Published online Sep 28, 2021. doi: 10.4329/wjr.v13.i9.307
Peer-review started: March 30, 2021
First decision: June 7, 2021
Revised: June 22, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: September 28, 2021
Processing time: 179 Days and 22.4 Hours
Symptomatic neonatal subdural hematomas usually result from head trauma incurred during vaginal delivery, most commonly during instrument assistance. Symptomatic subdural hematomas are rare in C-section deliveries that were not preceded by assisted delivery techniques. Although the literature is inconclusive, another possible cause of subdural hematomas is therapeutic hypothermia.
We present a case of a term neonate who underwent therapeutic whole-body cooling for hypoxic ischemic encephalopathy following an emergent C-section delivery for prolonged decelerations. Head ultrasound on day of life 3 demon
The aim of this report is to highlight the rarity and importance of mass-like subdural hematomas causing obstructive hydrocephalus, particularly in the setting of hypoxic ischemic encephalopathy and therapeutic whole-body cooling.
Core Tip: Screening head ultrasound during hypothermia protocols for hypoxic ischemic encephalopathy (HIE) warrant scrutiny for hemorrhage in unexpected locations. Symptomatic subdural hematomas warrant a high degree of clinical suspicion, particularly due to their rarity in children delivered by C-section. This report highlights the emerging association of HIE, therapeutic hypothermia, and perinatal intracranial hemorrhage. Prompt imaging and neurosurgical intervention may relieve hemorrhage induced obstructive hydrocephalus during therapeutic cooling with good neurological outcomes, preventing need for permanent cerebrospinal fluid diversion. Familiarity with the key imaging characteristics and clinical exam features of mass-like subdural hematomas can help the treatment team consider the diagnosis, and potentially enable a prompt recovery.
- Citation: Rousslang LK, Rooks EA, Meldrum JT, Hooten KG, Wood JR. Neonatal infratentorial subdural hematoma contributing to obstructive hydrocephalus in the setting of therapeutic cooling: A case report. World J Radiol 2021; 13(9): 307-313
- URL: https://www.wjgnet.com/1949-8470/full/v13/i9/307.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i9.307
Perinatal symptomatic subdural hematomas (SDH) are rare. They most commonly occur in the posterior fossa and are classically thought to result from venous disruption caused by birth trauma[1,2]. Although there are case reports of neonatal SDH after spontaneous vaginal delivery, or in-utero, it is still rare to observe a symptomatic SDH following an atraumatic C-section[3,4]. Hypoxic ischemic encephalopathy (HIE) has recently emerged as a potential cause of SDH, but the evidence is unclear and debated, with much of it based on autopsy[5-8]. Therapeutic hypothermia also appears to contribute to SDH, and whole-body cooling has been shown to impair hemostasis in vivo[9]. Additionally, Wang et al[10] recently reported a case of therapeutic cooling that is thought to have led to a massive SDH.
To the best of our knowledge, this is the first case in the literature to date of a neonate who developed a mass-like subdural hemorrhage of the posterior fossa while undergoing whole-body cooling causing obstructive hydrocephalus, following a non-traumatic C-section delivery.
A boy was born at 38 wk and 5 d to a gravida 3, aborta 2 mother via emergent C-section for prolonged decelerations and arrest of descent which was thought to be related to maternal difficulty in coordinating pushing efforts with contractions while receiving epidural anesthesia. The decelerations did not respond to changes in maternal positioning, or administration of supplemental oxygen and intravenous fluids. The mother had no pre-existing conditions, and was up to date with all vaccinations. His prenatal course was completely normal, including a 20-wk anatomy scan demonstrating normal brain imaging. Thick meconium was present at delivery, which was otherwise uncomplicated.
His birth weight was 4.0 kg, with APGAR scores of 11, 35, 410, and 615. At birth he was apneic, with a heart rate < 60, requiring chest compressions and intubation. Shortly after intubation he developed pulmonary hemorrhage and acute hypoxemic respiratory failure that responded well to endotracheal epinephrine. His respiratory issues were thought to be caused by the aspiration of the thick meconium.
Immediately after birth, his international normalized ratio (INR) was 2.3, with prothrombin time of 25.6 s, activated partial thromboplastin time of 65 s, and platelets of 81 × 103 platelets/uL. To correct his coagulopathy, he was given platelets, cryoprecipitate, and fresh frozen plasma for hemostasis, with downtrend in INR to 1.0 and uptrend in platelets to normal levels (> 150 × 103 platelets/uL) over the next four days.
Head ultrasound (HUS) on the first day of life (DOL) demonstrated left grade 1 germinal matrix hemorrhage, but no other intra-cranial hemorrhage. The patient was then started on whole-body cooling for HIE.
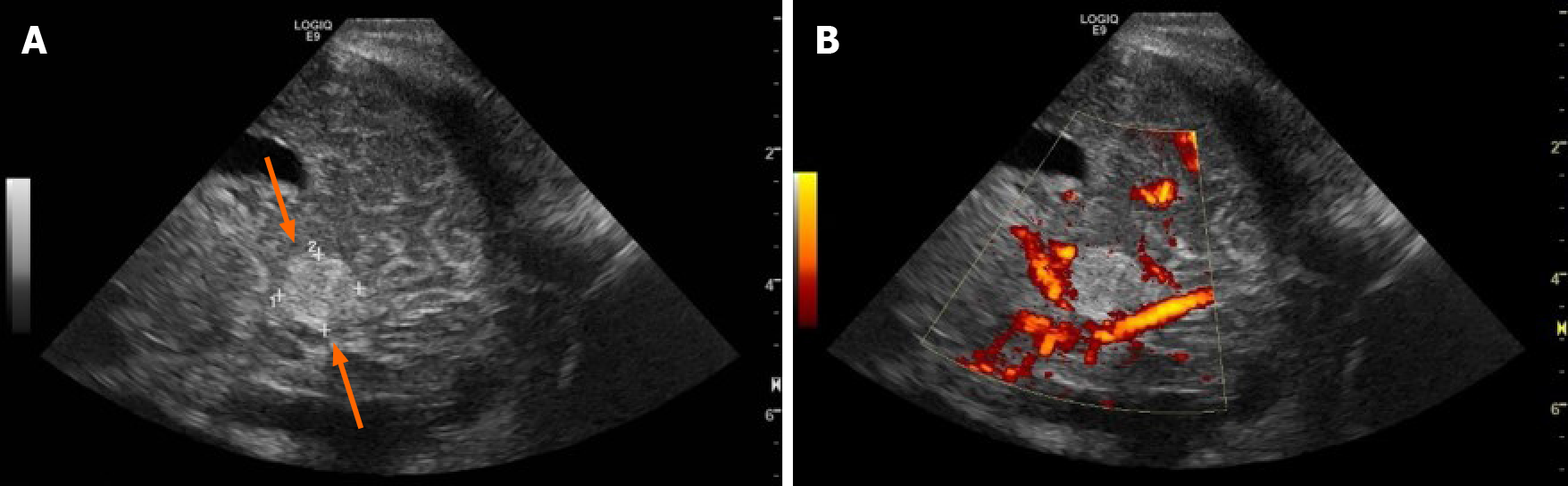
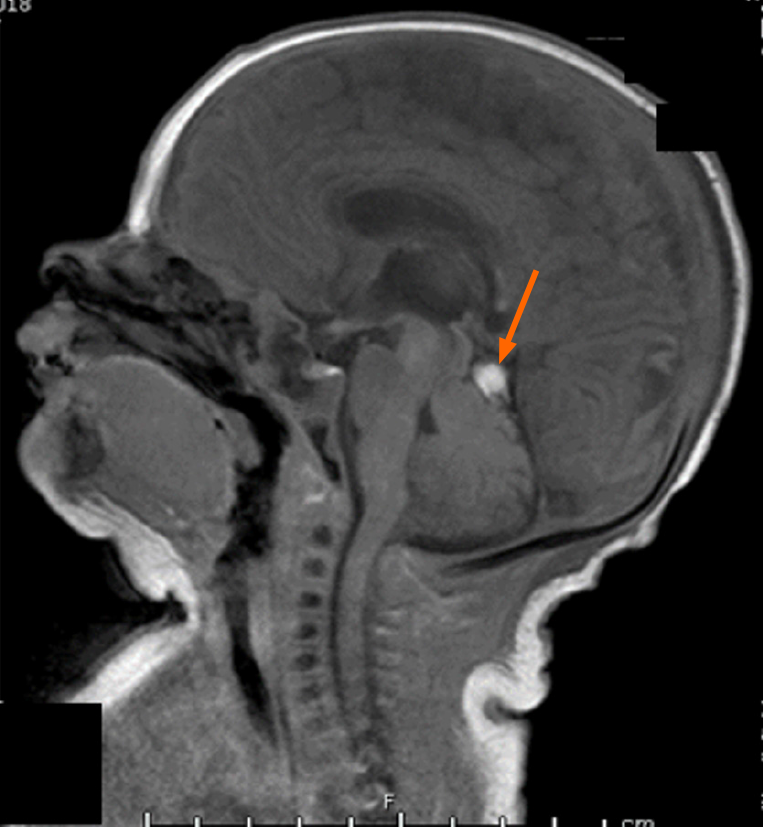
On his fourth day of whole-body cooling, the patient was found to have an increasing head circumference, increasing fontanelle size and fullness, and apneic events, suggestive of obstructive hydrocephalus. His exam further revealed a poor gag reflex and diminished response to stimuli with decreased spontaneous movement. Head ultrasound demonstrated a newly visualized mass in the infratentorial region, thought to represent a cerebellar or tentorial hemorrhage (Figure 1) and the patient was re-warmed.
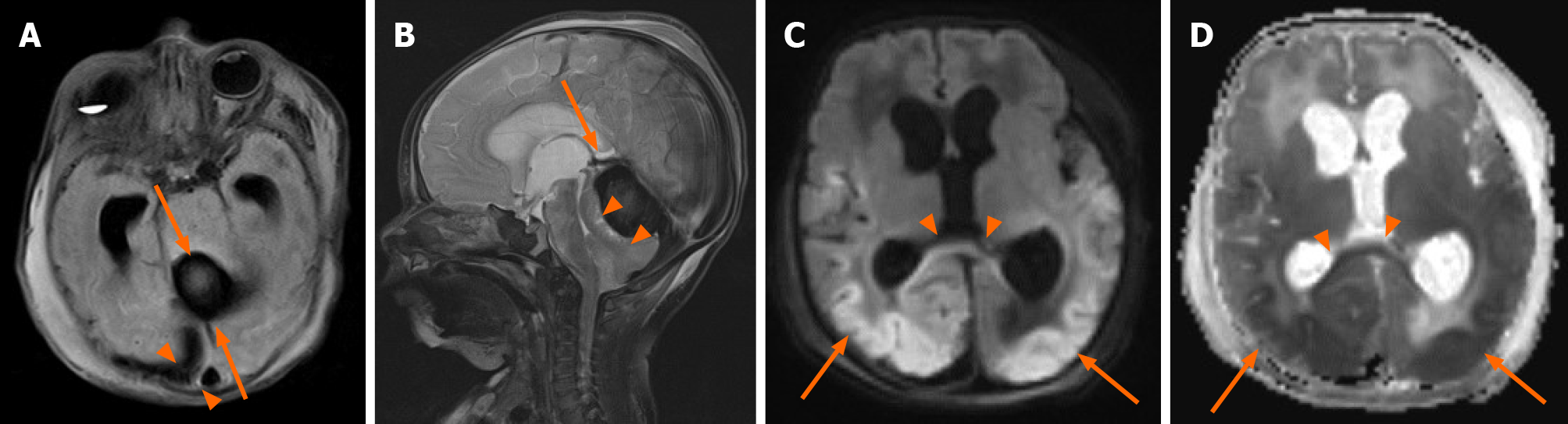
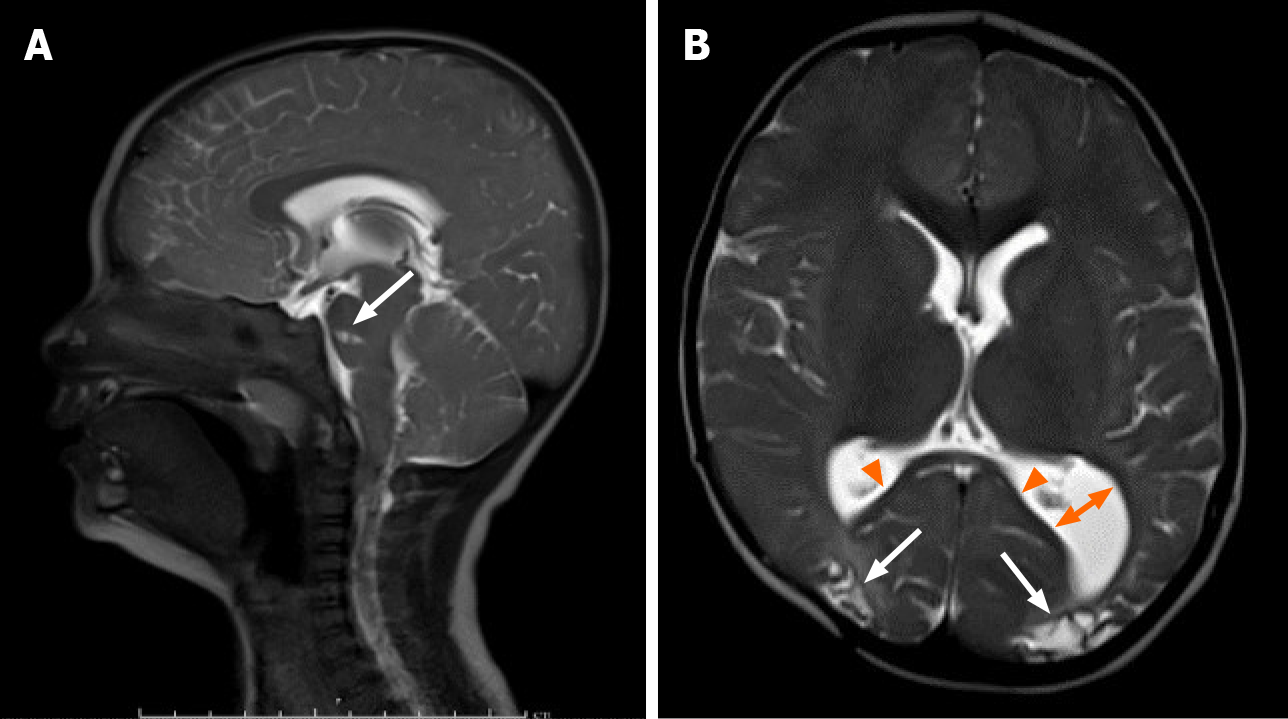
A same-day brain magnetic resonance imaging (MRI) was performed, revealing a 2.5 cm hematoma in the posterior fossa causing extensive mass effect on the cerebellum, and effacement of the fourth ventricle leading to an obstructive hydrocephalus. There was also widespread hypoxic ischemic injury (Figure 2 and Figure 3). A ventricular access device was placed that day for intermittent cerebrospinal fluid (CSF) diversion.
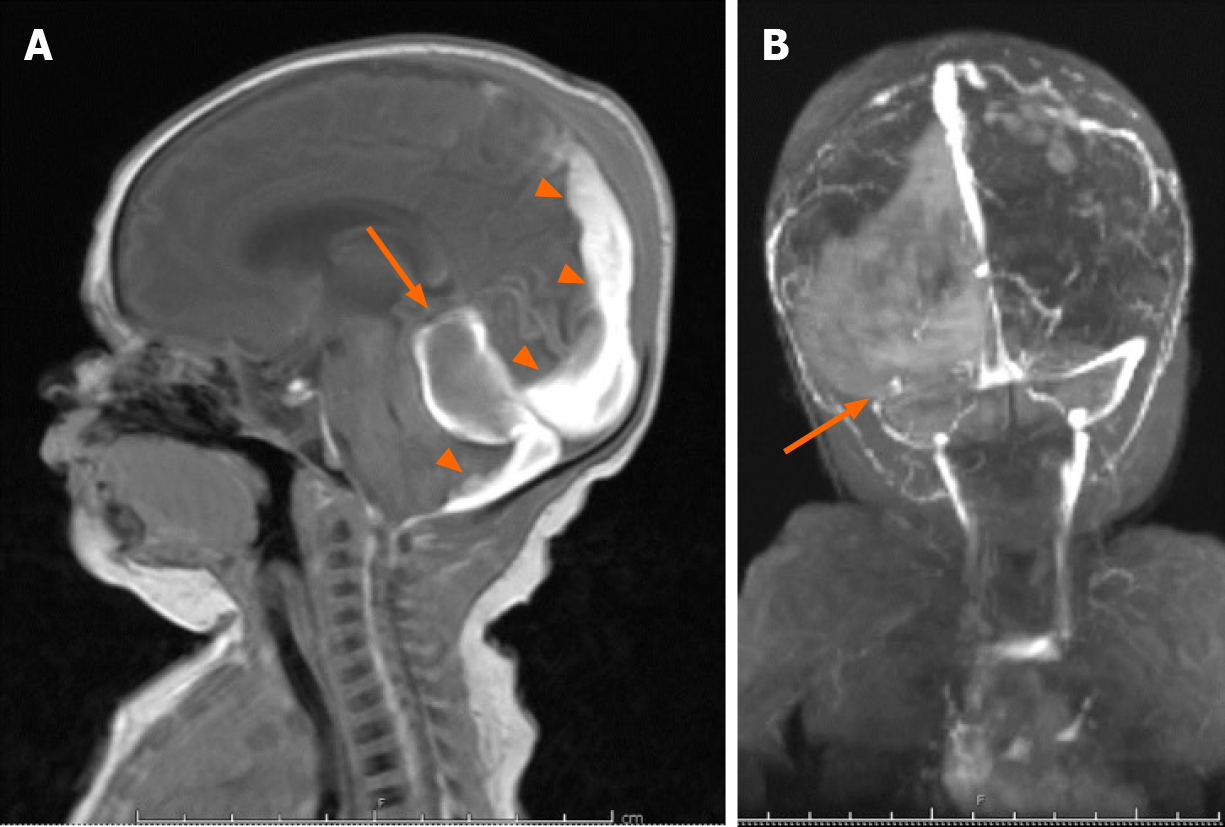
On DOL 20, due to an increase in apneic and bradycardic episodes, and increasing hydrocephalus on HUS, a repeat MRI was performed, and demonstrated acute on chronic bleeding into the subdural space (Figure 4).
Later on DOL 20, the patient underwent successful supratentorial burr-hole evacuation of the subdural hematoma as well as a sub-occipital craniectomy with an infratentorial, supracerebellar evacuation of the thrombus.
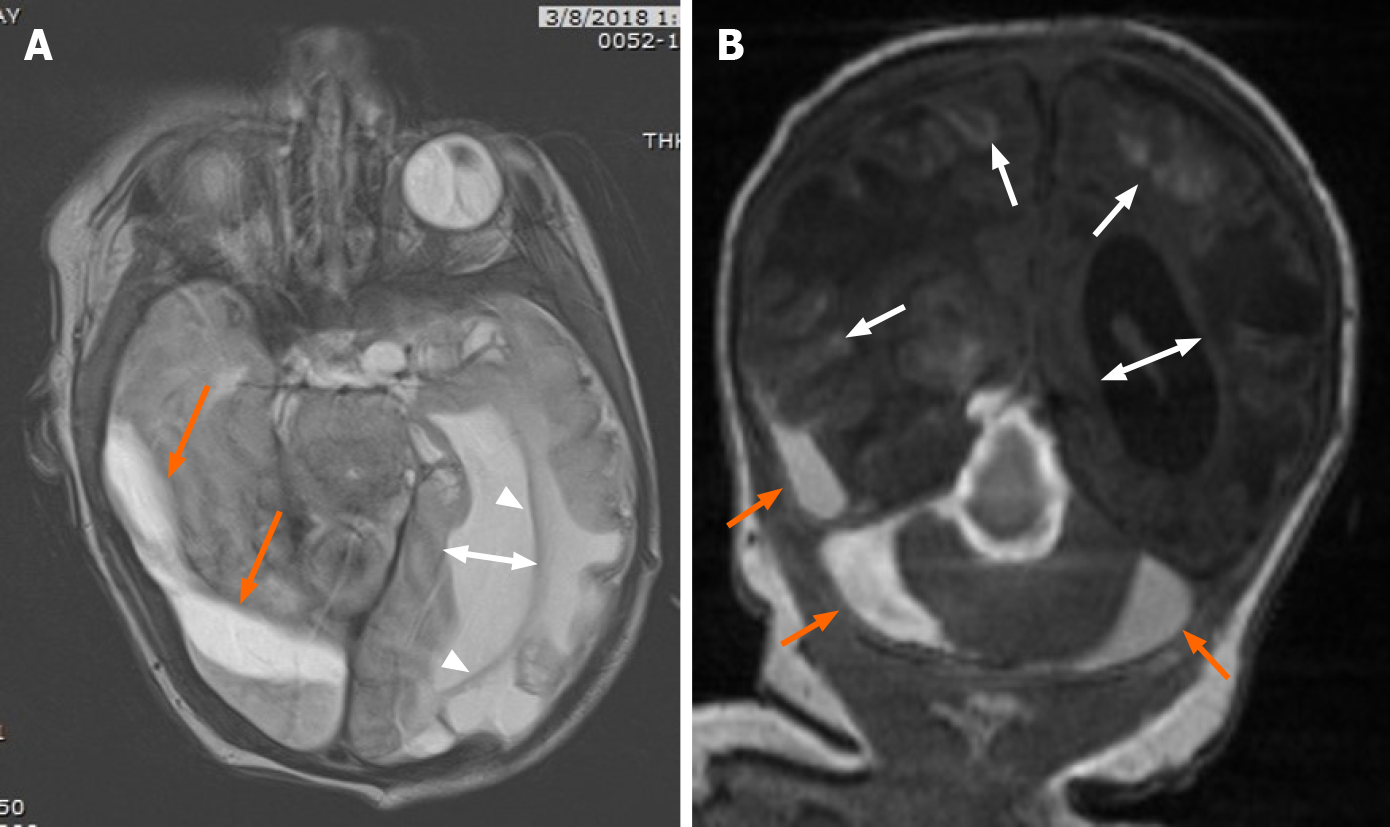
Post-operative imaging demonstrated near complete resolution of the subdural hematoma (Figure 5). MRI at 15 mo of age (Figure 6) demonstrated improved hydrocephalus. At the time of submission, the patient is 29 mo old, and suffers from right spastic hemiplegic cerebral palsy, an expressive aphasia, and strabismus.
While asymptomatic SDH are commonplace after delivery, symptomatic SDH are rare in neonates, with an incidence of approximately 3.8-5.2 of 10000 Live births[11-13]. SDH typically occur in the posterior fossa and are thought to arise from head trauma during vaginal delivery[1,2]. Infratentorial SDH most commonly results from falx or tentorial tears with bridging vein disruption and are worrisome because of their propensity to cause obstructive hydrocephalus, even with small volume bleeds[2]. Elective C-section deliveries are rarely associated with symptomatic SDH, likely due to lower rates of birth trauma.
Many researchers have conjectured that SDH can be secondary to cerebral ischemia[5-8]. The prevailing theory is that ischemia leads to damage of immature blood vessels, especially those of the richly vascularized falx cerebri, causing microvascular permeability that leads to intradural hemorrhage (IDH), which is then exacerbated by increased venous pressure[8]. IDH then leads to damage of the weak cell layer between the arachnoid and the dura, causing SDH[8]. However, other smaller studies still debate this theory[14].
The delayed presentation of the SDH in the setting of therapeutic cooling and HIE is what makes this case unique. Our patient’s HIE was likely due to meconium aspiration and pulmonary hemorrhage resulting in asphyxia and acute hypoxemic respiratory failure requiring intubation at birth. The presence of the germinal matrix hemorrhage on initial head ultrasound did not preclude the whole-body cooling protocol from being initiated. Although initial therapeutic hypothermia is not known to cause spontaneous SDH, in-vivo studies have shown that hypothermia can impair hemostasis[15]. Furthermore, many of the studies involved in evaluating whole-body cooling were not powered to assess for harm[10]. Given this case involved a C-section with no significant birth trauma, and a delay in the clinical and radiographic presentation of the hemorrhage, it is likely in this case as Cohen et al[8] suggests that the SDH occurred as a result of cerebral ischemia, and hypothermia exacerbated the condition.
Successful treatment of neonatal posterior fossa subdural hematomas has been reported in the literature as early as 1940. In the largest reported clinical series of 15 infants, Perrin et al[4] demonstrated that successful surgical evacuation of posterior fossa hemorrhages can relieve obstructive hydrocephalus and prevent the need for permanent CSF diversion with good neurologic outcomes. Generally, conservative management is recommended initially but in the presence of hydrocephalus, a worsening clinical exam, or an enlarging hematoma, surgical evacuation should be considered.
Screening HUS during hypothermia protocols for HIE warrant scrutiny for hemorrhage in unexpected locations. Symptomatic subdural hematomas warrant a high degree of clinical suspicion, particularly due to their rarity in children delivered by C-section. This report highlights the emerging association of HIE, therapeutic hypothermia, and perinatal intracranial hemorrhage. Prompt imaging and neurosurgical intervention may relieve hemorrhage induced obstructive hydrocephalus during therapeutic cooling with good neurological outcomes, preventing need for permanent CSF diversion. Familiarity with the key imaging characteristics and clinical exam features of mass-like SDH can help the treatment team consider the diagnosis, and potentially enable a prompt recovery.
The views expressed in this manuscript are those of the authors, and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States Government.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Radiology, No. 00888351.
Specialty type: Radiology, Nuclear Medicine and Medical Imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cazorla E S-Editor: Wang JL L-Editor: A P-Editor: Liu JH
| 1. | Menezes AH, Smith DE, Bell WE. Posterior fossa hemorrhage in the term neonate. Neurosurgery. 1983;13:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Perlman JM. Brain injury in the term infant. Semin Perinatol. 2004;28:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Gunn TR, Mok PM, Becroft DM. Subdural hemorrhage in utero. Pediatrics. 1985;76:605-610. [PubMed] |
| 4. | Perrin RG, Rutka JT, Drake JM, Meltzer H, Hellman J, Jay V, Hoffman HJ, Humphreys RP. Management and outcomes of posterior fossa subdural hematomas in neonates. Neurosurgery. 1997;40:1190-9; discussion 1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Steinbok P, Haw CS, Cochrane DD, Kestle JR. Acute subdural hematoma associated with cerebral infarction in the full-term neonate. Pediatr Neurosurg. 1995;23:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | McCubbin K, Thoma L, Mena H, Gill JR. Subdural Hemorrhage and Hypoxia in Children Less than Two Years Old. Acad Forensic Pathol. 2013;3:213-221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Henzi BC, Wagner B, Verma RK, Bigi S. Perinatal infratentorial haemorrhage: a rare but possibly life-threatening condition. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Cohen MC, Scheimberg I. Evidence of occurrence of intradural and subdural hemorrhage in the perinatal and neonatal period in the context of hypoxic Ischemic encephalopathy: an observational study from two referral institutions in the United Kingdom. Pediatr Dev Pathol. 2009;12:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 458] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Wang D, McMillan H, Bariciak E. Subdural haemorrhage and severe coagulopathy resulting in transtentorial uncal herniation in a neonate undergoing therapeutic hypothermia. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Chamnanvanakij S, Rollins N, Perlman JM. Subdural hematoma in term infants. Pediatr Neurol. 2002;26:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Gupta SN, Kechli AM, Kanamalla US. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr Neurol. 2009;40:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Rooks VJ, Eaton JP, Ruess L, Petermann GW, Keck-Wherley J, Pedersen RC. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol. 2008;29:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Byard RW, Blumbergs P, Rutty G, Sperhake J, Banner J, Krous HF. Lack of evidence for a causal relationship between hypoxic-ischemic encephalopathy and subdural hemorrhage in fetal life, infancy, and early childhood. Pediatr Dev Pathol. 2007;10:348-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Wang CH, Chen NC, Tsai MS, Yu PH, Wang AY, Chang WT, Huang CH, Chen WJ. Therapeutic Hypothermia and the Risk of Hemorrhage: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2015;94:e2152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |