiation exposure |
A 2009 study by Feuchter et al[24] to investigate the value of CCT for the assessment of valvular abnormalities included 37 consecutive patients (29 of whom went on to have surgery) with clinically suspected IE who underwent both CCT and TEE. CCT identified all pseudoaneurysms and abscesses in this study with sensitivity and specificity of 100%, which was superior to TEE (sensitivity of 89% and a specificity of 100%)[24]. CCT was also shown to be superior to TEE for perivalvular extension of the IE, identifying myocardial and pericardial extension more often than TEE[24]. In a 2009 prospective study, 19 patients with aortic valve endocarditis requiring surgical intervention underwent CCT pre-operatively[25]. The majority of patients (approx. 90%) had native valve IE. This study showed that CCT had sensitivity and specificity for diagnosing pseudoaneurysms of 100% and 92%, respectively, and CCT correctly identified all cases where there was extension of IE into the intervalvular fibrosa[25]. This paper did not report the TEE findings for their participants [25].
A paper by Fagman et al[23] reported in 2012 on 27 consecutive patients who had TEE findings of aortic valve PVE and investigated the strength of agreement between the TEE and CT results. They found a strength of agreement compared to TEE was 0.68 for abscesses and 0.75 for dehiscence[23]. However, using surgery as the reference standard (16 patients went on to have surgery), CCT had sensitivity of 91% to detect pseudoaneurysms /abscesses compared to 82% for TEE[23]. A 2013 study investigated the additional value of CCT beyond the usual evaluation with TEE in PVE in 28 patients, with a final diagnosis being either determined clinically or at the time of surgery as reported by Habets et al[35]. They reported that usual evaluation had sensitivity of 68% for detecting periannular complications (mycotic aneurysms and abscesses), which was increased to 100% with the use of CCT[35]. Koo et al[27] also compared CCT vs TEE using intra-operative findings as the reference standard in 2018. They enrolled 49 patients, 12 of whom had PVE[27]. The overall detection of IE by CCT was 94%, compared to 96% by TEE[27]. CCT performed better than TEE at detecting abscess/pseudoaneurysms, with sensitivities of 60% for CCT and 40% for TEE, respectively[27]. A retrospective study from 2018 by Sims et al[36] investigated the performance of CCT in the pre-operative evaluation of IE. In total, they had 251 patients undergoing TEE with 34 of these patients also having a CCT[36]. The sensitivity of CCT for detecting abscesses/pseudoaneurysms was 91%, which was superior to TEE at 78%[36]. CCT was reported to have a lower sensitivity for detecting fistulae at 50% vs 79% for TEE, and dehiscence at 57% vs 70% for TEE[36].
Two studies from 2018 (Ouchi et al[31] and Koneru et al[37]) retrospectively investigated the utility of CCT in IE with intra-operative findings as the reference standard. CCT performed better than TEE in detecting abscess/pseudoaneurysm in prosthetic valves with sensitivity of 81% (versus 64% for TEE) in the study by Koneru et al[31]. CCT had sensitivity of 100% sensitivity for detecting perivalvular complications, such as pseudoaneurysms in the paper by Ouchi et al[37]. A 2019 study by Hryniewiecki et al[26] investigated 53 consecutive patients who had perivalvular complications from IE, who also underwent CCT and TEE pre-operatively. They showed the sensitivity and specificity for detecting abscesses/pseudoaneurysms for CCT were 81% and 90%, respectively, compared to 63% and 90%, respectively for TEE[26]. A 2020 study of 68 patients reported by Sifaoui et al with definite left-sided IE who underwent CCT and TEE reported the comparison of CCT and TEE to detect perivalvular complications[38]. They showed again that CT had a higher sensitivity for detecting pseudoaneurysms at 100%, compared to TEE at 67%[38].
Overall, the current evidence base suggests that the diagnostic performance of CCT is likely superior to that of TEE for the detection of pseudoaneurysms and abscesses in appropriately selected cases. A recent meta-analysis reported pooled sensitivity and specificity for CCT for the detection of peri-annular complications of 88% and 93%, respectively, compared to TEE at 70% and 96%, respectively[39].
The identification of perivalvular complications is important for prognostic and management considerations. These sequelae of invasive IE, which are more common with aortic valve endocarditis and PVE, have been associated with increased rates of surgical management, and may confer an increased risk of mortality[32,33]. Therefore, a multimodality imaging strategy for IE that includes CCT would have the ability to identify more of these complications, compared to using TEE alone, and therefore impact on decision making for patients.
A vegetation is a mass-like lesion of infected material composed of fibrin, platelets and microorganisms attached to an endocardial structure or on an implanted cardiac device (CIED)[40,41]. On echocardiography, this appears as an oscillating or non-oscillating intracardiac echodensity, which can be attached to a valve, other endocardial surface or cardiac device[15]. A vegetation tends to move with the cardiac cycle, and is more frequently found on the atrial side of the atrioventricular valves and the ventricular side of the semi-lunar valves[15]. On CT, vegetations appear as hypodense homogeneous irregular masses, which can be attached to a valve or other cardiac structures[30].
In the 2009 paper by Feuchter et al[24] using surgical/pathological diagnosis as the reference standard, CCT had sensitivity of 96% and specificity of 97% for the diagnosis of vegetations. 5 vegetations were missed by CCT (11%) either due to artefact or small size (≤ 4mm)[24]. The performance of TEE was similar to CCT with sensitivity of 96% and specificity of 100%[24]. CCT was found to be inferior to TEE for detecting leaflet perforations[24]. The study by Gahide et al[25] on aortic valve IE showed CCT had a sensitivity of 71% and a specificity of 100% for detecting vegetations, though the sensitivity was increased to 100% for large vegetations (> 10 mm). The 2012 paper by Fagman et al[23] found that CCT detected vegetations in 7 out of 13 cases (54%), with a lower detection rate being potentially explained by artefact from the prosthetic valves obscuring the CCT images. The 2013 study by Habets et al[35] found additional benefit with CCT in addition to usual work-up with TEE in PVE, with a final diagnosis being either determined clinically or at the time of surgery. They reported that usual work-up had sensitivity of 63% for detecting vegetations, which was increased to 100% with the use of CCT[35]. The 2018 study by Koo et al[27] reported sensitivity for CCT to detect vegetations of 91%, compared to 100% by TEE. Missed vegetations were smaller, and the authors also listed motion artefact and beam hardening from mechanical valves as reasons for the failure of CCT to detect the vegetations[27]. Sims et al[36] reported the sensitivity for detecting vegetations to be 70% for CCT (34 patients) and 96% for TEE (251 patients). The study by Oucho et al[37] reported sensitivity of 92% for detecting vegetations for CCT, correctly identifying 12 of 13 cases who had vegetations confirmed at the time of surgery. The retrospective review on 122 patients by Koneru et al[31] showed TEE to have a statistically significantly higher sensitivity for detecting vegetations compared to CCT at 85% vs 16%, though CCT did have a higher specificity at 96% compared to 69% for TEE. The lower sensitivity in detecting vegetations by CCT in this study may be related to only reviewing single-phase images, and the fact that the slice thickness used was 3 mm which was thicker than the other studies referenced above[31]. In the 2019 paper by Hryniewiecki et al[26] the sensitivity and specificity for detecting vegetations by CT were 89% and 71%, respectively, compared to TEE at 97% and 42%, respectively. The 2020 study by Sifaoui et al[38] showed that TEE had a higher area under the curve (AUC) than CCT for detecting vegetations, with AUC for TEE of 0.86 vs AUC for CT of 0.69.
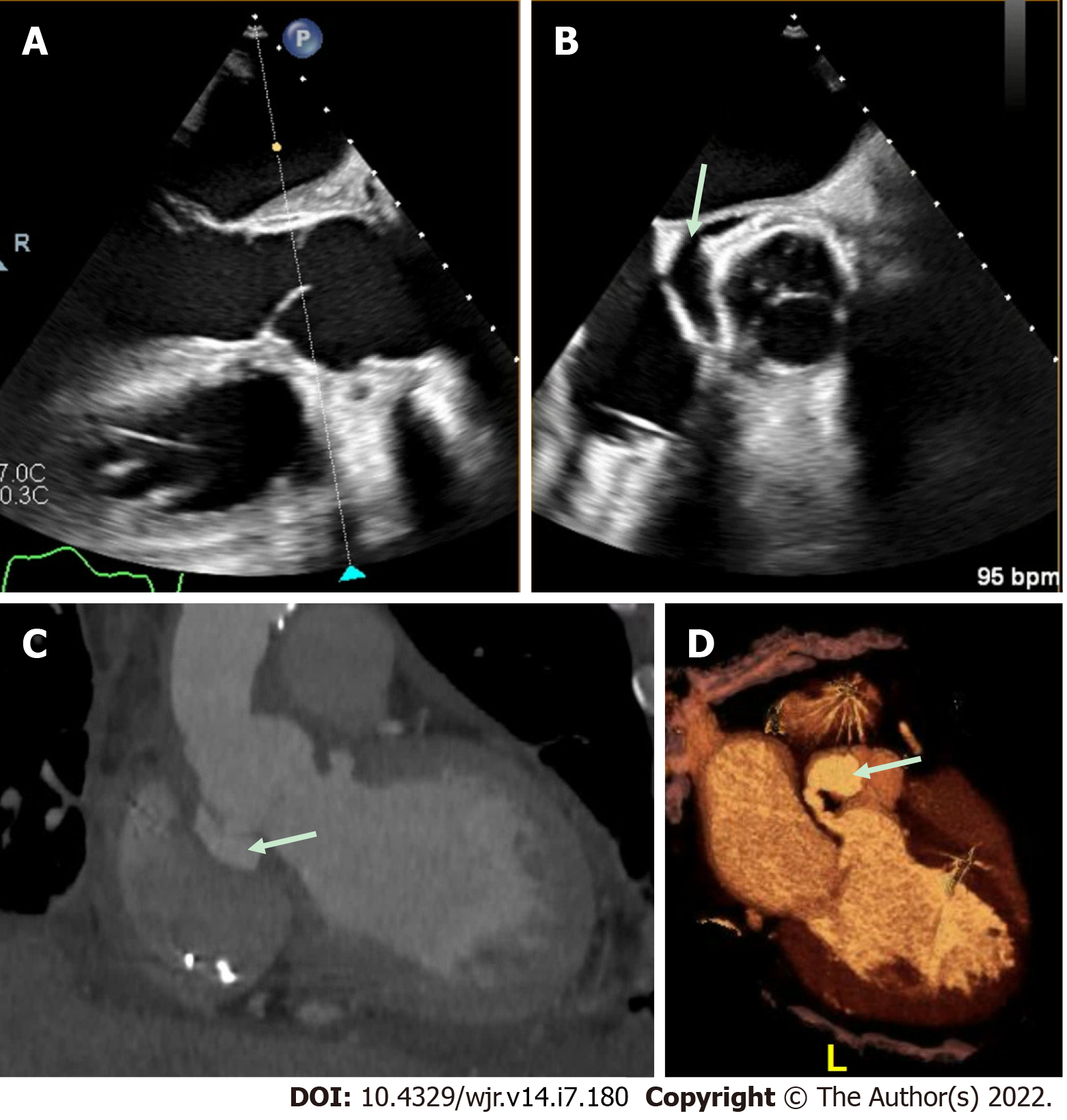
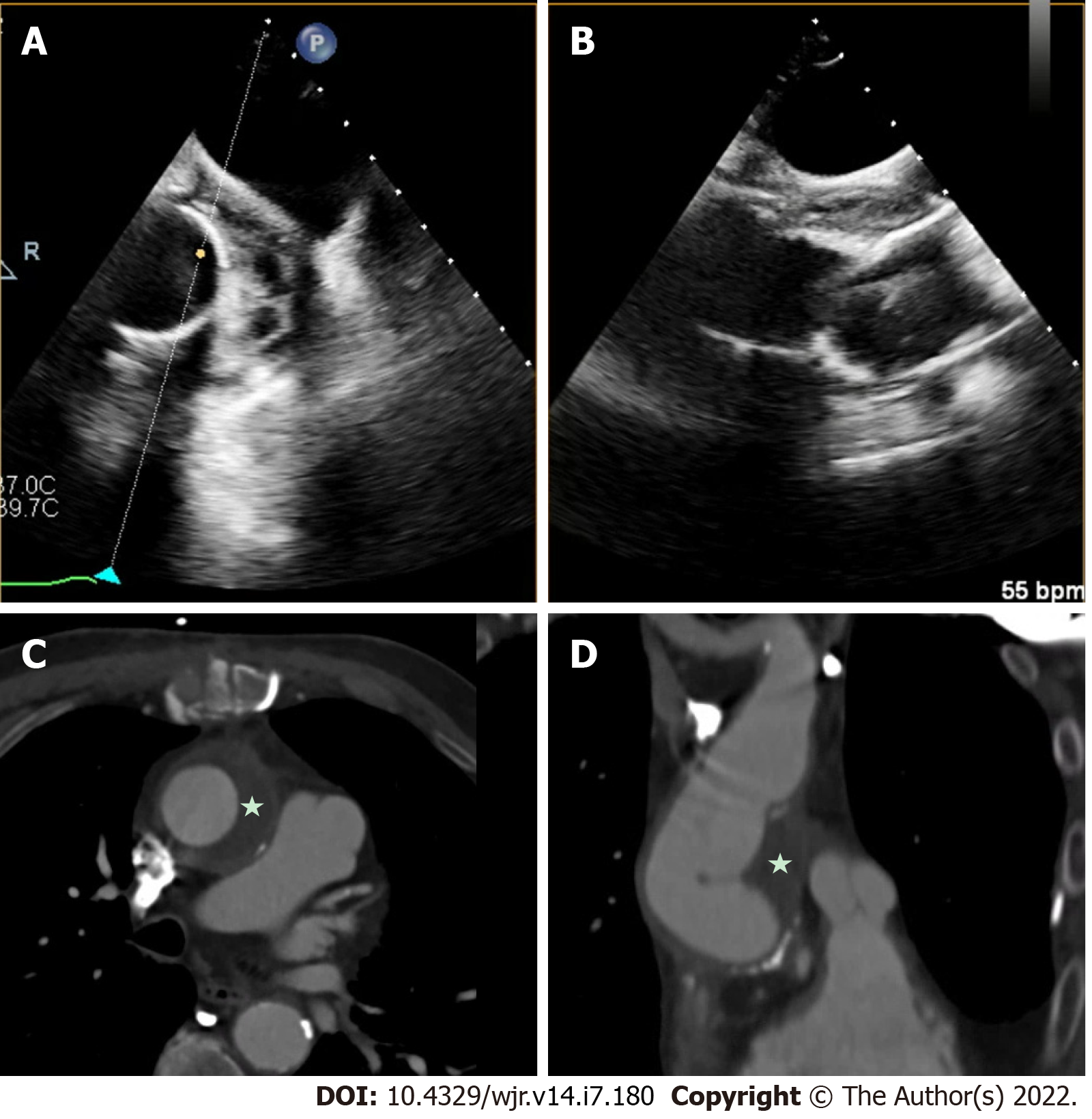
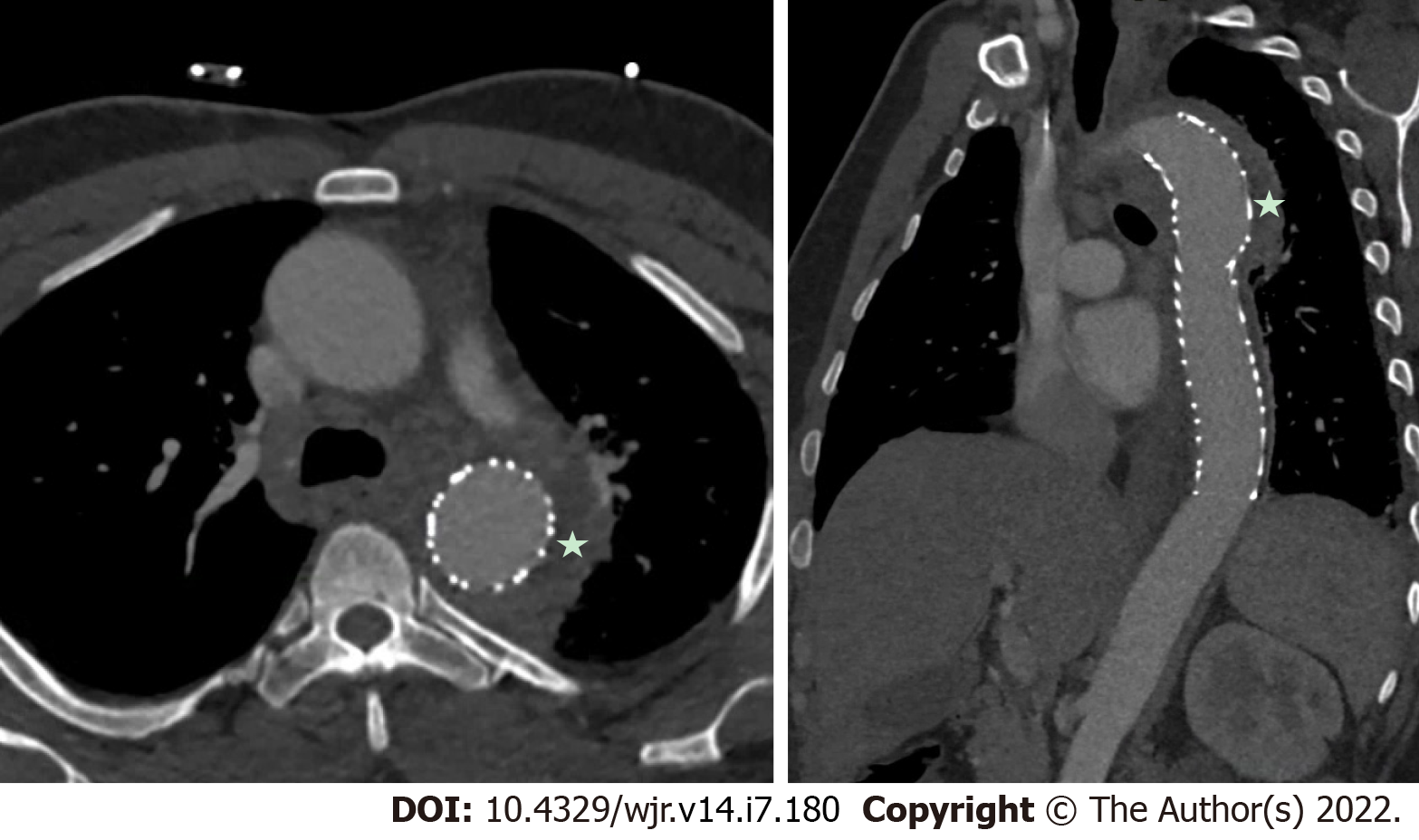
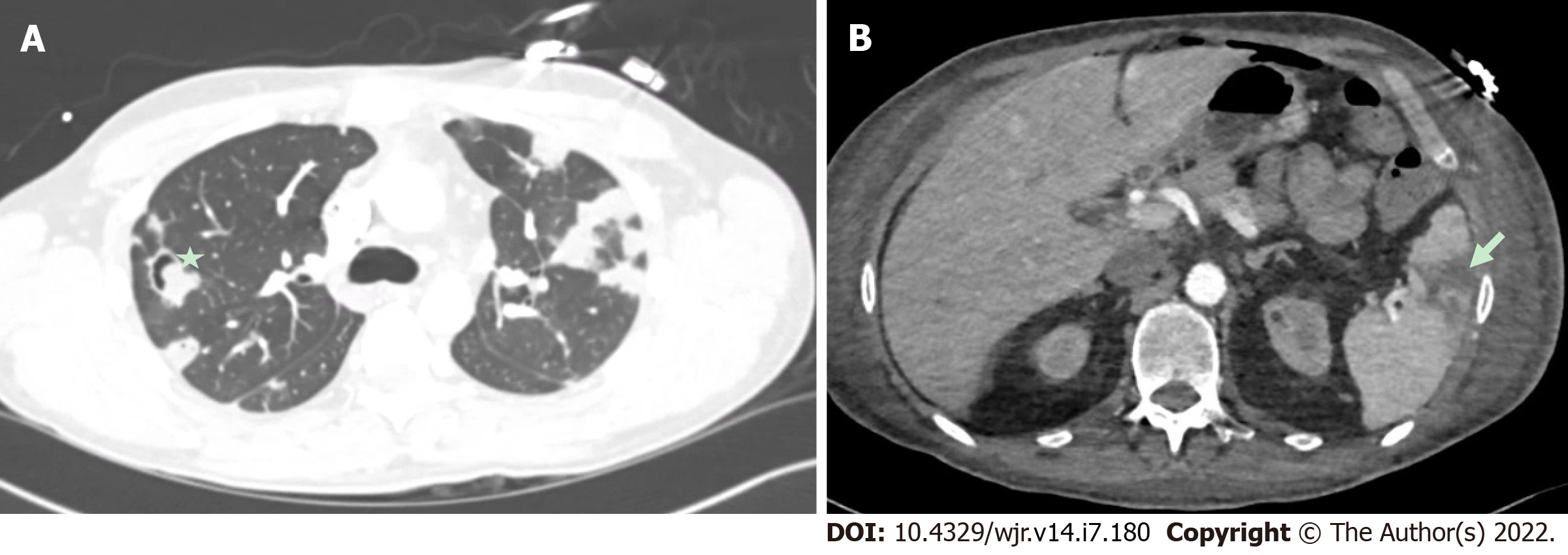
Overall, the current evidence base suggests that TEE is overall superior to CCT for the detection of vegetations, particular small vegetations, with pooled sensitivity for TEE from a recent meta-analysis of 94%[42]. CCT demonstrated a lower pooled sensitivity, at 64% for the detection of vegetations[42]. There was a wide range of results reported likely related to small sample sizes, differing patient populations and different protocols used for imaging. While CCT should not replace echocardiography as the first line imaging tool in the majority of patients primarily to detect vegetations, in a small subset of patients who could not undergo clinical indicated TEE (e.g., esophageal pathology), CCT may add diagnostic value. CCT has also been shown to improve the diagnostic accuracy overall, when used in combination with TEE[35]. For example Figure 5 shows a hypoattenuating lesion on a mechanical aortic valve that was more clearly defined on CCT. Figures 6 and 7 shows an examples of an aortic graft and aortic stent infections that can be difficult to image with TEE.
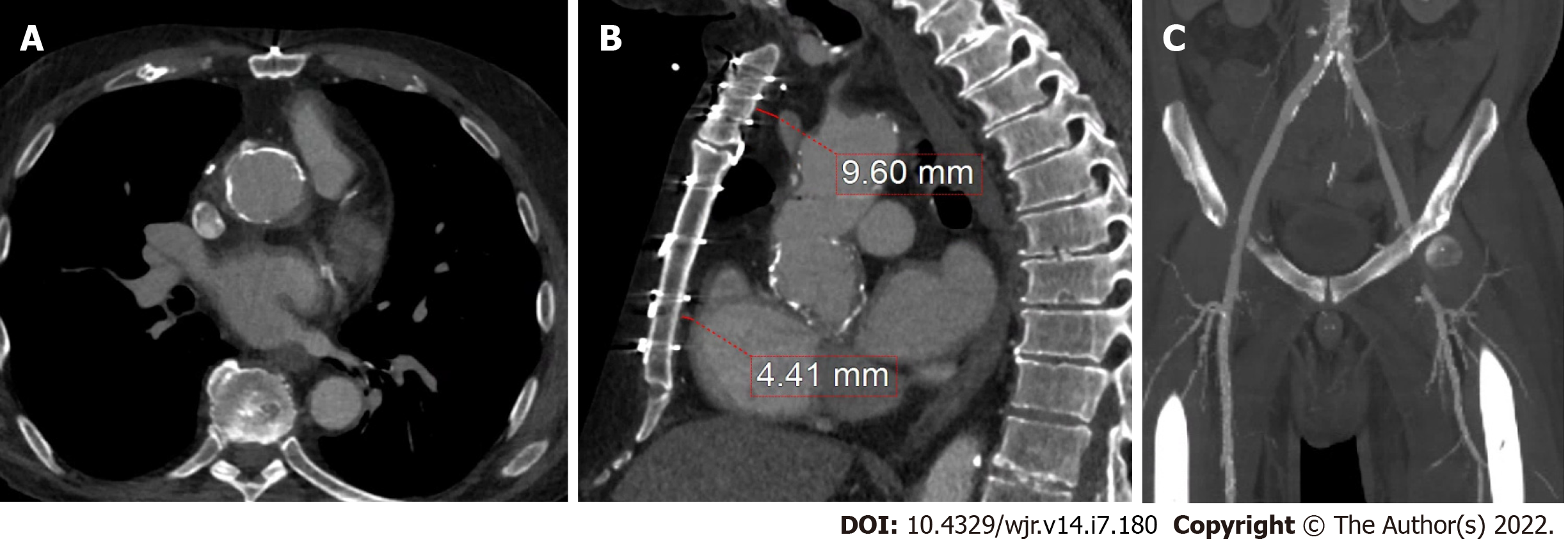
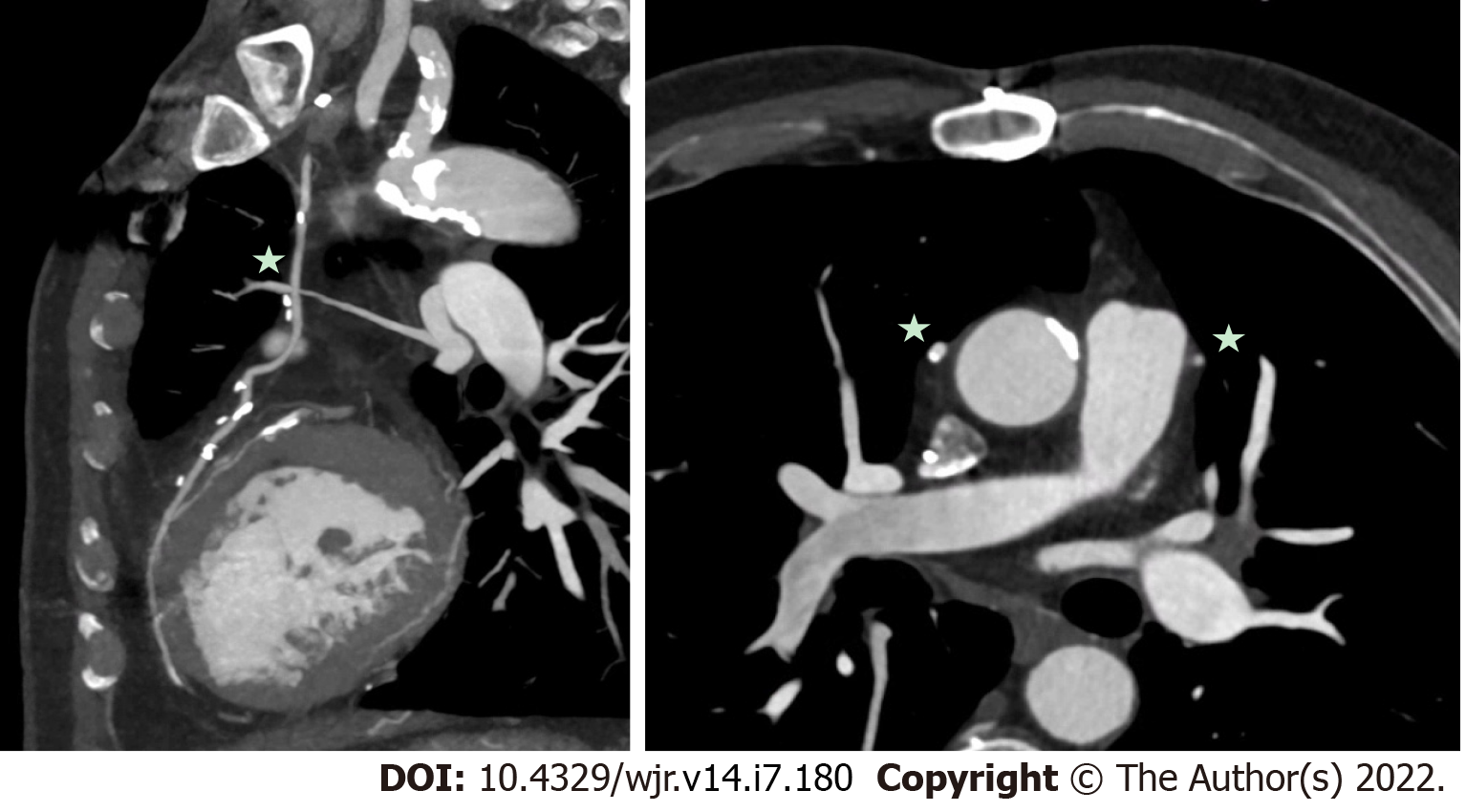
In addition to the advantages related to the management of IE as outlined above, CCT can also assist in the pre-operative planning of IE surgery. In patients with prior cardiothoracic surgery, CCT can delineate the relationship of cardiovascular structures to the sternum and the location of the coronary artery bypass grafts (CABG). See Figure 8 for CCT images of a patient with a prior CABG. For all patients, CCT can identify calcification of the ascending aorta (‘porcelain aorta’), which may preclude surgery as well as give precise anatomic location and extent of the degree of calcification of the subclavian, axillary and femoral arteries. The advantage of having a pre-operative CCT was described by Merlo et al[43] with reported lower rates of stroke and mortality in patients undergoing pre-operative CCT followed by primary cardiac surgery, vs those without pre-operative CCT imaging. Figure 9 shows a CCT in a patient with vascular calcification.
In addition to the advantages related to the management of IE as outlined above, CCT can also assist in the pre-operative planning of IE surgery[44-50]. In patients with prior cardiothoracic surgery, CCT can delineate the relationship of cardiovascular structures to the sternum and the location of the coronary artery bypass grafts (CABG). See Figure 8 for CCT images of a patient with a prior CABG[50-60]. For all patients, CCT can identify calcification of the ascending aorta (‘porcelain aorta’), which may preclude surgery as well as give precise anatomic location and extent of the degree of calcification of the subclavian, axillary and femoral arteries[61,62]. The advantage of having a pre-operative CCT was described by Merlo et al[43] with reported lower rates of stroke and mortality in patients undergoing pre-operative CCT followed by primary cardiac surgery, vs those without pre-operative CCT imaging. Figures 9 and 10 shows a CCT in a patient with vascular calcification.
With improvements in the temporal and spatial resolution of CCT technology, including the use of dedicated 4D CCT, there has been an expanding role of CCT imaging in IE. CCT has been shown to be superior to TEE for the identification of pseudoaneurysms and abscesses in appropriately selected cases, while the combination of both modalities results in the greatest sensitivity for detection. TEE is superior to CCT for small vegetations; however this advantage is less marked for larger vegetations. In addition, CCT has a number of adjunctive uses in IE beyond evaluation of valvular pathology. CCT can aid in the diagnosis of embolic events, such as pulmonary complications in RSIE. It can also be used to diagnose significant CAD in low to intermediate risk patients preoperatively, or when there is a contraindication to ICA, such as when there is a large aortic valve vegetation. CCT can also be helpful for pre-operative planning to assess the relationship of the cardiovascular structures in relation to the sternum, which is particularly helpful in re-do sternotomy cases. The addition of hybrid techniques such as positron emission computed tomography or SPECT/CT, has been shown to improve the diagnostic accuracy in challenging cases of PVE. Greater awareness of the strengths, weaknesses and appropriate applications of CCT in IE will assist in its optimal use for improved diagnosis and management of this challenging condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fan YW, China; Teragawa H, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J. 2010;31:1890-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Mansur AJ, Grinberg M, da Luz PL, Bellotti G. The complications of infective endocarditis. A reappraisal in the 1980s. Arch Intern Med. 1992;152:2428-2432. [PubMed] |
| 3. | Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 444] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 4. | Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in Infective Endocarditis in California and New York State, 1998-2013. JAMA. 2017;317:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 5. | Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1642] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 6. | Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, Levison ME, Korzeniowski OM, Kaye D. Risk factors for infective endocarditis: oral hygiene and nondental exposures. Circulation. 2000;102:2842-2848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 2102] [Article Influence: 210.2] [Reference Citation Analysis (1)] |
| 8. | Bayer AS. Infective endocarditis. Clin Infect Dis. 1993;17:313-20; quiz 321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1548] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 10. | Sekeres MA, Abrutyn E, Berlin JA, Kaye D, Kinman JL, Korzeniowski OM, Levison ME, Feldman RS, Strom BL. An assessment of the usefulness of the Duke criteria for diagnosing active infective endocarditis. Clin Infect Dis. 1997;24:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Hoen B, Béguinot I, Rabaud C, Jaussaud R, Selton-Suty C, May T, Canton P. The Duke criteria for diagnosing infective endocarditis are specific: analysis of 100 patients with acute fever or fever of unknown origin. Clin Infect Dis. 1996;23:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2699] [Cited by in RCA: 2827] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 13. | Fukuchi T, Iwata K, Ohji G. Failure of early diagnosis of infective endocarditis in Japan--a retrospective descriptive analysis. Medicine (Baltimore). 2014;93:e237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Naderi HR, Sheybani F, Erfani SS. Errors in diagnosis of infective endocarditis. Epidemiol Infect. 2018;146:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2661] [Cited by in RCA: 3356] [Article Influence: 335.6] [Reference Citation Analysis (0)] |
| 16. | Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 573] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 17. | Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M, Voigt JU, Sicari R, Cosyns B, Fox K, Aakhus S; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 18. | Haq IU, Haq I, Griffin B, Xu B. Imaging to evaluate suspected infective endocarditis. Cleve Clin J Med. 2021;88:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Daniel WG, Mügge A, Martin RP, Lindert O, Hausmann D, Nonnast-Daniel B, Laas J, Lichtlen PR. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med. 1991;324:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 446] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Zmaili MA, Alzubi JM, Kocyigit D, Bansal A, Samra GS, Grimm R, Griffin BP, Xu B. A Contemporary 20-Year Cleveland Clinic Experience of Nonbacterial Thrombotic Endocarditis: Etiology, Echocardiographic Imaging, Management, and Outcomes. Am J Med. 2021;134:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Lo Presti S, Elajami TK, Zmaili M, Reyaldeen R, Xu B. Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis: A contemporary narrative review. World J Cardiol. 2021;13:254-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 22. | Altiok E, Koos R, Schröder J, Brehmer K, Hamada S, Becker M, Mahnken AH, Almalla M, Dohmen G, Autschbach R, Marx N, Hoffmann R. Comparison of two-dimensional and three-dimensional imaging techniques for measurement of aortic annulus diameters before transcatheter aortic valve implantation. Heart. 2011;97:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Fagman E, Perrotta S, Bech-Hanssen O, Flinck A, Lamm C, Olaison L, Svensson G. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol. 2012;22:2407-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Feuchtner GM, Stolzmann P, Dichtl W, Schertler T, Bonatti J, Scheffel H, Mueller S, Plass A, Mueller L, Bartel T, Wolf F, Alkadhi H. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol. 2009;53:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 25. | Gahide G, Bommart S, Demaria R, Sportouch C, Dambia H, Albat B, Vernhet-Kovacsik H. Preoperative evaluation in aortic endocarditis: findings on cardiac CT. AJR Am J Roentgenol. 2010;194:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 26. | Hryniewiecki T, Zatorska K, Abramczuk E, Zakrzewski D, Szymański P, Kuśmierczyk M, Michałowska I. The usefulness of cardiac CT in the diagnosis of perivalvular complications in patients with infective endocarditis. Eur Radiol. 2019;29:4368-4376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Koo HJ, Yang DH, Kang JW, Lee JY, Kim DH, Song JM, Kang DH, Song JK, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW, Lim TH. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging. 2018;19:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Vrachliotis TG, Bis KG, Haidary A, Kosuri R, Balasubramaniam M, Gallagher M, Raff G, Ross M, O'neil B, O'neill W. Atypical chest pain: coronary, aortic, and pulmonary vasculature enhancement at biphasic single-injection 64-section CT angiography. Radiology. 2007;243:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Jakobs TF, Becker CR, Ohnesorge B, Flohr T, Suess C, Schoepf UJ, Reiser MF. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002;12:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 431] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 30. | Grob A, Thuny F, Villacampa C, Flavian A, Gaubert JY, Raoult D, Casalta JP, Habib G, Moulin G, Jacquier A. Cardiac multidetector computed tomography in infective endocarditis: a pictorial essay. Insights Imaging. 2014;5:559-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Koneru S, Huang SS, Oldan J, Betancor J, Popovic ZB, Rodriguez LL, Shrestha NK, Gordon S, Pettersson G, Bolen MA. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther. 2018;8:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Baumgartner FJ, Omari BO, Robertson JM, Nelson RJ, Pandya A, Milliken JC. Annular abscesses in surgical endocarditis: anatomic, clinical, and operative features. Ann Thorac Surg. 2000;70:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Graupner C, Vilacosta I, SanRomán J, Ronderos R, Sarriá C, Fernández C, Mújica R, Sanz O, Sanmartín JV, Pinto AG. Periannular extension of infective endocarditis. J Am Coll Cardiol. 2002;39:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Harris WM, Sinha S, Caputo M, Angelini GD, Ahmed EM, Rajakaruna C, Benedetto U, Vohra HA. Surgical outcomes and optimal approach to treatment of aortic valve endocarditis with aortic root abscess. J Card Surg. 2022;37:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Habets J, Tanis W, van Herwerden LA, van den Brink RB, Mali WP, de Mol BA, Chamuleau SA, Budde RP. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging. 2014;30:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Sims JR, Anavekar NS, Chandrasekaran K, Steckelberg JM, Wilson WR, Gersh BJ, Baddour LM, DeSimone DC. Utility of cardiac computed tomography scanning in the diagnosis and pre-operative evaluation of patients with infective endocarditis. Int J Cardiovasc Imaging. 2018;34:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Ouchi K, Sakuma T, Ojiri H. Cardiac computed tomography as a viable alternative to echocardiography to detect vegetations and perivalvular complications in patients with infective endocarditis. Jpn J Radiol. 2018;36:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Sifaoui I, Oliver L, Tacher V, Fiore A, Lepeule R, Moussafeur A, Huguet R, Teiger E, Audureau E, Derbel H, Luciani A, Kobeiter H, Lim P, Ternacle J, Deux JF. Diagnostic Performance of Transesophageal Echocardiography and Cardiac Computed Tomography in Infective Endocarditis. J Am Soc Echocardiogr. 2020;33:1442-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Jain V, Wang TKM, Bansal A, Farwati M, Gad M, Montane B, Kaur S, Bolen MA, Grimm R, Griffin B, Xu B. Diagnostic performance of cardiac computed tomography vs transesophageal echocardiography in infective endocarditis: A contemporary comparative meta-analysis. J Cardiovasc Comput Tomogr. 2021;15:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL; ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1259] [Article Influence: 78.7] [Reference Citation Analysis (1)] |
| 41. | Fava AM, Xu B. Tricuspid valve endocarditis: Cardiovascular imaging evaluation and management. World J Clin Cases. 2021;9:8974-8984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Oliveira M, Guittet L, Hamon M. Comparative Value of Cardiac CT and Transesophageal Echocardiography in Infective Endocarditis: A Systematic Review and Meta-Analysis. Radiol Cardiothorac Imaging. 2020;2:e190189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Merlo A, Chen K, Deo S, Markowitz A. Does routine preoperative computed tomography imaging provide clinical utility in patients undergoing primary cardiac surgery? Interact Cardiovasc Thorac Surg. 2017;25:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O'Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368-e454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 45. | SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 750] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 46. | SCOT-HEART Investigators, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 961] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 47. | Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, Jüni P, Windecker S, Bax JJ, Wijns W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39:3322-3330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 48. | Opolski MP, Staruch AD, Jakubczyk M, Min JK, Gransar H, Staruch M, Witkowski A, Kepka C, Kim WK, Hamm CW, Möllmann H, Achenbach S. CT Angiography for the Detection of Coronary Artery Stenoses in Patients Referred for Cardiac Valve Surgery: Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1059-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 3261] [Article Influence: 815.3] [Reference Citation Analysis (0)] |
| 50. | Hekimian G, Kim M, Passefort S, Duval X, Wolff M, Leport C, Leplat C, Steg G, Iung B, Vahanian A, Messika-Zeitoun D. Preoperative use and safety of coronary angiography for acute aortic valve infective endocarditis. Heart. 2010;96:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, Casalta JP, Gouriet F, Riberi A, Avierinos JF, Collart F, Mundler O, Raoult D, Thuny F. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 52. | Wang TKM, Sánchez-Nadales A, Igbinomwanhia E, Cremer P, Griffin B, Xu B. Diagnosis of Infective Endocarditis by Subtype Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: A Contemporary Meta-Analysis. Circ Cardiovasc Imaging. 2020;13:e010600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 53. | Mahmood M, Kendi AT, Ajmal S, Farid S, O'Horo JC, Chareonthaitawee P, Baddour LM, Sohail MR. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J Nucl Cardiol. 2019;26:922-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 54. | Swart LE, Gomes A, Scholtens AM, Sinha B, Tanis W, Lam MGEH, van der Vlugt MJ, Streukens SAF, Aarntzen EHJG, Bucerius J, van Assen S, Bleeker-Rovers CP, van Geel PP, Krestin GP, van Melle JP, Roos-Hesselink JW, Slart RHJA, Glaudemans AWJM, Budde RPJ. Improving the Diagnostic Performance of 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography in Prosthetic Heart Valve Endocarditis. Circulation. 2018;138:1412-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 55. | Erba PA, Conti U, Lazzeri E, Sollini M, Doria R, De Tommasi SM, Bandera F, Tascini C, Menichetti F, Dierckx RA, Signore A, Mariani G. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med. 2012;53:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 56. | Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, Iung B, Vahanian A, Le Guludec D, Hyafil F. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. 2014;55:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 57. | Di Salvo G, Habib G, Pergola V, Avierinos JF, Philip E, Casalta JP, Vailloud JM, Derumeaux G, Gouvernet J, Ambrosi P, Lambert M, Ferracci A, Raoult D, Luccioni R. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol. 2001;37:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 58. | Rossi SE, Goodman PC, Franquet T. Nonthrombotic pulmonary emboli. AJR Am J Roentgenol. 2000;174:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Huang JS, Ho AS, Ahmed A, Bhalla S, Menias CO. Borne identity: CT imaging of vascular infections. Emerg Radiol. 2011;18:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Thuny F, Avierinos JF, Tribouilloy C, Giorgi R, Casalta JP, Milandre L, Brahim A, Nadji G, Riberi A, Collart F, Renard S, Raoult D, Habib G. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study. Eur Heart J. 2007;28:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 61. | García-Cabrera E, Fernández-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A, Lomas JM, Gálvez-Acebal J, Hidalgo-Tenorio C, Ruíz-Morales J, Martínez-Marcos FJ, Reguera JM, de la Torre-Lima J, de Alarcón González A; Group for the Study of Cardiovascular Infections of the Andalusian Society of Infectious Diseases; Spanish Network for Research in Infectious Diseases. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013;127:2272-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 62. | Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160:2781-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |