Published online Apr 28, 2022. doi: 10.4329/wjr.v14.i4.70
Peer-review started: February 25, 2021
First decision: May 3, 2021
Revised: May 16, 2021
Accepted: April 8, 2022
Article in press: April 8, 2022
Published online: April 28, 2022
Processing time: 423 Days and 17.2 Hours
Contrast-enhanced ultrasound (CEUS) represents a great innovation for the evaluation of focal liver lesions (FLLs). The main advantage of CEUS is the real-time imaging examination and the very low toxicity in patients with renal failure. Liver cirrhosis has been recognized as a major risk factor for the onset of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). HCC in liver cirrhosis develops as the last step of a complex that leads to the gradual transformation from regenerative nodule through dysplastic nodule to HCC. In patients with liver cirrhosis, a surveillance program is recommended consisting of ultrasound (US) for detecting small focal lesions. A wide spectrum of benign and malignant lesions other than HCC may be found in the cirrhotic liver and their differentiation is important to avoid errors in staging diseases that may preclude potentially curative therapies. Several published studies have explored the value of CEUS in liver cirrhosis and they have been shown to have excellent diagnostic and prognostic performances for the evaluation of non-invasive and efficient diagnosis of FLLs in patients at high risk for liver malignancies. The purpose of this article is to describe and discuss CEUS imaging findings of FLLs including HCC and ICC, all of which occur in cirrhotic livers with varying prevalence.
Core Tip: Contrast-enhanced ultrasound (CEUS) represents a breakthrough in the evaluation of focal liver lesions (FLLs). Currently, CEUS is included as a part of the suggested diagnostic work-up of FLLs in cirrhotic patients and in their follow-up for an accurate assessment of therapeutic response. After a brief description of the basis of different CEUS techniques, several liver lesions that can be found in the cirrhotic liver including benign, malignant or pseudo-lesions, will be described and discussed on the basis of our experience and literature data.
- Citation: Bartolotta TV, Randazzo A, Bruno E, Taibbi A. Focal liver lesions in cirrhosis: Role of contrast-enhanced ultrasonography. World J Radiol 2022; 14(4): 70-81
- URL: https://www.wjgnet.com/1949-8470/full/v14/i4/70.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i4.70
Liver cirrhosis represents the final stage of chronic inflammation through the establishment of necrosis and fibrogenesis up to a total subversion of the hepatic parenchyma and it has systemic repercussions and a fatal outcome in the absence of a liver transplant. Liver cirrhosis is the 14th most common cause of death worldwide[1].
Etiologically, liver cirrhosis recognizes infectious causes (hepatitis B, hepatitis C, schistosoma japonicum), autoimmune (primary biliary cirrhosis, autoimmune hepatitis, primary sclerosing cholangitis), alcohol abuse, metabolic causes (Wilson disease, hemochromatosis) and vascular or cryptogenic causes[2]. The combination of imaging and serological investigation (transaminases and cholestasis indices) is often sufficient for the diagnosis; however, the gold standard remains the liver biopsy which also allows physicians to identify the noxa that led to the stage of cirrhosis[1]. In the clinical setting, ultrasound (US) allows a morphological assessment of the liver and portal circulation. US also plays a major role as the recommended tool for surveillance every 6 mo at early detection of small hepatocellular carcinoma (HCC)[3].
Imaging characterization of focal lesions in cirrhosis is crucial for appropriate patient management[4,5]. To this end, US is a non-specific technique used to characterize focal liver lesions (FLLs).
At the end of the 1990s, the introduction of contrast agents based on intravenous microbubbles to contrast-specific gray-scale US techniques has enabled contrast-enhanced ultrasonography (CEUS) to represent macro-vascularity and also microcirculation ( vessels up to 40 μm). Starting in the 2000s, the advent of low-solubility gas bubbles (like sulfur hexafluoride) with phospholipid shells for their flexibility has led to a full real time CEUS examination[6].
CEUS, throughout the vascular phase with its blood-pool contrast agent, allows real-time recording with non-invasive assessment of liver perfusion without resorting to expensive and not very common equipment such as Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) that require the use of ionizing radiation or nephrotoxic contrast agents. OF note, when gas microbubbles are injected into the vein, they remain in the intravascular space (blood-pool agents), Only one of marketed contras agents shows a late phase with uptake by hepatic Kupffer cells (Table 1)[7].
| Agent | SonoVue | Sonazoid |
| Gas | Sulfurhexafluoride (SF6) | Perfluorobutane (C4F10) |
| Envelope | Monolayer of phospholipid (DSPC, DPPG-Na) | Monolayer of phospholipid (Hydrogenated egg phosphatidyl serine Na) |
| MI | Low MI (< 0.1) | Intermediate MI (> 0.2) |
| Mean size | 1.5-2.5 µm | 2.3-2.9 µm |
| Distribution after injection | Pure blood pool agent | Blood pool agent with uptake by hepatic Kupffer cells after 1 min by injection |
CEUS is safe and well tolerated: Renal or pulmonary diseases do not present contraindications for this use and no blood tests are needed to check kidney function. In a study of about 23000 patients, less than 0.01% of the patient population reported a serious adverse event with no death events[8].
Actually, CEUS is included in the diagnostic work-up of FLLs detected in the healthy population and to study metastases in patients with cancer and to identify HCC in cirrhotic patients, allowing for better management of the disease with effective and advantageous therapies[9-10]. A recent meta-analysis showed that specificity and sensitivity for CEUS in the characterization of FLLs were respectively 87% and 92%[10]. CEUS is gaining an increasing role in the imaging work-up of HCC and many international guidelines are now considering CEUS as a diagnostic tool for HCC as well as CT and MRI with encouraging results and is positive in terms of the cost-benefit analysis[11-12]. Based on literature data and our experience in our center, the recent innovations in the CEUS of FLLs in cirrhotic patients will be presented and discussed.
The US cases illustrated in this article are acquired through various ultrasound equipment provided with multifrequency convex array probes and contrast-specific imaging software: MyLab Twice (Esaote, Genova, Italy), RS80A and RS85A (Samsung Medison, Co. Ltd., Seoul, Korea) and iU22 unit (Philips Ultrasound, Bothell, WA, USA). Before the injection of bolus contrast, a standard exam together with color/power and pulsed Doppler valuation was always performed to optimize lesion images and define the best plane for its visualization. The contrast agent used was composed of gas microbubbles filled with sulfur hexafluoride (SonoVue, Bracco, Milan, Italy) that was injected using a 20- or 22-gauge needle in a cubital vein and a 2.4-mL bolus with a 5-10 ml of saline flush. Low mechanical index (MI) from 0.05 to 0.08 and low frame rate (5 Hz) were used for real-time imaging to avoid microbubble breakdown. The level of the lesion was the focus of examination and the duration of each exam was about 5 min after contrast agent injection.
The digital cine-loops were acquired before and after performing the contrast at different times in the arterial phase (from 10 s to 35 s after the injection), portal phase (from 55 s to 80 s after the injection) and delayed phase (from 235 s to 260 s after the injection).
Basal echogenicity and the dynamic modality of enhancement of each lesion in all vascular phases and among the near liver parenchyma were compared.
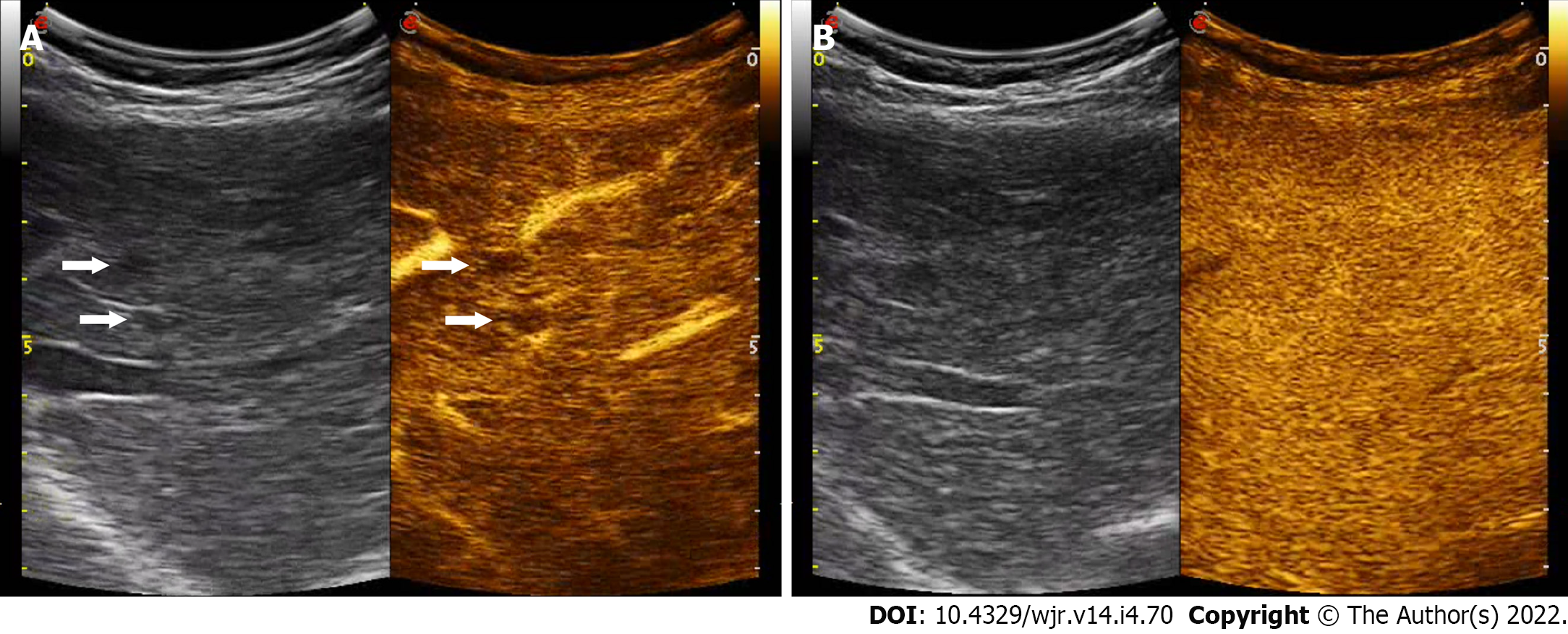
Liver cirrhosis has been recognized as a major risk factor for the onset of HCC and intrahepatic cholangiocarcinoma (ICC) compared to the non-cirrhotic population, of 30 and 20 times, respectively[13]. In the management of hepatic nodules in liver cirrhosis, early diagnosis and treatment is mandatory. HCC in liver cirrhosis develops as the last step of a complex, multi-step hepatocarcinogenesis process during several molecular and tissue alterations leading to the gradual transformation from regenerative nodule (RN) through low- and high-grade dysplastic nodule (DN) to HCC[14]. Changes of intranodular blood supply is the main transformation for imaging diagnosis: RN show similar blood supply to a normal liver. As a consequence, RNs are typically non-hypervascular. They can be seen as numerous tiny hypoechoic or hyperechoic nodules throughout the liver on grayscale US whereas at CEUS they usually are iso-enhancing to the adjacent liver parenchyma throughout the vascular phase, even if they may show transient hypo-vascularity in the arterial phase[4] (Figure 1).
DN are the next step towards HCC. Often multiple, DNs are classified as low or high grade according to the presence of cytological atypia. These borderline lesions show wide variations of blood supply with overlaps of vascular supply between DN and well-differentiated HCC, with the vast majority of RN and DN being isoechoic to the adjacent liver parenchyma in portal venous and late phase at CEUS[15].
Of note, in a study encompassing 215 FLLs in cirrhotic patients and comparing the CEUS features of RN and DN, 95.1% of RN lesions showed delayed or simultaneous enhancement in the arterial phase in comparison to surrounding liver parenchyma. On the other hand, DN lesions resembled this contrast-enhancement pattern only partially, due to the presence of intralesional areas of arterial enhancement followed by a wash out in the late phase. In pathology, these areas of arterial contrast-enhancement within the DN have proven to be early HCC[16]. Hence, any enhancement in the arterial phase within a nodule should be regarded as suspicious for HCC, resembling a “nodule in a nodule” appearance.
HCC is the fifth most common cancer in men and the ninth in women showing a greater incidence in developing countries where over 80% of all estimated new cases worldwide occurred in 2012[17].
Almost 90% of HCCs originate through a stepway progression from RN to HCC which may take place in a quite variable period, even though it may take only a few months[18]. On the other hand, the estimated doubling time of HCC ranges between 4 and 6 mo[19].
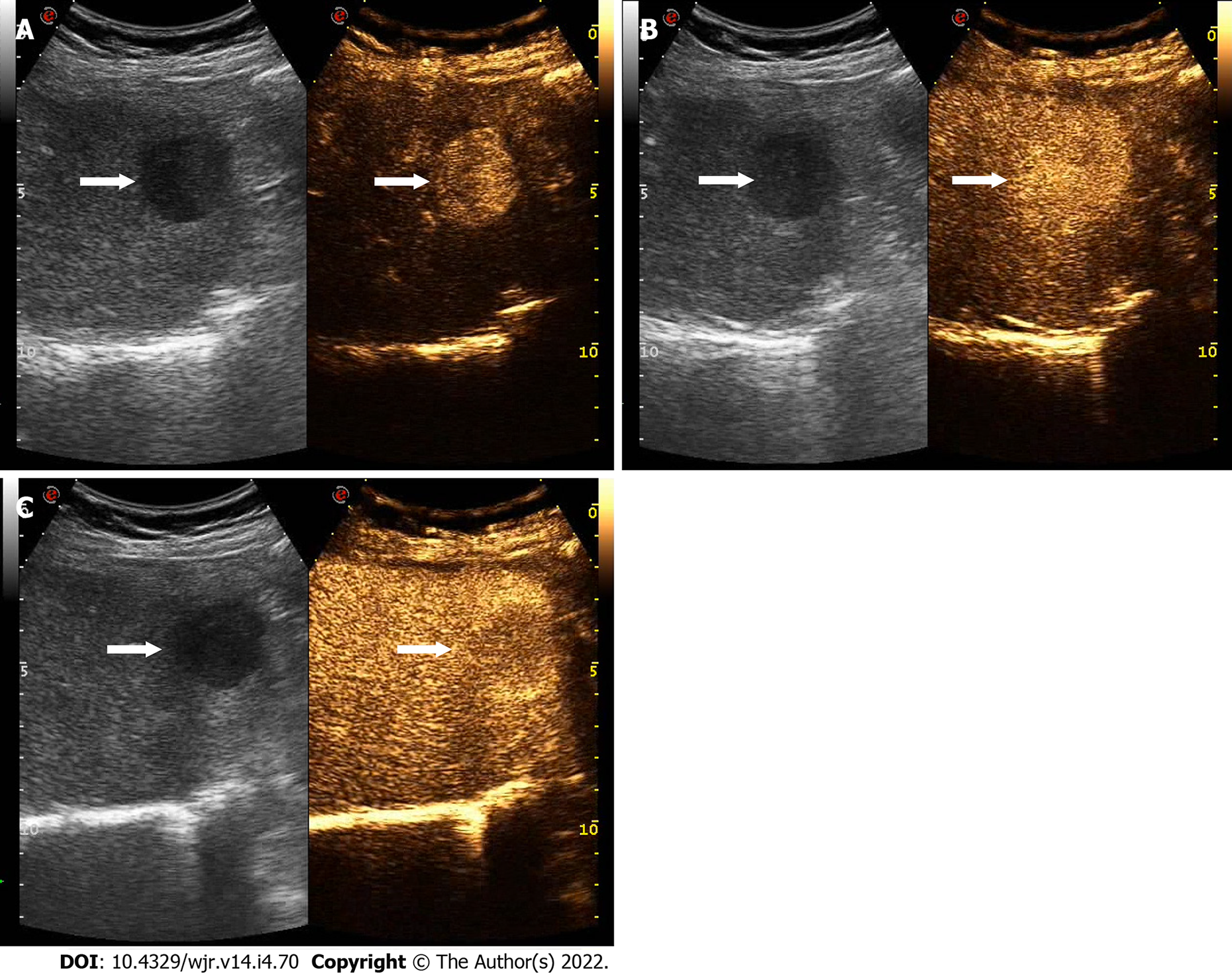
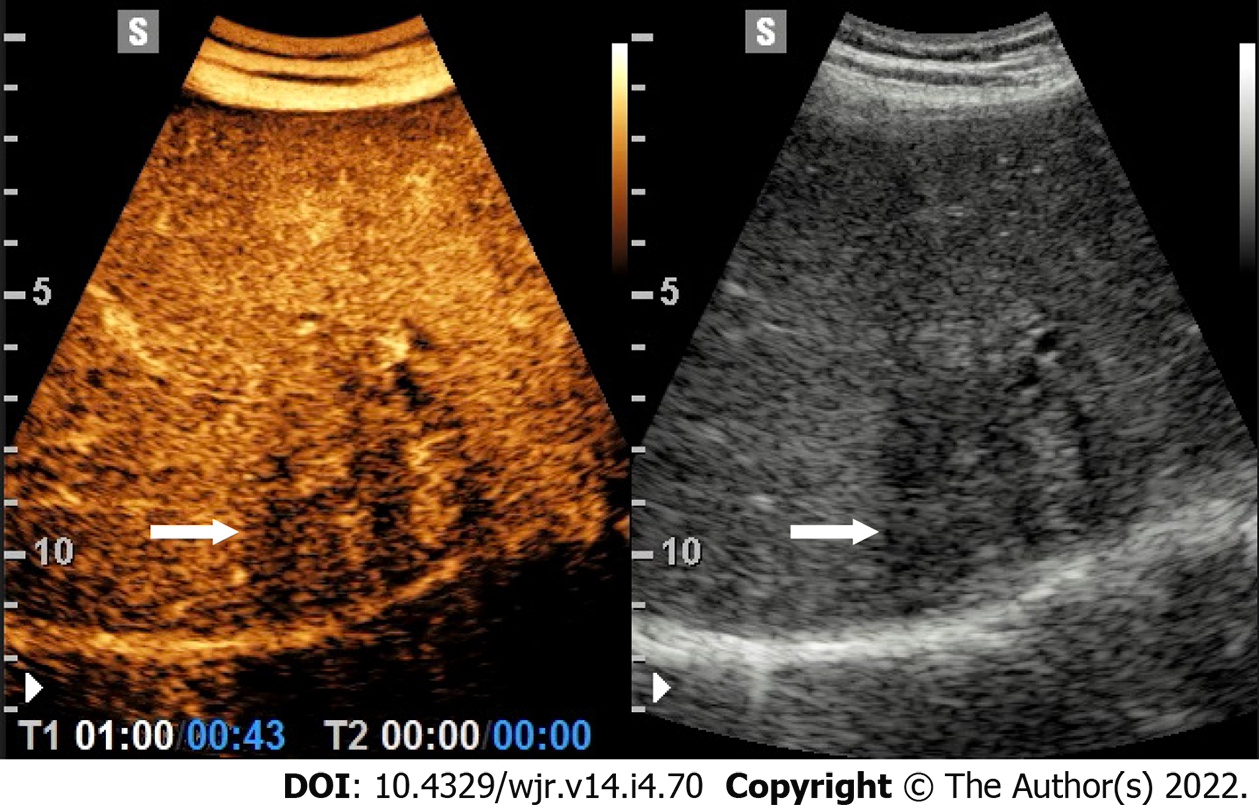
At CEUS, the typical enhancement pattern of HCC is hyperenhancement in the arterial phase followed by gradual and mild wash-out in the portal venous and/or late phases[10] (Figure 2). Washout is represented as a relatively hypoechoic aspect compared to healthy liver parenchyma in the later stages of the study with any type of contrast-enhancement in the arterial phase. In general, at CEUS, the presence of the wash-out sign is highly suggestive of malignancy. In HCC, washout begins over 60-90 s after injection of contrast agent, whereas metastases or intrahepatic cholangiocarcinoma usually show a rapid washout (< 60 s) (Table 2) (Figure 3)[20]. Therefore, in CEUS, an observation period of up to approximately 5 min is required to easily visualize the typically subtle and late (> 1 min) washout of HCC (Figure 2).
| Lesion | Arterial phase | Portal venous phase | Late phase | Post-vascular phase |
| RN | Hypo-enhance | Iso-enhance | Iso-enhance | Iso-enhance |
| DN | Hypo-enhance or partial hyper-enhanced within lesion (early-HCC) | Iso-enhance | Iso-enhance | Iso-enhance |
| HCC | Hyper-enhance | Hypo-enhance or iso-enhance | Hypo-enhance or iso-enhance1 | Hypo-enhance (mild and late washout) or iso-enhance1 |
| ICC | Rim-enhance or Hyper-enhance with early washout (< 60 seconds) | Hypo-enhance | Hypo-enhance | Hypo-enhance |
| Metastasis | Rim-enhance or Hyper-enhance with early washout (< 60 seconds) | Hypo-enhance | Hypo-enhance | Hypo-enhance |
Noteworthy, a study showed that arterial enhancement patterns of HCC at CEUS are related to the degree of histologic differentiation: moderately differentiated HCC exhibits a classic behavior after contrast agent injection compared to well-differentiated HCC. Extended observation in the portal phase is important for reporting late washout that in HCC occurs more frequently later than in the portal venous phase[21]. As a caveat, well-differentiated HCC may appear iso-enhancing in the portal-venous or late phase[9].
On the other hand, in a study by Tada et al[22], 63 of 68 (92.6%) small HCCs (< 3 cm in size) showed a mainly diffuse and homogeneous enhancement in the arterial-phase whereas large HCCs presented a heterogeneous arterial-phase enhancement pattern mainly related to non-enhancing areas of fibrosis, necrosis or internal hemorrhage.
In general, thanks to the real-time nature of CEUS, its high spatial and temporal resolution, the sensitivity of CEUS in the detection of hypervascularization of cirrhotic nodules was found to be higher compared to CT/MRI[23].
Overall, CEUS showed a sensitivity of 88.8%, a specificity of 89.2% and a PPV of 91.3% in the characterization of HCC[24].
Although it is still a matter of debate, several international guidelines are now endorsing the use of CEUS as a first or second-line diagnostic tool for the diagnosis of HCC[12,25]. In 2016, the American College of Radiology included CEUS in its comprehensive Liver Imaging Reporting and Data System (LI-RADS): a unique scoring system for CEUS examinations in patients with increased risk of HCC. A systematic review comparing the cost-effectiveness of CEUS with CT and MRI confirmed that CEUS is cost-effective in the surveillance of patients with liver cirrhosis[11].
Table 3 shows the main recommendations on the use of CEUS in cirrhotic patients according to the World Federation for Ultrasound in Medicine & Biology[26].
| Recommendations | Notes |
| Characterization FLLs found in patients with liver cirrhosis to establish a diagnosis of malignancy | CT or MR imaging is required for a complete staging |
| In nodules not suitable for biopsy | When CT or MR are inconclusive |
| Selection of FLLs with different contrast pattern in a cirrhotic liver to be biopsied | |
| Monitoring changes in enhancement patterns in FLLs in cirrhotic liver requiring follow-up | |
| Guiding percutaneous biopsies to increase the diagnostic outcome | To compare to B-mode US |
CEUS has shown high sensitivity for the evaluation of portal vein patency and in the differential diagnosis between benign and malignant portal vein thrombosis, this latter occurring in cirrhotic patients at various stages[27]. A thrombus showing hypervascularity in the arterial phase, irrespective of the presence of subsequent washout, is deemed to be malignant[10].
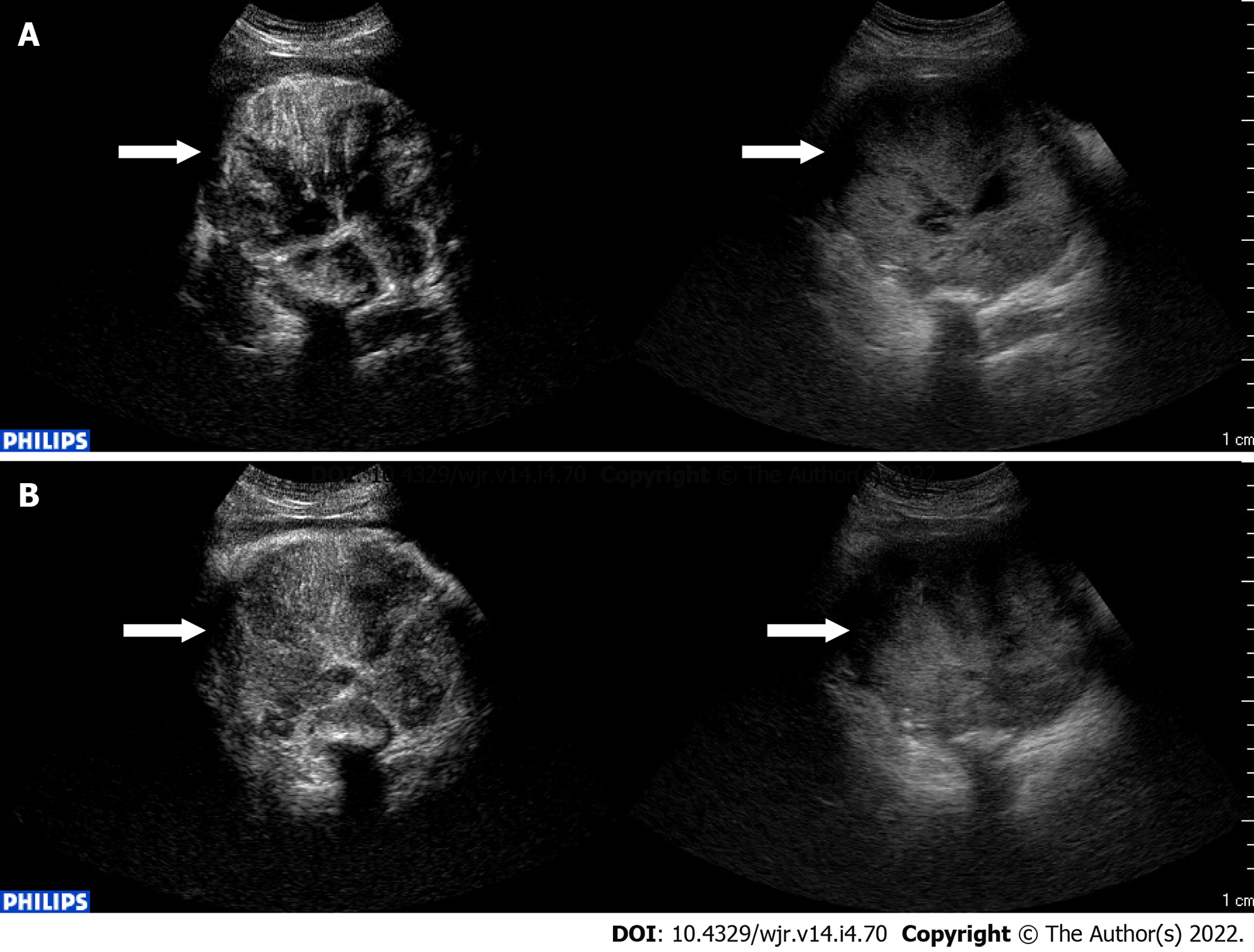
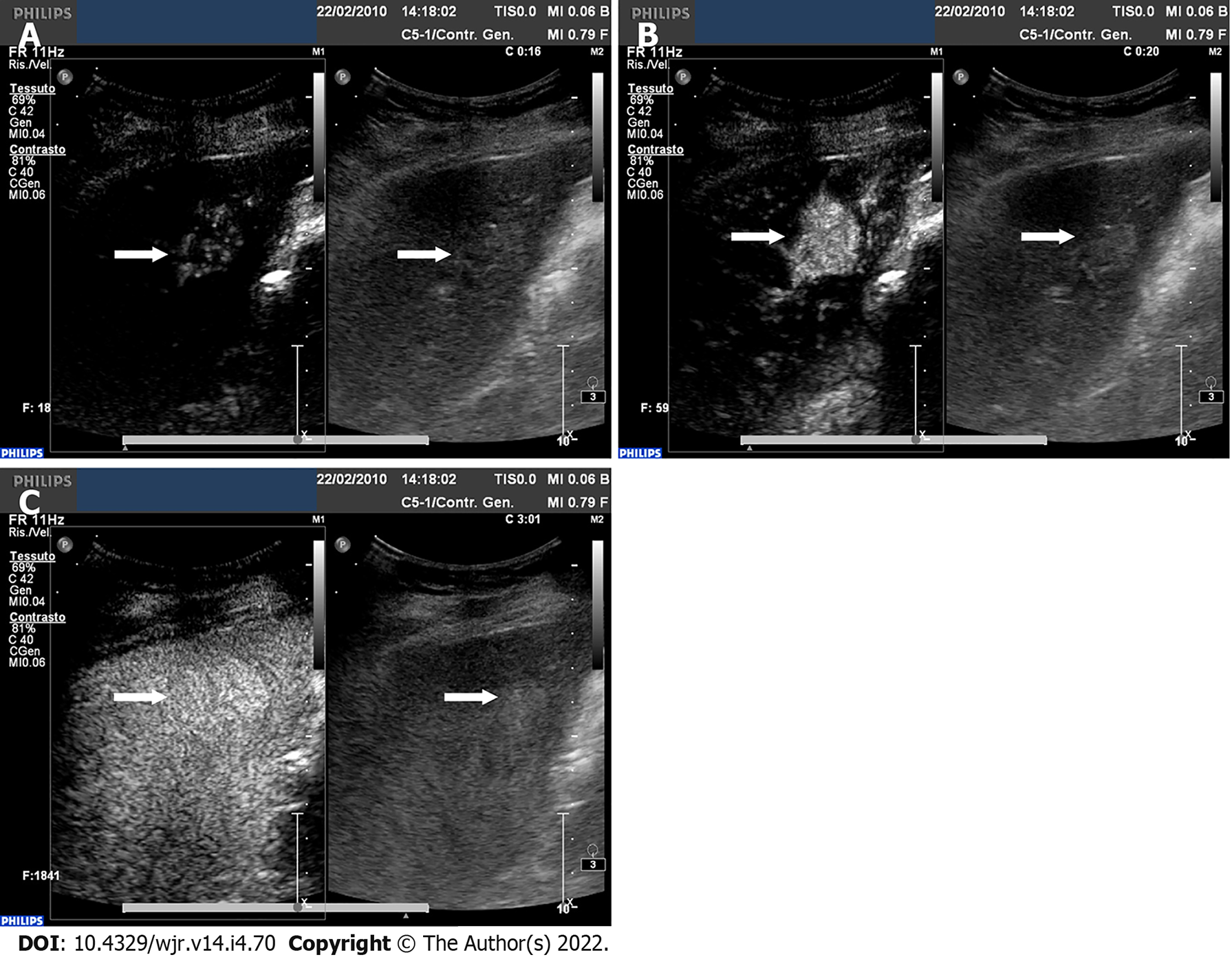
CEUS can also be used with valid results in guidance, response and detection of complications of interventional procedures[28] (Figure 4). CEUS may be of help during or after the interventional procedure[29]. Intraprocedural use of CEUS showed a relevant clinical impact, reducing the number of re-treatments and the related costs per patient[30].
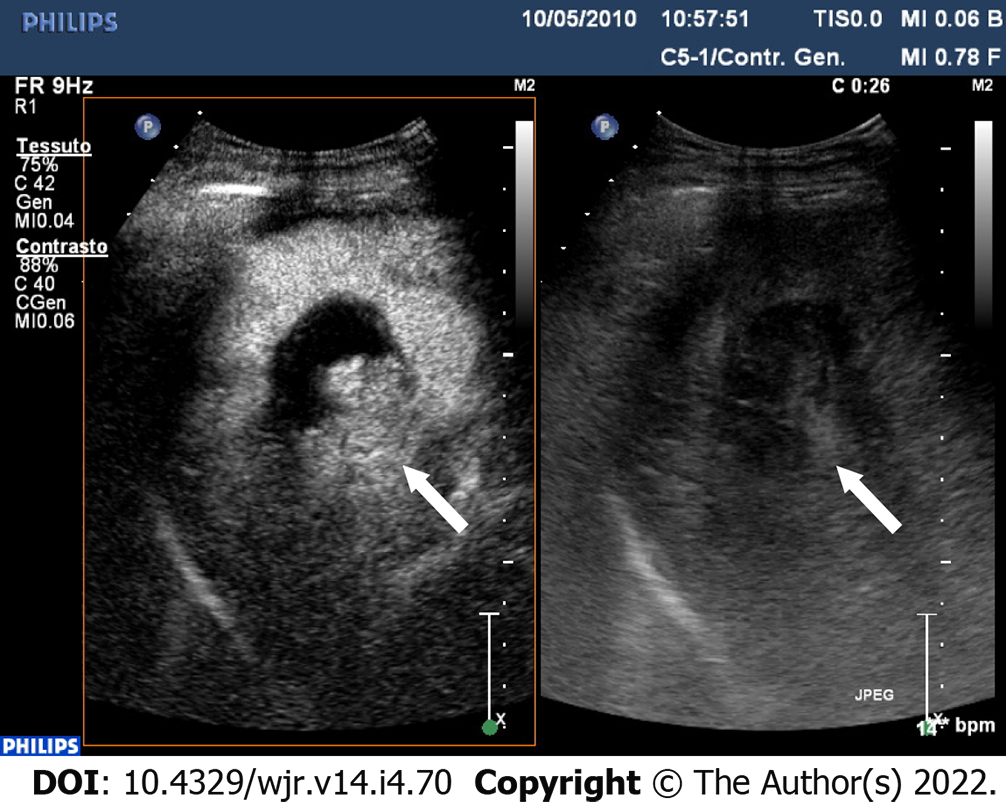
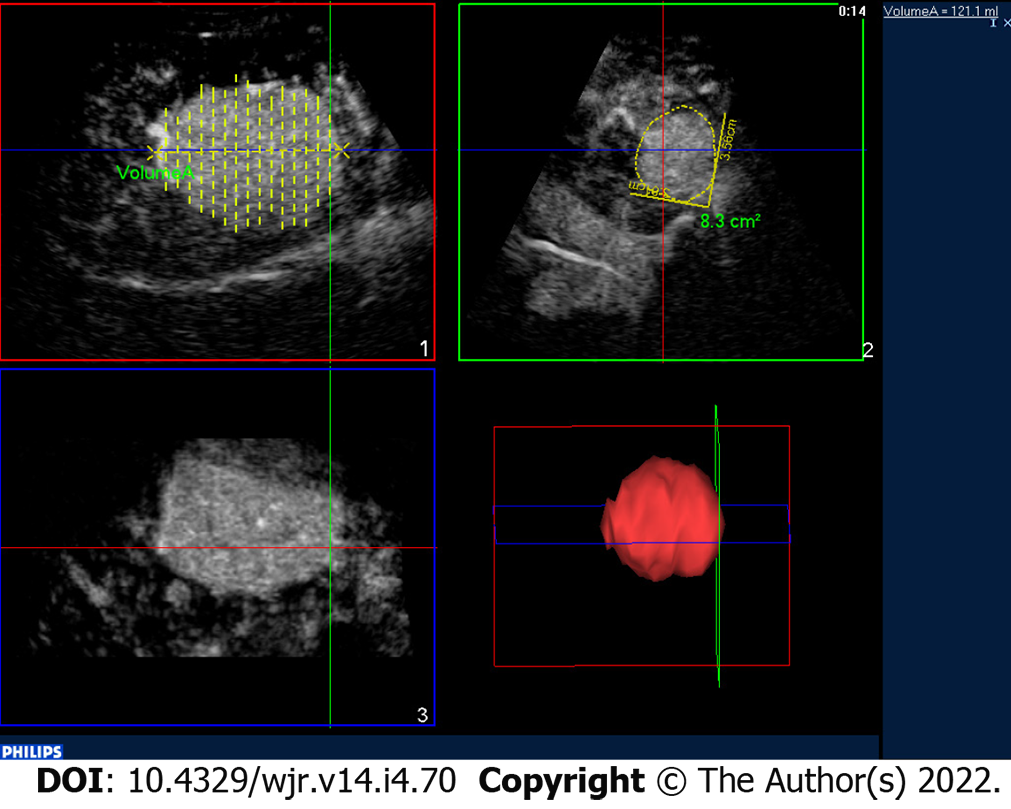
The three-dimensional evaluation through the CEUS of the tumor lesion allows more accurate planning and the treatment with locoregional therapies[31,32] (Figure 5).
Intrahepatic peripheral cholangiocarcinoma (ICC) constitutes the second most common primary liver malignant tumor in cirrhotic patients and accounts for 1%–3% of newly developed tumors[32,33]. Differentiating ICC from HCC is of clinical relevance since liver transplantation is contraindicated in patients with ICC given poorly reported outcomes[34].
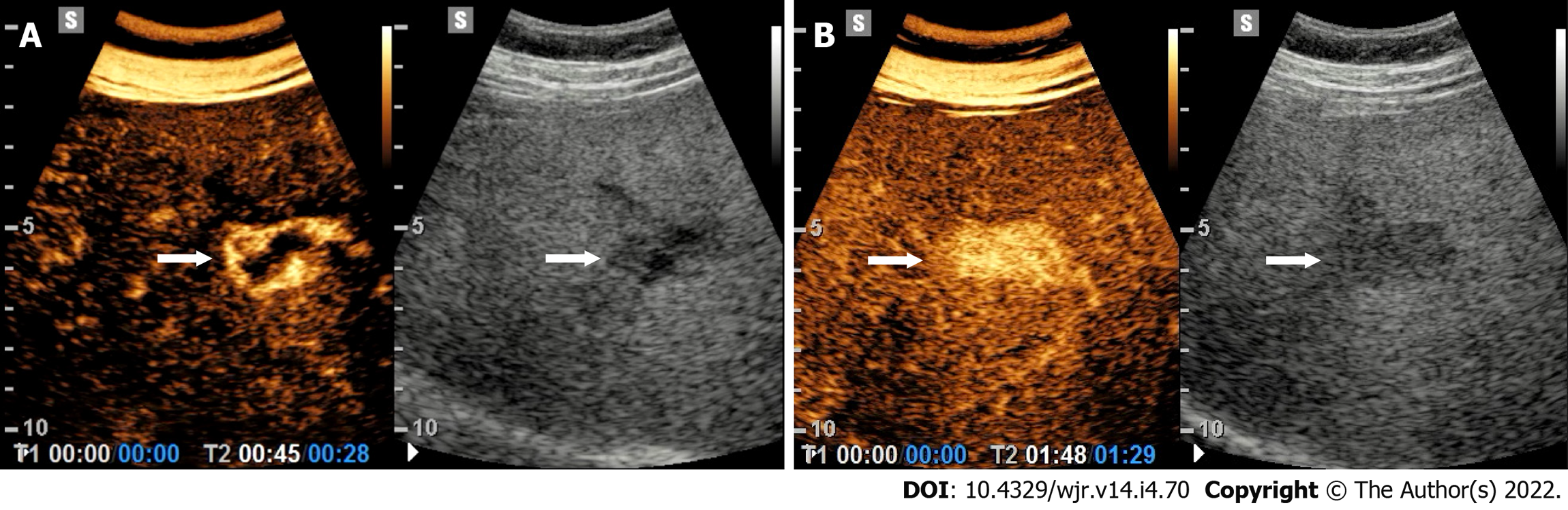
At CEUS, ICC shows heterogeneous contrast enhancement in the arterial phase with a substantially hypoechoic appearance in the extended portal-venous phase[35]. A rim-like contrast-enhancement has been reported but with a quite variable range (8-51% of cases)[9]. The presence and the quantity of fibrotic tissue and necrotic areas may strongly influence the CEUS appearance of ICC. This latter may present at CEUS overlapping features with HCC[36]. At CEUS, a clue suggestive for ICC is the presence of a wash out occurring earlier than 60 s, whereas HCC usually washes out later on (Figure 6)[37,38]. The same temporal difference in wash-out between HCC and other malignancies, including ICC, is also used by the CEUS LI-RADS lexicon for the diagnosis of ICC[10].
In a multicenter study of 1,006 nodules from 848 patients, the use of CEUS LI-RADS criteria for HCC - namely, arterial phase hyperenhancement and late washout (onset ≥ 60 s after contrast injection) of mild degree - was 98.5% predictive of HCC with no risk of misdiagnosis for pure cholangiocarcinoma[39]. To this purpose, contrast-enhanced CT and MRI may provide useful information due to the different contrast agent kinetic. Microbubbles are essentially blood pool agents and remain confined to the vascular space, whereas iodinated contrast agent and gadolinium chelates are essentially extra-cellular contrast agents and progressively accumulate in the fibrotic spaces of ICC[39].
The presence of both ICC and HCC components in the same lesion can make the lesion even more difficult and biopsy may be eventually needed in equivocal cases.
Metastatic liver deposits are relatively uncommon in the cirrhotic liver. This finding may probably be due to alteration of hemodynamics and the microstructural environment in the liver[40]. In particular, the hepatofugal portal venous flow may prevent neoplastic cells from seeding and flourishing in the liver[41]. Liver metastases from colorectal carcinoma are infrequently reported to spread to the cirrhotic liver[42]. Metastases from non-Hodgkin B-cell lymphoma may also involve the liver in patients with hepatitis C virus and typically consist of multiple small nodules[43].
On CEUS, liver metastases show a sharp and early washout within 60 s of contrast administration, irrespective of the contrast enhancement type in the arterial phase (Figure 3)[44]. This latter may present various patterns, such as rim-like, dotted, heterogeneous or even homogeneous, depending on the size and the grade of cellularity, vascularity, fibrosis and necrosis accompanying the development of the lesion.
A wide spectrum of benign lesions may arise in a cirrhotic liver. Hence, it is crucial to avoid the misdiagnosis of benign liver lesions as HCC (i.e. minimize false positives) because this diagnostic interpretation may incorrectly increase the tumor burden[43].
Generally, at CEUS, a benign lesion presents a progressive and sustained enhancement in all phases of the study[45] (Table 4, Figures 7 and 8). Although tumor lesions may have similar characteristics, a clinical context of oncological or cirrhotic pathology allows differentiating the nature of the lesions[21]. Further aspects that are decisive for the diagnosis are detected by observing the arterial phase[4].
| Lesion | Arterial phase | Portal venous phase | Late phase | Post-vascular phase |
| Hepatic cysts | Non-enhance | Non-enhance | Non-enhance | Non-enhance |
| Cystic hydatid disease | Non-enhance cysts and septa | Non-enhance cysts and septa | Non-enhance cysts and septa | Non-enhance cysts and septa |
| Abscess | Rim-enhance with enhanced septa; no central enhancement | Rim-enhance with enhanced septa; no central enhancement | Hypo-enhance rim; no central enhancement | Hypo-enhance rim; no central enhancement |
| Hemangioma | Peripheral, discontinuous and globular hyper-enhance | Peripheral, globular iso enhance and fill-in | Iso-enhance or hypo-enhance | Iso-enhance or hypo-enhance |
| FNH | Hyper-enhance from the center to peripheral region spoke-wheel vascularity | Hyper-enhance with/without un-enhanced central scar | Iso-enhance or hyper-enhance with/without un-enhanced central scar | Iso-enhance or hypo-enhance |
| HA | Hyper-enhance | Iso-enhance | Iso-enhance | Iso-enhance or hypo-enhance |
| Pseudo lesions | Hyper-enhance | Hyper-enhance or iso-enhance | Iso-enhance | Iso-enhance |
Hemangioma is seen less frequently in cirrhotic patients than in the general population. In general, imaging features remain similar to those of hemangiomas observed in non-cirrhotic patients[46].
At CEUS, hemangioma has a characteristic globular, progressive, peripheral and discontinuous enhancement (Figure 7). However, with progressive cirrhosis, hemangiomas are likely to decrease in size, become more fibrotic and may appear as a hypo vascular lesion with a lack of peripheral globular contrast-enhancement[47,48]. Furthermore, flash filling hemangiomas may pose a diagnostic dilemma with well-differentiated HCC not showing wash-out, thus needing further radiological workup with CT or MRI for the final diagnosis.
Although Focal nodular hyperplasia (FNH) is the second most common benign liver tumor after hemangioma, the report of FNH-like nodules in the cirrhotic liver is only sporadic and imaging appearance is similar to FNH arising in the non-cirrhotic liver[43,49].
At CEUS, the typical findings of FNH are a centrifugal contrast-enhancement pattern with a spoke-wheel appearance in the arterial phase followed by sustained contrast-enhancement and iso or hyperechoic appearance in portal-venous and late phase[50] (Figure 8). A central avascular area in the arterial phase is often appreciable in FNH larger than 3 cm with a hypoechoic appearance.
The incidence of hepatocellular adenoma (HA) in the cirrhotic liver is exceedingly rare with a few reports in the literature[51].
At CEUS, a peripheral enhancement with centripetal filling and sustained hypervascularization, suggests the diagnosis of HA[10,52]. However, as a warning, HA may show a hypoechoic appearance in the portal-venous and late phase[52].
Simple biliary and peribiliary cysts have similar features in cirrhotic and noncirrhotic livers. They present a homogenous anechoic appearance, a very thin wall and through transmission with posterior acoustic enhancement and no contrast enhancement at CEUS[43]. CEUS may be a problem-solving technique in diagnosing complicated non-anechoic cyst or a rare form of Co-existence of hepatocellular carcinoma and cystic echinococcosis[53]. Usually, CEUS shows a lack of enhancement of septa separating daughter cysts[54].
Hepatic abscesses, pyogenic, fungal and amebic have similar CEUS features in cirrhotic and non-cirrhotic livers. Abscesses do not have a significant internal enhancement after contrast ultrasound administration but septations within the lesion may enhance as well as an irregular peripheral rim[55].
Focal fatty changes or confluent hepatic fibrosis can mimic malignancies. Focal fatty changes are an increase or decrease in fat content in a focal area of the liver parenchyma owing to an aberrant portal-venous vascularization[55].
Confluent hepatic fibrosis is usually shown in patients with alcohol-related cirrhosis. It involves peripheral parenchymal replacement by thick fibrotic bands that appear as focal wedge-shaped areas with thick fibrotic bands causing retraction of the overlying capsule; the presence of inflammation can lead to inhomogeneous arterial phase hyperenhancement[40].
At CEUS, these pseudo lesions present isoenhanced in comparison with the surrounding liver parenchyma during the extended portal-venous phase[55], furthermore, fibrosis is usually seen in a typical position (medial segment of the left lobe or anterior segment of the right lobe)[40].
A wide spectrum of benign and malignant lesions other than HCC may be found in the cirrhotic liver. More than several years after its release, CEUS is being used for safe diagnostic imaging which enables real-time recognition of enhancement characteristics of focal liver lesions arising in cirrhotic patients. Currently, CEUS is increasingly being performed on a routine basis and is included as a part of the recommended diagnostic work-up of HCC as well as in the follow-up.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ren J, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1310] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 2. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 907] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 3. | Taibbi A, Petta S, Matranga D, Caruana G, Cannella R, Busè G, Marco VD, Midiri M, Bartolotta TV. Liver stiffness quantification in biopsyproven nonalcoholic fatty liver disease patients using shear wave elastography in comparison with transient elastography. Ultrasonography. 2020;40:407-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Ronot M, Dioguardi Burgio M, Purcell Y, Pommier R, Brancatelli G, Vilgrain V. Focal lesions in cirrhosis: Not always HCC. Eur J Radiol. 2017;93:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kim MJ, Lee S, An C. Problematic lesions in cirrhotic liver mimicking hepatocellular carcinoma. Eur Radiol. 2019;29:5101-5110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Barr RG, Huang P, Luo Y, Xie X, Zheng R, Yan K, Jing X, Xu H, Fei X, Lee JM. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical evidence for SonoVue and Sonazoid. Abdom Radiol (NY). 2020;45:3779-3788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Piscaglia F, Bolondi L; Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 532] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Durot I, Wilson SR, Willmann JK. Contrast-enhanced ultrasound of malignant liver lesions. Abdom Radiol (NY). 2018;43:819-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Bartolotta TV, Vernuccio F, Taibbi A, Lagalla R. Contrast-Enhanced Ultrasound in Focal Liver Lesions: Where Do We Stand? Semin Ultrasound CT MR. 2016;37:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, Gloy V, Raatz H, Misso K, Severens J, Kleijnen J. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17:1-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Cassinotto C, Aubé C, Dohan A. Diagnosis of hepatocellular carcinoma: An update on international guidelines. Diagn Interv Imaging. 2017;98:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3591] [Article Influence: 276.2] [Reference Citation Analysis (4)] |
| 14. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 15. | Kim TK, Lee KH, Khalili K, Jang HJ. Hepatocellular nodules in liver cirrhosis: contrast-enhanced ultrasound. Abdom Imaging. 2011;36:244-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Wu W, Chen M, Yan K, Dai Y, Yin S, Yang W, Fan Z. Evaluation of contrast-enhanced ultrasound for diagnosis of dysplastic nodules with a focus of hepatocellular carcinoma in liver cirrhosis patients. Chin J Cancer Res. 2015;27:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20496] [Article Influence: 2049.6] [Reference Citation Analysis (20)] |
| 18. | Sato T, Kondo F, Ebara M, Sugiura N, Okabe S, Sunaga M, Yoshikawa M, Suzuki E, Ogasawara S, Shinozaki Y, Ooka Y, Chiba T, Kanai F, Kishimoto T, Nakatani Y, Fukusato T, Yokosuka O. Natural history of large regenerative nodules and dysplastic nodules in liver cirrhosis: 28-year follow-up study. Hepatol Int. 2015;9:330-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | An C, Choi YA, Choi D, Paik YH, Ahn SH, Kim MJ, Paik SW, Han KH, Park MS. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol. 2015;21:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Bhayana D, Kim TK, Jang HJ, Burns PN, Wilson SR. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Kaneoka Y, Maeda A, Okuda S, Otobe K, Takahashi K. Utility of Contrast-enhanced Ultrasonography with Perflubutane for Determining Histologic Grade in Hepatocellular Carcinoma. Ultrasound Med Biol. 2015;41:3070-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Maruyama H, Takahashi M, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound for characterization of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br J Radiol. 2012;85:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Zheng SG, Xu HX, Liu LN. Management of hepatocellular carcinoma: The role of contrast-enhanced ultrasound. World J Radiol. 2014;6:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Kang HJ, Lee JM, Yoon JH, Han JK. Role of Contrast-Enhanced Ultrasound as a Second-Line Diagnostic Modality in Noninvasive Diagnostic Algorithms for Hepatocellular Carcinoma. Korean J Radiol. 2021;22:354-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D'Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 27. | Tarantino L, Ambrosino P, Di Minno MN. Contrast-enhanced ultrasound in differentiating malignant from benign portal vein thrombosis in hepatocellular carcinoma. World J Gastroenterol. 2015;21:9457-9460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Francica G, Meloni MF, Riccardi L, Giangregorio F, Caturelli E, Terracciano F, de Sio I. Role of Contrast-Enhanced Ultrasound in the Detection of Complications After Ultrasound-Guided Liver Interventional Procedures. J Ultrasound Med. 2020;40:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Mauri G, Porazzi E, Cova L, Restelli U, Tondolo T, Bonfanti M, Cerri A, Ierace T, Croce D, Solbiati L. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Bartolotta TV, Taibbi A, Matranga D, Midiri M, Lagalla R. 3D vs 2D contrast-enhanced sonography in the evaluation of therapeutic response of hepatocellular carcinoma after locoregional therapies: preliminary findings. Radiol Med. 2015;120:695-704. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Rimola J, Forner A, Tremosini S, Reig M, Vilana R, Bianchi L, Rodríguez-Lope C, Solé M, Ayuso C, Bruix J. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol. 2012;56:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Sersté T, Barrau V, Ozenne V, Vullierme MP, Bedossa P, Farges O, Valla DC, Vilgrain V, Paradis V, Degos F. Accuracy and disagreement of computed tomography and magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma and dysplastic nodules: role of biopsy. Hepatology. 2012;55:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Sapisochín G, Fernández de Sevilla E, Echeverri J, Charco R. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol. 2015;7:2396-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Loria F, Loria G, Basile S, Crea G, Frosina L, Di Carlo I. Contrast-enhanced ultrasound appearances of enhancement patterns of intrahepatic cholangiocarcinoma: correlation with pathological findings. Updates Surg. 2014;66:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Vilana R, Forner A, Bianchi L, García-Criado A, Rimola J, de Lope CR, Reig M, Ayuso C, Brú C, Bruix J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 37. | Li R, Yuan MX, Ma KS, Li XW, Tang CL, Zhang XH, Guo DY, Yan XC. Detailed analysis of temporal features on contrast enhanced ultrasound may help differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS One. 2014;9:e98612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Wildner D, Bernatik T, Greis C, Seitz K, Neurath MF, Strobel D. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med. 2015;36:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F; CEUS LI-RADS Italy study group collaborators:. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol. 2018;68:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 40. | Elsayes KM, Chernyak V, Morshid AI, Tang A, Kielar AZ, Bashir MR, Sirlin CB. The spectrum of Pitfalls, Pseudolesions, and Potential Misdiagnoses in Cirrhosis. AJR Am J Roentgenol. 2018;211:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Seymour K, Charnley RM. Evidence that metastasis is less common in cirrhotic than normal liver: a systematic review of post-mortem case-control studies. Br J Surg. 1999;86:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Gervaz P, Pak-art R, Nivatvongs S, Wolff BG, Larson D, Ringel S. Colorectal adenocarcinoma in cirrhotic patients. J Am Coll Surg. 2003;196:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Galia M, Taibbi A, Marin D, Furlan A, Dioguardi Burgio M, Agnello F, Cabibbo G, Van Beers BE, Bartolotta TV, Midiri M, Lagalla R, Brancatelli G. Focal lesions in cirrhotic liver: what else beyond hepatocellular carcinoma? Diagn Interv Radiol. 2014;20:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Bartolotta TV, Taibbi A, Picone D, Anastasi A, Midiri M, Lagalla R. Detection of liver metastases in cancer patients with geographic fatty infiltration of the liver: the added value of contrast-enhanced sonography. Ultrasonography. 2017;36:160-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Zarzour JG, Porter KK, Tchelepi H, Robbin ML. Contrast-enhanced ultrasound of benign liver lesions. Abdom Radiol (NY). 2018;43:848-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 46. | Duran R, Ronot M, Di Renzo S, Gregoli B, Van Beers BE, Vilgrain V. Is magnetic resonance imaging of hepatic hemangioma any different in liver fibrosis and cirrhosis compared to normal liver? Eur J Radiol. 2015;84:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Brancatelli G, Federle MP, Blachar A, Grazioli L. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology. 2001;219:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Wu XF, Bai XM, Yang W, Sun Y, Wang H, Wu W, Chen MH, Yan K. Differentiation of atypical hepatic hemangioma from liver metastases: Diagnostic performance of a novel type of color contrast enhanced ultrasound. World J Gastroenterol. 2020;26:960-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Lee YH, Kim SH, Cho MY, Shim KY, Kim MS. Focal nodular hyperplasia-like nodules in alcoholic liver cirrhosis: radiologic-pathologic correlation. AJR Am J Roentgenol. 2007;188:W459-W463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Giambelluca D, Taibbi A, Midiri M, Bartolotta TV. The "spoke wheel" sign in hepatic focal nodular hyperplasia. Abdom Radiol (NY). 2019;44:1183-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Seo JM, Lee SJ, Kim SH, Park CK, Ha SY. Hepatocellular carcinoma arising from hepatocellular adenoma in a hepatitis B virus-associated cirrhotic liver. Clin Radiol. 2012;67:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Garcovich M, Faccia M, Meloni F, Bertolini E, de Sio I, Calabria G, Francica G, Vidili G, Riccardi L, Zocco MA, Ainora ME, Ponziani FR, De Gaetano AM, Gasbarrini A, Rapaccini GL, Pompili M. Contrast-enhanced ultrasound patterns of hepatocellular adenoma: an Italian multicenter experience. J Ultrasound. 2019;22:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Bo R, Yasen A, Shao Y, Zhang W, Lin R, Jiang T, Wen H, Xiao H, Aji T. Co-existence of hepatocellular carcinoma and cystic echinococcosis. Infect Agent Cancer. 2020;15:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Pakala T, Molina M, Wu GY. Hepatic Echinococcal Cysts: A Review. J Clin Transl Hepatol. 2016;4:39-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 55. | Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4021] [Cited by in RCA: 4267] [Article Influence: 474.1] [Reference Citation Analysis (0)] |