Published online Jan 28, 2021. doi: 10.4329/wjr.v13.i1.19
Peer-review started: November 23, 2020
First decision: December 7, 2020
Revised: December 13, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: January 28, 2021
Processing time: 64 Days and 21.1 Hours
Coronavirus disease 2019 (COVID-19) is caused by the novel coronavirus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Systemic complications include cardiovascular, neurological, hepatic, renal and altered coagulation. Derangements in haemostasis with SARS-CoV-2 infection have been termed COVID-19 associated coagulopathy (CAC). CAC is postulated to be one of the significant causes for sudden deaths in this pandemic, with infection of endothelial cells and subsequent endotheliitis through angiotensin-converting enzyme-2 receptors playing a key role in the pathogenesis. In this pictorial review, we describe the imaging findings in a multitude of extrapulmonary arterial (aorta, cerebral, mesenteric, renal and peripheral arterial system) and venous thrombotic phenomena detected on contrast-enhanced computed tomography and magnetic resonance imaging of COVID-19 patients which could not be attributed to any other causes. Knowledge of incidence of these complications, lowering the threshold for diagnostic imaging in symptomatic patients and timely radiological detection can play a vital role in subsequent management of these critically ill patients.
Core Tip: Coronavirus disease 2019 (COVID-19) disease is a systemic illness with multi-organ system manifestations. Coagulopathy in the setting of COVID-19 has a unique pathophysiology with a propensity for both arterial and venous thrombosis. These phenomena may be clinically occult with imaging playing a vital role in detection and management. A high degree of clinical suspicion with a low threshold for cross sectional imaging can positively alter outcomes during this ongoing pandemic.
- Citation: Shankar A, Varadan B, Ethiraj D, Sudarsanam H, Hakeem AR, Kalyanasundaram S. Systemic arterio-venous thrombosis in COVID-19: A pictorial review. World J Radiol 2021; 13(1): 19-28
- URL: https://www.wjgnet.com/1949-8470/full/v13/i1/19.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i1.19
Coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) began as no more than a cluster of pneumonia cases first reported in the Hubei province of China in December 2019. From its origin till date however, it has swept across the globe; emerging as one of the most far-reaching pandemics in human history. The most common presentation of COVID-19 is related to infection of the respiratory epithelial cells by the virus and ranges from mild upper or lower respiratory tract symptoms to hypoxic respiratory failure requiring oxygen therapy and in some instances, mechanical ventilation. Systemic complications include cardiovascular, neurological, hepatic and renal dysfunction, as well as altered coagulation[1]. Derangements in haemostasis occurring in patients with SARS-CoV-2 infection have been termed COVID-19 associated coagulopathy (CAC). CAC is postulated to be one of the significant causes for sudden deaths in this pandemic especially those occurring out of hospitals[2]. Literature is still emerging regarding the epidemiology and pathophysiology behind CAC with reported incidence of venous and arterial thromboembolism between 10%-25% among the COVID-19 admitted patients, with increase in incidence up to 31%-59% amongst those in intensive care[3-5]. The pro-coagulant state has been attributed to macrophage and endothelial cell mediated processes culminating in the acceleration of fibrin synthesis and suppression of its degradation[2]. Infection of endothelial cells through angiotensin-converting enzyme-2 (ACE-2) receptors is believed to be a characteristic unique to corona viruses and this plays a key role in pathogenesis[2,6]. Although CAC shares some common underlying mechanisms causing widespread micro/macro thrombi with conditions like sepsis induced coagulopathy, disseminated intravascular coagulation, hemophagocytic and hemolytic uremic syndromes; it has a few distinctive features not previously described in these conditions; and has emerged as a new category of coagulopathy[2]. The most common alterations in coagulation parameters in CAC include markedly elevated D-dimer levels; mild to moderate thrombocytopenia and prolonged prothrombin time[2,7].
Initially, a possible association between SARS-CoV-2 viral infection and pulmonary vascular thromboembolism was proposed in multiple case reports emerging from global hotspots when patients who developed sudden onset cardiac or respiratory deterioration or both at any time during the course of the disease, also had elevated D-dimer levels and a positive pulmonary angiography[8-12]. Subsequently, in a research article by Kaminetzky et al[13], a higher incidence of pulmonary embolism was recorded amongst the COVID-19 positive cohort. The study concluded that pulmonary embolism could indeed be a cause for acute deterioration in these patients. In addition, it suggested that D-dimer levels could be used for risk categorization.
In this pictorial review, we describe the imaging findings in a multitude of extrapulmonary arterial and venous thrombotic phenomena detected in cross sectional imaging (computed tomography/magnetic resonance imaging, CT/MRI) of COVID-19 patients which could not be attributed to other causes. Knowledge of incidence of these complications and early radiological detection can play a key role in subsequent management of these critically ill patients, often determining the outcomes.
Stroke is characterised by neuronal injury with a manifest clinical deficit secondary to a vascular cause. It encompasses parenchymal infarction, intracerebral and subarachnoid haemorrhage[14]. Stroke is an important cause of morbidity and mortality world over. The causal relationship between infections and stroke has been researched in the past and has been deemed probable. Bacteria, viruses, fungi and a few parasites have been recognised as primary etiological or contributory factors of stroke[15]. The most important mechanism of stroke in infections is the stimulation of a systemic inflammatory response and consequent generalised procoagulant state or a localised effect on atherosclerotic plaques making them prone to rupture[16]. Other means of pathogenesis include effects on vasculature — vessel wall inflammation (e.g., Varicella- Zoster, Epstein-Barr, Cytomegalovirus) and/or vessel wall remodelling (e.g., human immunodeficiency virus), emboli from cardiac causes including valves (e.g., infectious endocarditis due to staphylococcus, streptococcus, HACEK group of bacteria) and from dilated chambers (dilated cardiomyopathy in Chagas disease)[15].
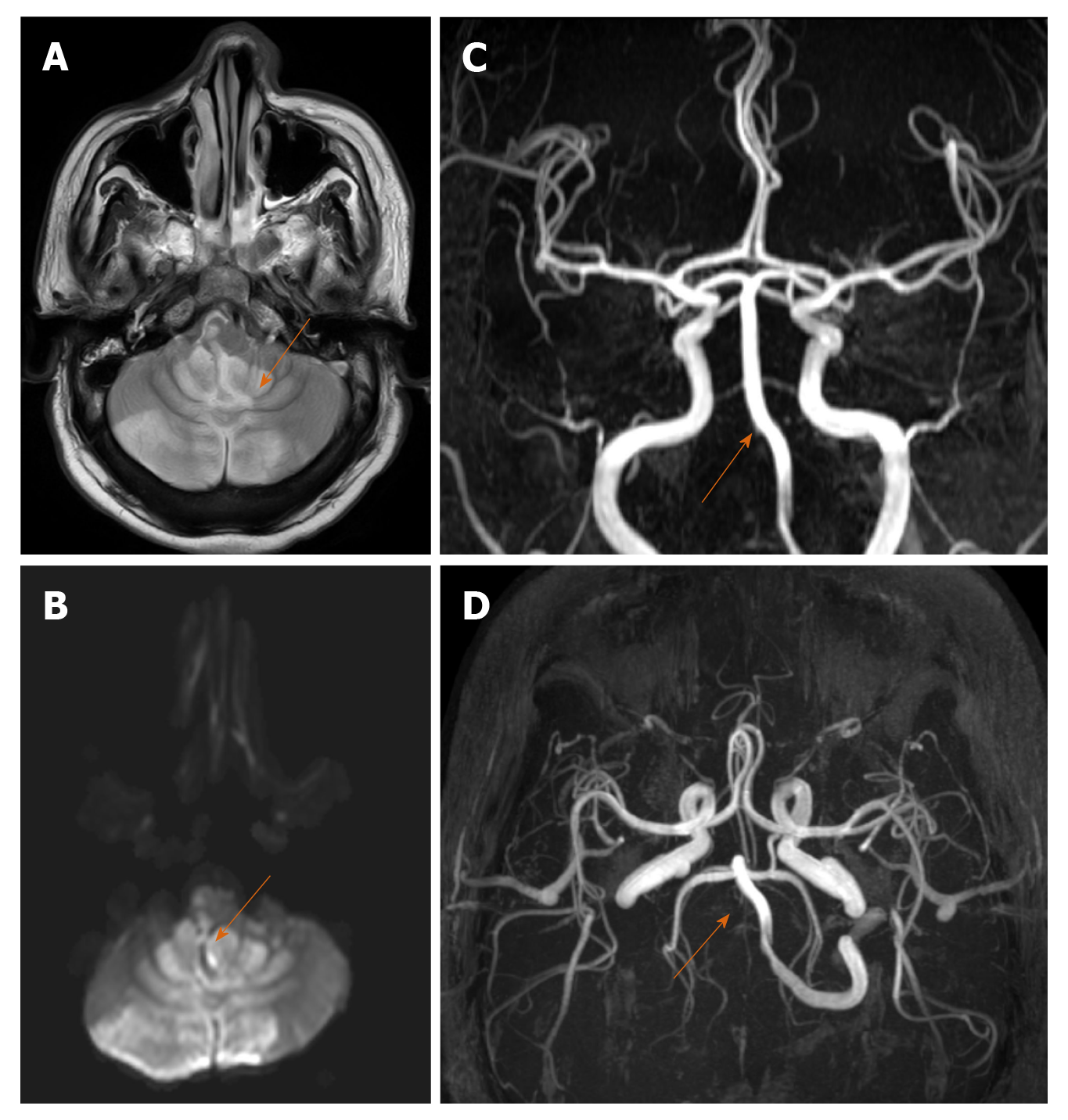
Stroke is one of the neurological complications associated with SARS-CoV-2 attributed to CAC (Figure 1A-D). One of the interesting mechanisms includes binding with and depletion of ACE-2 receptors reducing its vasodilatory and anti-inflammatory effects[17]. Literature regarding epidemiology of stroke in COVID-19 is still emerging and the exact incidence is yet to be established. Some interesting imaging observations include large vessel involvement in a relatively younger population even in the absence of established risk factors, concordant multiple vessel (both cerebral and systemic) thrombosis, unusual sites of thrombi, greater thrombus load and poorer functional outcomes due to contributory effects of hypoxia from lung and myocardial involvement[18].
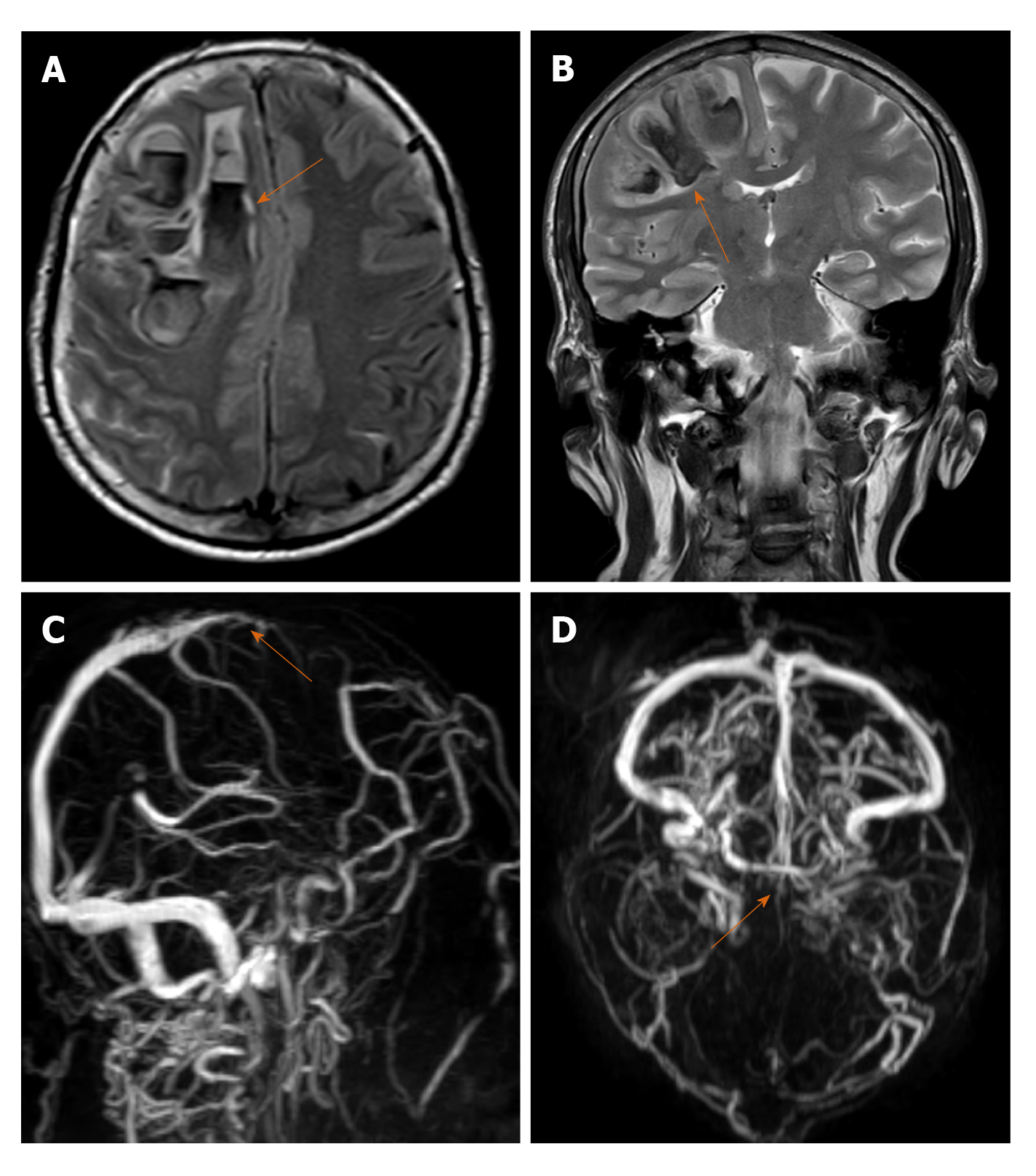
Cerebral venous thrombosis (CVT) is less common when compared to other types of stroke and affects a different patient demographic, those of a comparatively younger age group with a notable female preponderance. The most important risk factors for CVT are genetic and acquired causes of thrombophilia including pregnancy, puerperium, intake of contraceptive pills, infections and neoplasms (CNS, systemic). Most common infections associated with CVT include oto-mastoiditis, sinusitis and facial infections with an overall declining trend in the modern antibiotic era[19]. CVT seldom presents with focal neurological deficit like typical stroke. The symptoms vary depending on the site of thrombosis, chronicity and patient age. Headache is the most common initial and in many instances, the only presenting symptom[20]. MRI with venography is the investigation of choice to confirm the diagnosis (Figure 2A-D).
Cases of CVT are being increasingly reported during this pandemic, CAC most likely being the underlying mechanism. Clinicians need to maintain a high index of suspicion while treating COVID-19 patients with persistent headache irrespective of presence of other neurological symptoms[21]. A low threshold for ordering radiological investigations in these patients can potentially alter therapeutic decision making. Imaging in CVT includes demonstration of the thrombus as a loss of flow void in baseline images and absence of flow related signal in venography[19]. Associated complications including venous infarcts, intra and extra-axial haemorrhages.
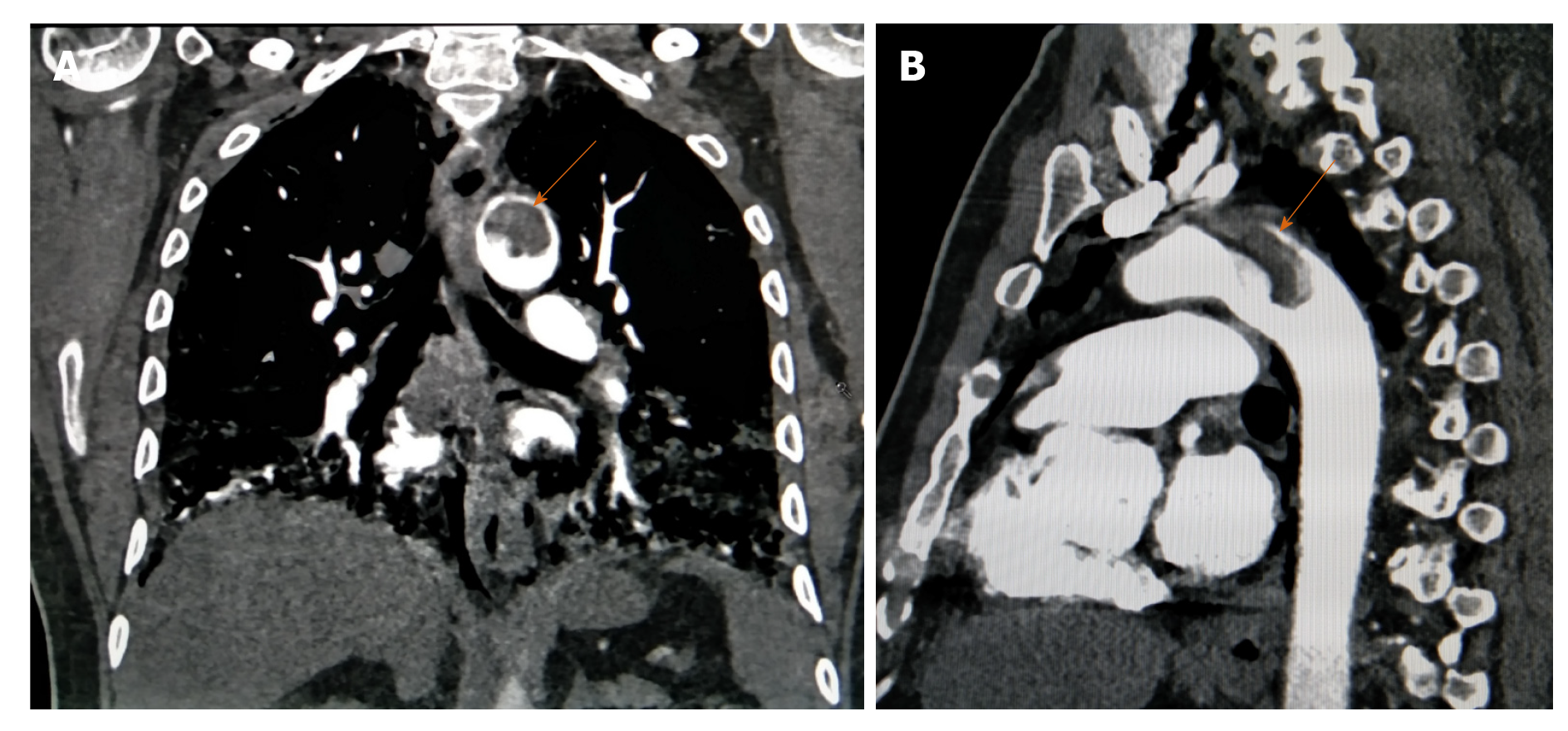
Aortic mural thrombus (AMT) is a rare entity defined by an intraluminal filling defect with an attachment to the intima. Two types have been described namely sessile and pedunculated with the latter having a higher incidence of peripheral embolization and related complications. AMT is usually associated with regional vessel wall abnormalities like atherosclerosis, aneurysm, vasculitis and dissection. Primary AMT without underlying wall pathology is extremely rare and one multicentre study including more than 10000 autopsies reported its incidence at approxiately 0.45% and that of major vessel occlusion contributive to mortality up to 6%[22]. CAC is one cause of such aortic thrombosis likely due to endothelial inflammation[2]. It is usually the radiologist who first comes across this finding and alerts the clinicians, aiding in subsequent management depending on the site, size of thrombus and patients' hemodynamic and respiratory status (Figure 3A and B).
Mesenteric ischemia is an uncommon, potentially fatal abdominal emergency. It is characterised by interrupted blood supply to the gastrointestinal tract with resulting mural ischemia progressing from a reversible mucosal stage to irreversible transmural necrosis and subsequently more adverse outcomes. Hence, a very important prognostic factor is the temporal relation between symptom onset and initiation of revascularization with mortality rate increasing from 12% in the initial 12 h to nearly a 100% when there is a delay of more than 48 h[23]. Contrast-enhanced CT (CECT) is the investigation of choice for diagnosis and exclusion of other causes of acute abdomen. The presentation can be nonspecific with abdominal pain being the most frequent and consistent symptom[24].
Causes of ischemia are most commonly arterial, either embolic (approxiately 40%-50% cases) or in situ thrombosis of a vessel with pre-existing luminal narrowing (approxiately 25%-30%), the latter being more common in the elderly (> 70 years). Mesenteric venous occlusion as a cause of ischemia is less common (approxiately 5%-10%), and usually occurs in a much younger population with hypercoagulable states. Non-occlusive mesenteric ischemia is a condition with diffuse small and large bowel involvement without identifiable focal stenotic or occlusive vascular pathology usually occurring in generalised low flow states like cardiogenic or hypovolemic shock[23,25].
The role of imaging in mesenteric ischemia is two-fold and includes diagnosis and prognostication. A systematic approach should be followed including evaluation of vasculature (assessment of presence and extent of occlusive/partial filling defects, mural atherosclerotic changes), bowel (for presence of dilation, mural enhancement, thickening/thinning, pneumatosis), mesentery and additionally signs of perforation (wall discontinuity, pneumoperitoneum)[23,25].
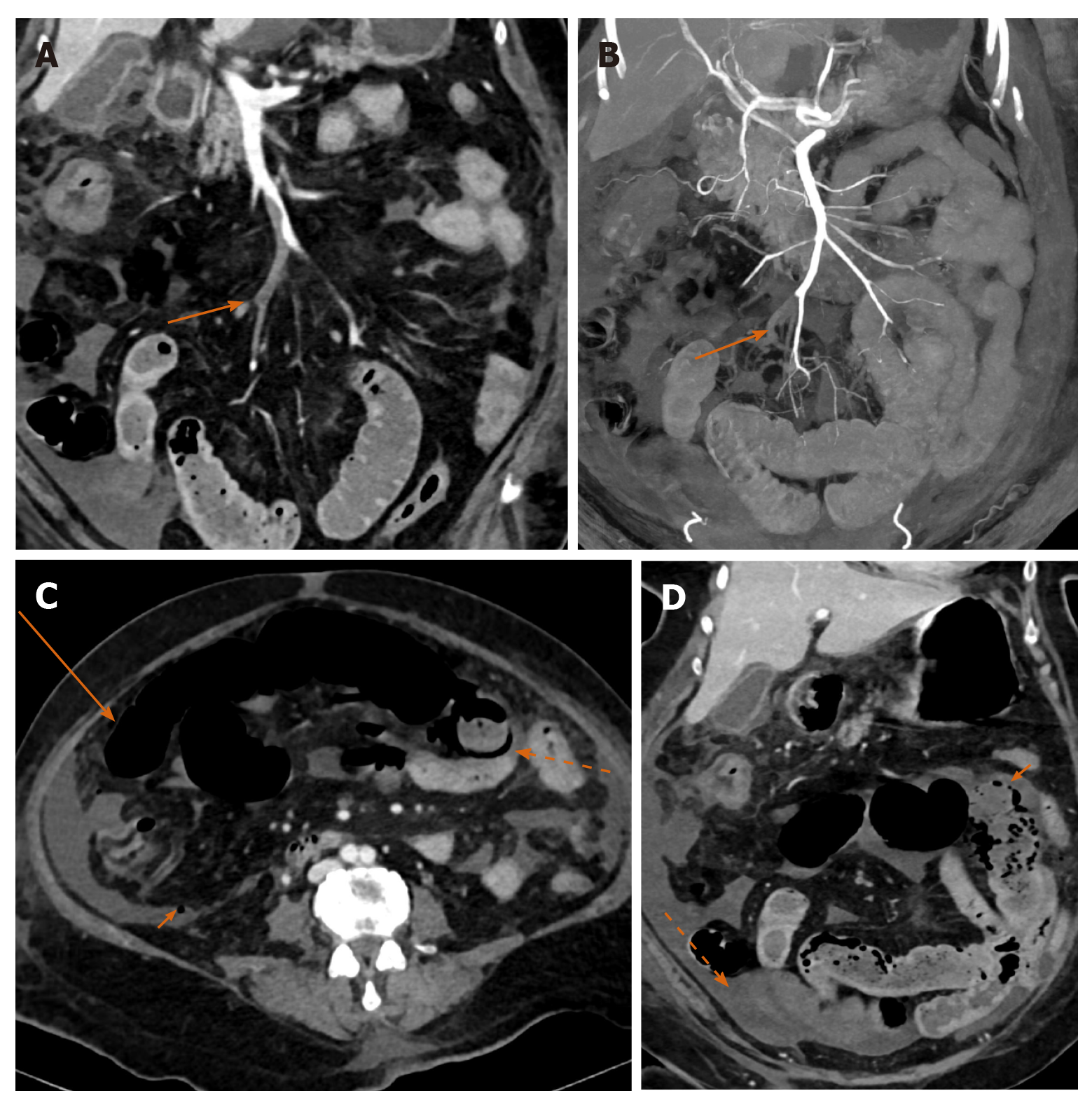
Mesenteric ischemia has been reported in patients with severe COVID-19 disease with underlying causative mechanisms including CAC, direct enterocyte infection, microvascular thrombosis in the gut wall and non-occlusive ischemia[26]. Concomitant arterial and venous mesenteric thrombosis has been reported with COVID-19 disease[27] (Figure 4A-D). Knowledge of this complication and timely investigation followed by intervention can help reduce associated mortality from this condition.
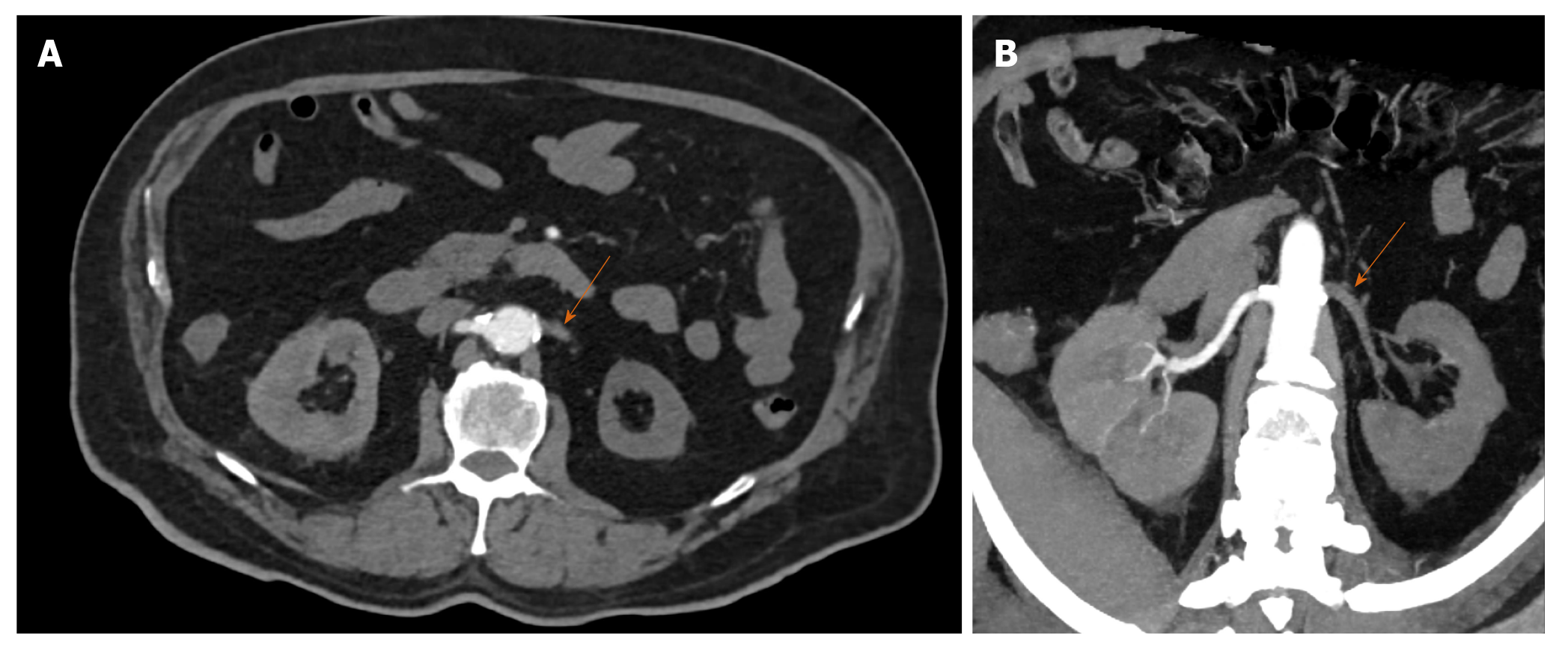
Renal artery thrombosis may be secondary to embolic phenomena or in situ thrombosis. The most common source of emboli are cardiac, usually secondary to either structural (valvular abnormalities, cardiomyopathy) or functional abnormalities (arrhythmias, myocardial infarction) or from the aorta (aneurysms, atherosclerosis). In situ thrombosis could be secondary to vasculitis, trauma or dissection[28]. Absence of these predisposing factors raises the possibility of de-novo thrombosis secondary to CAC (Figure 5A and B). The importance of identification of renal artery thrombosis lies in the fact that it is a treatable cause of renal dysfunction. Indeed because of its non-specific clinical presentation (most commonly unilateral flank pain) radiologists play a key role in detection and management which entails anticoagulation measures and endovascular intervention as indicated.
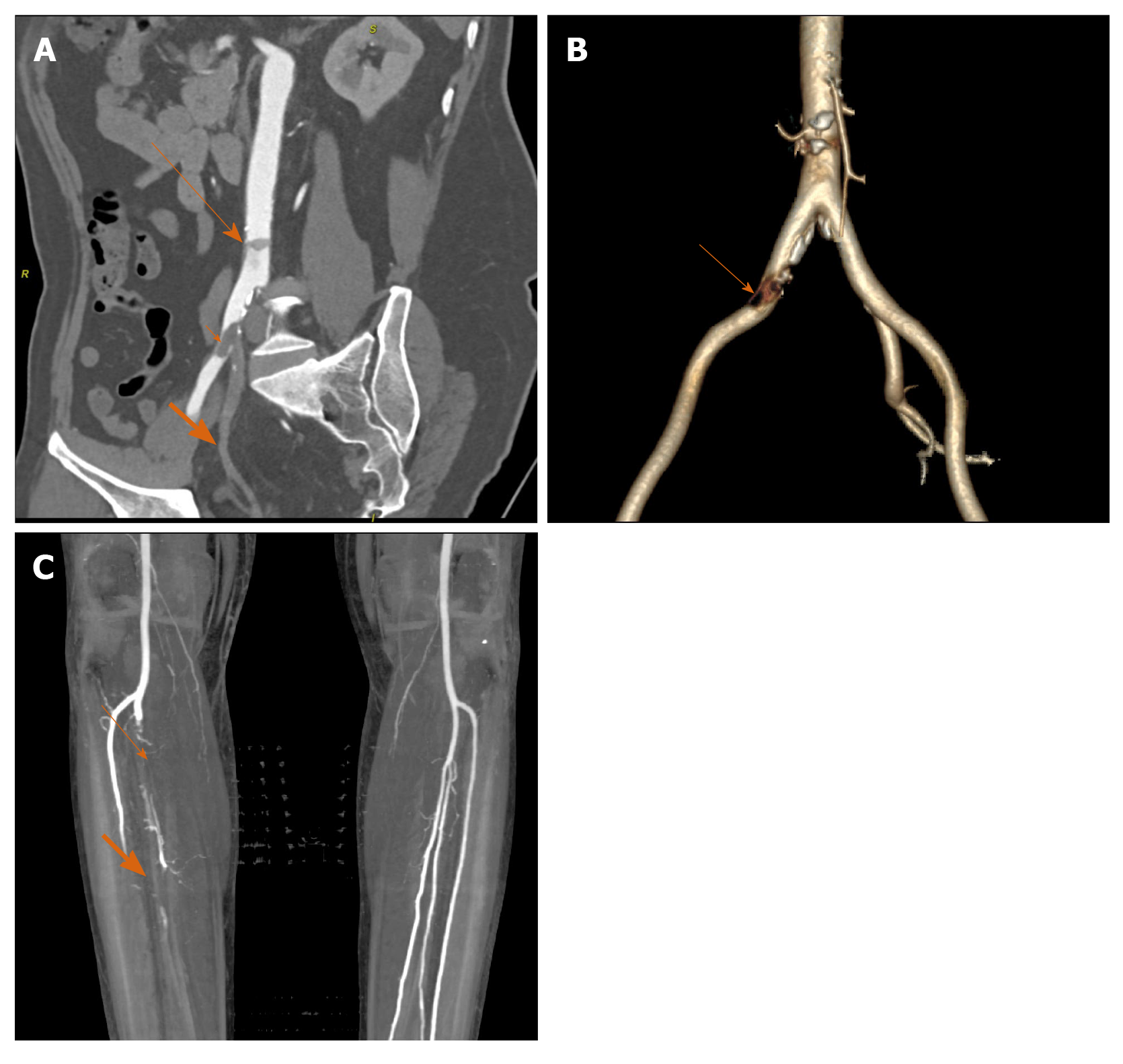
Peripheral arterial disease (PAD) is characterised by reduced or absent forward flow in major systemic vessels excluding the cerebrovascular and coronary circulations. It affects the lower limb arteries more frequently with the most common cause being atherosclerosis. Other less common causes include thromboembolism, vasculitis, degenerative and dysplastic conditions of vessel wall. Risk factors for PAD include diabetes, obesity, hypertension, hyperlipidemia, smoking (strongest association) and a positive family history. Clinically PAD is classified based on patient presentation into four categories: Asymptomatic, intermittent claudication, acute and chronic limb ischemia on the basis of American Heart Association/American College of Cardiology guidelines[29]. Amongst this acute limb ischemia due to any cause is an emergency since the rapidity of developing occlusion precludes collateral pathway formation, thereby threatening limb viability. The most common mechanism of acute limb ischemia is rupture of pre-existing atheromatous plaque with thrombus formation and vessel occlusion.
CT angiography plays an important role in management by classification of PAD based on location, lesion length [short (< 5 cm) vs long], degree of luminal narrowing and status of distal vessels (most important consideration in revascularisation procedures)[30] (Figure 6A-C). Functional classification (Fontaine/Rutherford) along with radiological investigations help guide the course of treatment planning between conservative, endovascular and surgical[31]. A retrospective study by Goldman et al[32]. during the pandemic situation (January to April 2020) witnessed an elevated positivity rate amongst CT angiographic studies performed for claudication symptoms in COVID-19 patients with a higher clot burden and worse prognosis (higher incidence of amputation and/or death) in test population when compared with control group.
A recently published meta-analysis of literature with reference to the prevalence of deep vein thrombosis and venous thromboembolism in COVID-19 patients estimated these at approxiately 20% and 30% respectively[33]. Prevalence was higher amongst patients with a higher BMI, those belonging to an older age group and with a more severe illness. The prothrombotic state induced by SARS-CoV-2 has led to the question of whether pulmonary thrombi in this disease originate from peripheral veins or develop in situ, the significance being the difference in composition and subsequently choice of anticoagulation. This article brings attention to the requirement of appropriate screening protocols in all COVID-19 patients. Therapeutic strategies including choice of anticoagulant, dosage and duration are beyond the scope of this review.
Although SARS-CoV-2 is primarily a respiratory virus, COVID-19 is more of a systemic illness with multiorgan involvement. Coagulopathy associated with this condition can affect both arterial and venous systems with catastrophic effects depending on the site and severity of thrombosis. Many of these phenomena can be clinically silent or obscure in presentation. Imaging therefore remains the cornerstone in arriving at the appropriate diagnosis with a potential to alter the course of disease progression by advocating timely management.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deng B S-Editor: Gao CC L-Editor: A P-Editor: Wang LYT
| 1. | Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2691] [Cited by in RCA: 3174] [Article Influence: 634.8] [Reference Citation Analysis (0)] |
| 2. | Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 329] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 3. | Mezalek ZT, Khibri H, Ammouri W, Bouaouad M, Haidour S, Harmouche H, Maamar M, Adnaoui M. COVID-19 Associated Coagulopathy and Thrombotic Complications. Clin Appl Thromb Hemost. 2020;26:1076029620948137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1092] [Article Influence: 218.4] [Reference Citation Analysis (0)] |
| 5. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3407] [Article Influence: 681.4] [Reference Citation Analysis (0)] |
| 6. | Woehl B, Lawson B, Jambert L, Tousch J, Ghassani A, Hamade A. 4 Cases of Aortic Thrombosis in Patients With COVID-19. JACC Case Rep. 2020;2:1397-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438-e440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 1060] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 8. | Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 474] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 9. | Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. RSNA. 2020;2:e200067. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID-19 Complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep. 2020;2:1379-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Rotzinger DC, Beigelman-Aubry C, von Garnier C, Qanadli SD. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb Res. 2020;190:58-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Hékimian G, Lebreton G, Bréchot N, Luyt CE, Schmidt M, Combes A. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care. 2020;24:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Kaminetzky M, Moore W, Fansiwala K, Babb JS, Kaminetzky D, Horwitz LI, McGuinness G, Knoll A, Ko JP. Pulmonary Embolism on CTPA in COVID-19 Patients. RSNA. 2020;2:e200308. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV; American Heart Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition; Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2156] [Cited by in RCA: 2241] [Article Influence: 186.8] [Reference Citation Analysis (0)] |
| 15. | Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis. 2014;14:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 304] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Hess DC, Eldahshan W, Rutkowski E. COVID-19-Related Stroke. Transl Stroke Res. 2020;11:322-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 18. | John S, Kesav P, Mifsud VA, Piechowski-Jozwiak B, Dibu J, Bayrlee A, Elkambergy H, Roser F, Elhammady MS, Zahra K, Hussain SI. Characteristics of Large-Vessel Occlusion Associated with COVID-19 and Ischemic Stroke. AJNR Am J Neuroradiol. 2020;41:2263-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG. Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76:1084-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Poillon G, Obadia M, Perrin M, Savatovsky J, Lecler A. Cerebral venous thrombosis associated with COVID-19 infection: Causality or coincidence? J Neuroradiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 22. | Yang S, Yu J, Zeng W, Yang L, Teng L, Cui Y, Shi H. Aortic floating thrombus detected by computed tomography angiography incidentally: five cases and a literature review. J Thorac Cardiovasc Surg. 2017;153:791-803. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Garzelli L, Nuzzo A, Copin P, Calame P, Corcos O, Vilgrain V, Ronot M. Contrast-Enhanced CT for the Diagnosis of Acute Mesenteric Ischemia. AJR Am J Roentgenol. 2020;215:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Nuzzo A, Corcos O. [Management of mesenteric ischemia in the era of intestinal stroke centers: The gut and lifesaving strategy]. Rev Med Interne. 2017;38:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Navas-Campo R, Moreno-Caballero L, Ezponda Casajús A, Muñoz DI. Acute mesenteric ischemia: a review of the main imaging techniques and signs. Radiologia. 2020;62:336-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Parry AH, Wani AH, Yaseen M. Acute Mesenteric Ischemia in Severe Coronavirus-19 (COVID-19): Possible Mechanisms and Diagnostic Pathway. Acad Radiol. 2020;27:1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 27. | de Barry O, Mekki A, Diffre C, Seror M, El Hajjam M, Carlier RY. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep. 2020;15:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Kawashima A, Sandler CM, Ernst RD, Tamm EP, Goldman SM, Fishman EK. CT evaluation of renovascular disease. Radiographics. 2000;20:1321-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Conte SM, Vale PR. Peripheral Arterial Disease. Heart Lung Circ. 2018;27:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Fleischmann D, Hallett RL, Rubin GD. CT angiography of peripheral arterial disease. J Vasc Interv Radiol. 2006;17:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Sena G, Gallelli G. An increased severity of peripheral arterial disease in the COVID-19 era. J Vasc Surg. 2020;72:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Goldman IA, Ye K, Scheinfeld MH. Lower-extremity Arterial Thrombosis Associated with COVID-19 Is Characterized by Greater Thrombus Burden and Increased Rate of Amputation and Death. Radiology. 2020;297:E263-E269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID-19 and Venous Thromboembolism: A Meta-analysis of Literature Studies. Semin Thromb Hemost. 2020;46:763-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |