Peer-review started: November 2, 2017
First decision: November 30, 2017
Revised: December 3, 2017
Accepted: January 25, 2018
Article in press: January 25, 2018
Published online: January 28, 2018
Processing time: 88 Days and 13.2 Hours
To evaluate upper abdominal computed tomography (CT) scan as primary follow-up after laparoscopic Roux-en-Y gastric bypass (LRYGB).
This prospective study was approved by the Ethical Committee of the State of Zurich, and informed consent was obtained from all patients. Sixty-one patients who underwent LRYGB received upper abdominal CT on postoperative day 1, with the following scan parameters: 0.6 mm collimation, 1.2 mm pitch, CareKV with reference 120 mAs and 120 kV, and 0.5 s rotation time. Diluted water-soluble radiographic contrast-medium (50 mL) was administered to achieve gastric pouch distension without movement of the patient. 3D images were evaluated to assess postoperative complications and the radiation dose received was analysed.
From the 70 patients initially enrolled in the study, 9 were excluded from analysis upon the intraoperative decision to perform a sleeve gastrectomy and not a LRYGB. In all of the 61 patients who were included in the analysis, CT was feasible and there were no instances of aspiration or vomiting. In 7 patients, two upper abdominal scans were necessary as the pouch was not distended by contrast medium in the first acquisition. Radiologically, no leak and no relevant stenosis were found on the first postoperative day. These early postoperative CT findings were consistent with the findings at clinical follow-up 6 wk postoperatively, with no leaks, stenosis or obstructions being diagnosed. The average total dose length product in CT was 536.6 mGycm resulting in an average effective dose of 7.8 mSv. The most common surgical complication, superficial surgical site infections (n = 4), always occurred at the upper left trocar site, where the circular stapler had been introduced.
Early LRYGB postoperative multislice spiral CT scan is feasible, with low morbidity, and provides more accurate anatomical information than standard upper gastrointestinal contrast study.
Core tip: In most bariatric centres, a routine upper gastrointestinal (UGI) study is performed in the early postoperative period. Yet, the real value of a standard postoperative radiological exam after laparoscopic Roux-en-Y gastric bypass is debatable. From the available data, an UGI is not necessary as a standard postoperative exam and, similarly, a routine computed tomography (CT) scan might not be indicated. For patients who experience unexpectedly difficult surgery or abnormal postoperative clinical course, CT is the diagnostic tool of choice, especially considering that a number of patients with pathological findings in UGI contrast studies will additionally undergo CT scan.
- Citation: Delko T, Mattiello D, Koestler T, Zingg U, Potthast S. Computed tomography as primary postoperative follow-up after laparoscopic Roux-en-Y gastric bypass. World J Radiol 2018; 10(1): 1-6
- URL: https://www.wjgnet.com/1949-8470/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i1.1
Morbid obesity has become a major worldwide health problem, with increasing prevalence[1-3]. The most effective treatment to reduce weight in obese patients is bariatric surgery. Consequently, the number of bariatric procedures has increased dramatically over the last decade[4]. The laparoscopic Roux-en-Y gastric bypass (LRYGB) is the most common bariatric procedure worldwide. Early morbidity and mortality of LRYGB is generally very low[5]. In a majority of bariatric centres, postoperative upper gastrointestinal (UGI) contrast studies are performed routinely in order to evaluate complications that may arise in the early postoperative period, such as anastomotic leaks or strictures[6,7]. The usefulness of UGI examination is debatable, considering its radiation dose and the diagnostic information obtained[6,8-10].
UGI examinations are insufficient to accurately assess certain postoperative surgical problems, such as hematoma or fluid collections, and have only limited sensitivity (i.e., 25% for correctly diagnosing a leak)[11]. Furthermore, in case of UGI indicating an early postoperative complication, a multislice computed tomography (CT) will be additionally performed to better delineate the pathologic features[12,13] and to provide guidance for possible interventional procedures such as aspiration or drainage of fluid collections. Several studies have suggested the usefulness of CT to investigate postoperative complications after LRYGB; however, data on routinely performed CT after bariatric surgery are scarce[13-15]. Hence, the prospective study described herein was conducted to assess the feasibility and usefulness of CT scan as a primary early postoperative radiological measure in patients who underwent LRYGB.
Informed consent was obtained from all patients. This prospective study was performed at Limmattal Hospital and approved by the Ethical Committee of Zurich, Switzerland. All patients were preoperatively assessed according to the guidelines of the Swiss Study Group for Morbid Obesity. A total of 70 patients undergoing LRYGB were enrolled during the 10-mo study period (July 2014 to May 2015). Surgery was performed by two surgeons, each having individual experience of over 500 LRYGB procedures.
A laparoscopic six-port technique with prior creation of the pneumoperitoneum by Veress needle was used. The greater omentum was split and the ligament of Treitz identified. The length of the biliopancreatic limb was measured to 50 cm and the jejunum divided using an Echelon Flex™ Powered Endopath® Stapler (60 mm, white; Ethicon, Somerville, NJ, United States). Next, the proximal stomach was divided using the Echelon Flex™ Powered Endopath® Stapler (60 mm, blue; Ethicon). The first cartridge was fired perpendicular to the lesser curvature and one to two additional cartridges parallel to the lesser curvature up to the angle of His, lateral to the Besley’s fat pad.
Once the small pouch was created, an end-to-side, ante-colic and ante-gastric gastro-jejunal anastomosis was created using a Premium Plus CEEA™ 25 mm circular stapler (Covidien, Dublin, Republic of Ireland). Then, the jejuno-jejunal anastomosis was created 150 cm distal to the gastro-jejunal anastomosis by using the Echelon Flex™ Powered Endopath® Stapler (60 mm, white; Ethicon), with closure of the stapler entry defect using absorbable PDS® (polydioxanone) 3-0 suture (Ethicon). All mesenteric defects were closed systematically with non-absorbable interrupted Prolene® 3-0 sutures (Ethicon).
CT scanning was performed on the first morning postoperatively. All patients were kept as nil per os until the upper abdomen CT had been performed. A clinical scanner equipped with modern dose reduction software was used (Somatom Definition 64; Siemens Healthineers, Erlangen, Germany), with the following scan parameters: collimation, 0.6 mm; pitch, 1.2 mm; CareKV with reference 120 mAs and reference 120 kV; rotation time, 0.5 s. The scan was acquired in supine position and a scout scan was acquired. Diluted water-soluble radiographic contrast-medium (50 mL) (Telebrix Gastro®, Guerbet, France) was given in a spout cup to achieve gastric pouch distension without movement of the patient.
The scan was performed in cranio-caudal direction, starting at the level of the distal oesophagus and stopping just distal to the pouch (Figure 1). The average total dose length product was registered and radiation dose was calculated. Image data was transferred to a 3D workstation and 3D images were created for evaluation of leaks and stenosis. The procedure was performed in all patients by the same senior radiologist with more than 15 years of experience in body radiology. If no significant stenosis or leak was diagnosed, a liquid diet was started.
Basic demographic, pre-, peri- and postoperative data was collected prospectively and entered into a database (Excel®, Office, Microsoft). Postoperative morbidity was separated into surgical and non-surgical categories. Surgical morbidity included anastomotic leak, deep surgical site infection (i.e., intra-abdominal), superficial surgical site infection, re-laparoscopy and re-laparotomy. Non-surgical morbidity included pulmonary or cardiac complications as well as renal failure. Special attention was given to CT-related morbidity, such as aspiration of contrast fluid, and the presence of leakage, slow transit or stenosis in the postoperative CT scan.
A total of 70 patients were enrolled in the study. Nine of those were excluded upon intraoperative decision to perform a sleeve gastrectomy and not a LRYGB; the main reason for procedure switch was suspected tension at the gastro-jejunostomy due to short and fatty mesentery. Among the 61 included patients, 50 were female and 11 were male, with a mean age of 40.3 years. The basic demographic and clinical data are presented in Table 1.
| Feature | Data, n (%) |
| Patients | 61 |
| Age, yr | 40.3 (19-62) |
| BMI, kg/m2 | 41 (35-48.1) |
| Female | 50 (82) |
| Male | 11 (18) |
| ASA I | 0 (0) |
| ASA II | 30 (49.1) |
| ASA III | 31 (50.9) |
| ASA IV | 0 (0) |
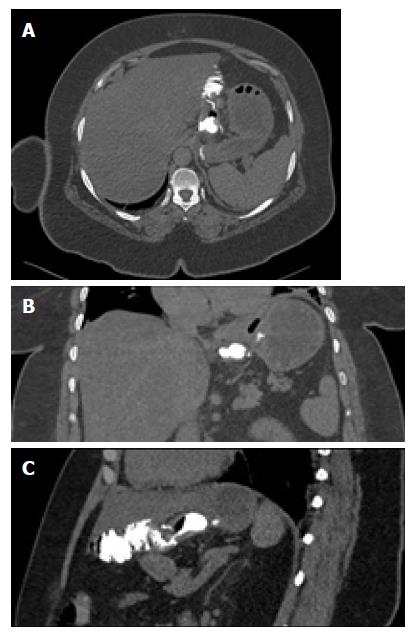
All patients tolerated early CT well and no vomiting occurred. 3D analysis of imaging was feasible in all patients and the proximal anastomosis was depicted very well (Figure 2). In 7 patients, two upper abdominal scans were necessary as the pouch was not distended by contrast medium in the first acquisition. The average total dose length product in CT was 536.6 mGycm and 7.8 mSv, respectively. Radiologically, no cases of leak or relevant stenosis were found on the first postoperative day. These early postoperative CT findings were consistent with the clinical follow-up at 6 wk postoperatively, for which no leaks, stenosis or obstructions were diagnosed. The morbidity data are presented in Table 2.
| Surgical | Non-surgical, n (%) | ||
| Anastomotic leak | 0 | Atelectasis | 52 (85.3) |
| Superficial hematoma | 1 (1.6) | Pleural effusion | 25 (41.0) |
| Superficial surgical site infection | 4 (6.6) | Pneumonia | 1 (1.6) |
| Re-laparoscopy | 0 | Cardiac complications | 1 (1.6) |
| Intraluminal bleeding | 1 (1.6) | Renal failure | 0 |
| Steatosis hepatitis | 7 (11.5) | ||
| Kidney stone | 1 (1.6) | ||
Non-surgical morbidity was very low. CT diagnosed atelectases in 85.3% of the patients and pleural effusions in 41% of the patients. In these patients, physiotherapy, which is part of the standard postoperative protocol, was intensified and fluid volume treatment was optimized, but no interventional treatment was required. In 1 patient with pneumonia, an antibiotic therapy was started. Moderate to severe diffuse hepatic steatosis was seen in 11 patients. One patient showed a kidney stone on the left side with no clinical symptoms.
The most common surgical complication, superficial surgical site infections (n = 4), occurred exclusively at the upper left trocar site, where the circular stapler had been introduced. No intra-abdominal re-operation due to complications was necessary. No radiological drain placement was needed within 6 wk postoperatively.
The present study of a large prospective cohort of patients who underwent LRYGB shows that CT scan performed on the first postoperative day is feasible and safe, and has high diagnostic accuracy. The CT was able to diagnose pulmonary changes that had been missed using UGI.
The usefulness of postoperative imaging after LRYGB, especially UGI studies, is under debate. Several studies have questioned the use of routine imaging, in particular UGI, in the early postoperative phase due to lack of sensitivity, high cost and the substantial radiation exposure to patients[5-7]. Most bariatric surgeons consider UGI useful to assess the gastric pouch and gastro-jejunostomy for anastomotic leaks or strictures in the early postoperative phase[12]. However, evaluation of a possible leak at the level of the biliopancreatic anastomosis is not possible with a routine UGI[16]. The positive predictive value and the sensitivity of diagnosing a leak by UGI were 31% and 25%, respectively, and the procedure has limited or no value in finding extra-luminal problems, such as hematoma, and is of no use for evaluating the biliopancreatic limb.
The effectiveness of UGI contrast studies has been evaluated in a number of research studies[8-10,17,18]. Brockmeyer et al[6] reported a 100% specificity for detection of a stenosis in a total of 319 patients. However, no leaks were detected on day 1 or 2 after surgery; although, 10 leaks occurred during further follow-up (all diagnosed by CT). Therefore, these authors stopped performing routine UGI on postoperative day 1 after a bariatric surgery.
Dallal et al[17] found that using clinical indicators of a leak or bleeding was sufficient for diagnosis, and no additional data from drains or an UGI study were necessary. The reported anastomotic leakage rate following LRYGB varies from 0.1%-5.6%, depending on the definition of a leak and the volume of the reporting surgical unit[19]. Leaks are the most significant factor for postoperative mortality in those patients[19,20]. Diagnosis usually takes place within the first 10 days after surgery[21]. A large prospective study[22] with 3018 patients diagnosed anastomotic leaks in 2.1% at a median of 3 d after LRYGB.
The earlier an anastomotic leak diagnosis is made, the better a patient’s prospects. This is mainly because early detection allows for an early therapeutic intervention, such as re-operation, surgical drainage or the application of stents. Delayed diagnosis of a leak leads to peritonitis and subsequently to a significantly higher mortality. In contrast to Dallal et al[17], we believe that radiological examination is an important adjunct since the clinical evaluation of these morbidly obese patients is difficult. The only clinical symptoms may be tachycardia and abdominal discomfort. In most bariatric centres, an UGI swallow study is performed on the first postoperative day[21] to assess for anastomotic stenosis or slow contrast transit, as well as to exclude an early postoperative leak primarily to allow the start of liquid intake. Therefore, it was decided to perform a CT scan on the first postoperative scan.
In UGI contrast studies, extra-luminal contrast extending into the upper left abdomen is regarded as highly suspicious with respect to a potential leak at the gastro-jejunostomy. Major leaks can be identified in up to 100% of patients by using UGI, but minor leaks are often missed. The latter are preferably diagnosed on CT scans[12,23-25]. Sometimes, the only sign of extra-luminal contrast in UGI studies is an opacification of the surgical drain that was placed nearby. But, some surgeons tend to omit a drain, and the opportunity of detecting this discrete sign is lost[26]. If no complication is detected by radiological means, oral intake is started. If UGI contrast studies are suspicious of a leak, stenosis or any other obstruction, usually, a CT scan is performed[12]. An anastomotic leak can show various manifestations on CT, including gas or fluid collections adjacent to the pouch, a tract of enteric contrast extending through the anastomotic defect, staple line dehiscence or diffuse peritoneal fluid[14]. Assessment of the jejuno-jejunostomy is easily feasible by CT.
Anastomotic stenosis or obstruction can be due to oedema, hematoma, ischemia or a blood clot at the circular stapled anastomosis[16]. These phenomena are more common at the gastro-jejunostomy (3%-9%) compared to the jejuno-jejunostomy (0.8%-2%)[16]. UGI contrast studies allow finding of an obstructed flow of contrast. But, in order to identify the underlying cause, again, a CT is usually performed. Although CT does not allow dynamic imaging, abnormal pouch distension and a contrast-filled oesophagus are indicators of an obstruction at the proximal anastomosis. CT can differentiate between any transient cause of obstruction, such as hematoma or oedema within the bowel wall, and a more permanent cause, such as kinking. Whereas the former will usually resolve within a few days and mandates only a slower increase of the oral diet, the latter will lead to further invasive procedures, such as endoscopy or re-operation. Furthermore, in case of suspected bleeding, a CT scan with an arterial phase can be performed to exclude surgical bleeding that needs to be addressed by re-operation.
Concerning the radiation dose, the individual dose received was 7.8 mSv for a limited CT scan in the obese patients of the present study. UGI radiation doses of 4 mSv per exam are known from the literature[9]. However, this is a very high radiation dose considering its limited use of diagnosing relevant pathologies. In contrast, CT scans, despite having a higher radiation dose, provide detailed depiction of the proximal and distal anastomosis and of any complication within the soft-tissue and lung base. CT scans also provide guidance for any possible interventional procedures. In addition, bariatric surgery patients may receive radiation doses from further postoperative radiological exams that could potentially increase their lifetime risk of cancer[9]. All these factors need to be considered when implementing postoperative protocols.
Our present study showed that CT is feasible and safe, and that a number of extra-gastrointestinal pathologies were detectable by this imaging modality. However, the real value as a standard postoperative radiological exam after LRYGB is debatable. From the available data, an UGI is not necessary as a standard postoperative exam, and similarly, a routine CT might not be indicated. However, if a patient undergoes unexpectedly difficult surgery or displays an abnormal postoperative clinical course, CT is the diagnostic tool of choice and the indication should be given liberally, especially when one considers the fact that a number of patients with pathological findings in UGI contrast studies will additionally undergo a CT scan.
The main limitation of this study is the fact that no surgical complications, such as leaks or obstructions, occurred in our study population. Therefore, no statement can be made on the sensitivity or specificity of CT regarding these pathologies on the first postoperative day. However, in our opinion, this technique is superior to a UGI study, since distinctive features such as fluid collection or free air surrounding the anastomosis, which are evidence for more serious complications, can be seen on CT images. Such has been demonstrated by Yu et al[14] in a retrospective study. While the risk-to-benefit ratio may be justified, strategies to minimize radiation dose should be emphasized and the appropriate technique in radiology should be selected judiciously.
In conclusion, early postoperative CT scan is feasible and safe. It has advantages over a standard UGI study as it provides more accurate information of the early postoperative anatomy after LRYGB and detects extra-gastrointestinal pathologies.
The laparoscopic Roux-en-Y gastric bypass (LRYGB) is the most common bariatric procedure worldwide. In a majority of bariatric centres, postoperative upper gastrointestinal (UGI) contrast studies are performed routinely in order to evaluate for complications. The usefulness of UGI examinations is debatable considering its related radiation dose and the obtained diagnostic information.
Several studies have suggested the usefulness of computed tomography (CT) to investigate postoperative complications after LRYGB, but data on routinely performed CT after bariatric surgery are scarce. UGI contrast studies are insufficient to accurately assess certain postoperative surgical problems, such as hematoma or fluid collections, and have only limited sensitivity for correctly diagnosing a leak, whereas CT can clearly diagnose these complications.
The prospective study was conducted to assess the feasibility and usefulness of CT scan as a primary early postoperative radiological measure in patients who underwent LRYGB.
Sixty-one patients who underwent LRYGB received upper abdominal CT on postoperative day 1. Diluted water-soluble radiographic contrast-medium (50 mL) was administered to achieve gastric pouch distension without movement of the patient. 3D images were evaluated to assess postoperative complications and the radiation dose received was analysed.
In 61 patients who were included in the analysis, CT was feasible and there were no instances of aspiration or vomiting. In 7 patients, two upper abdominal scans were necessary as the pouch was not distended by contrast medium in the first acquisition. Radiologically, no leak and no relevant stenosis were found on the first postoperative day. These early postoperative CT findings were consistent with the findings at clinical follow-up 6 wk postoperatively, with no leaks, stenosis or obstructions being diagnosed. The average total dose length product in CT was 536.6 mGycm resulting in an average effective dose of 7.8 mSv.
Early LRYGB postoperative CT scan is feasible, with low morbidity, and provides more accurate anatomical information than standard upper gastrointestinal contrast study.
The main limitation of this study is the fact that no surgical complications, such as leaks or obstructions, occurred in our study population. Therefore, no statement can be made on the sensitivity or specificity of CT regarding these pathologies on the first postoperative day. From this point of view large trials are necessary to enable a statement on the sensitivity and specificity of CT in this patient cohort.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gao BL, Mastoraki A S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6297] [Cited by in RCA: 5885] [Article Influence: 309.7] [Reference Citation Analysis (0)] |
| 2. | Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184:9S-16S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1220] [Cited by in RCA: 1071] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 4. | Blachar A, Federle MP, Pealer KM, Ikramuddin S, Schauer PR. Gastrointestinal complications of laparoscopic Roux-en-Y gastric bypass surgery: clinical and imaging findings. Radiology. 2002;223:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Doraiswamy A, Rasmussen JJ, Pierce J, Fuller W, Ali MR. The utility of routine postoperative upper GI series following laparoscopic gastric bypass. Surg Endosc. 2007;21:2159-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Brockmeyer JR, Simon TE, Jacob RK, Husain F, Choi Y. Upper gastrointestinal swallow study following bariatric surgery: institutional review and review of the literature. Obes Surg. 2012;22:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Singh R, Fisher BL. Sensitivity and specificity of postoperative upper GI series following gastric bypass. Obes Surg. 2003;13:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Blanchet MC, Mesmann C, Yanes M, Lepage S, Marion D, Gelas P, Gouillat C. 3D gastric computed tomography as a new imaging in patients with failure or complication after bariatric surgery. Obes Surg. 2010;20:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Oei TN, Shyn PB, Govindarajulu U, Flint R. Diagnostic medical radiation dose in patients after laparoscopic bariatric surgery. Obes Surg. 2010;20:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Mittermair R, Sucher R, Perathoner A, Wykypiel H. Routine upper gastrointestinal swallow studies after laparoscopic sleeve gastrectomy are unnecessary. Am J Surg. 2014;207:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Rawlins L, Penn R, Schirmer B, Hallowell P. Accuracy of routine postoperative swallow study in predicting leak or obstruction after gastric bypass. Surg Obes Relat Dis. 2015;11:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Blachar A, Federle MP, Pealer KM, Abu Abeid S, Graif M. Radiographic manifestations of normal postoperative anatomy and gastrointestinal complications of bariatric surgery, with emphasis on CT imaging findings. Semin Ultrasound CT MR. 2004;25:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Alva S, Eisenberg D, Duffy A, Roberts K, Israel G, Bell R. Virtual three-dimensional computed tomography assessment of the gastric pouch following laparoscopic Roux-Y gastric bypass. Obes Surg. 2008;18:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Yu J, Turner MA, Cho SR, Fulcher AS, DeMaria EJ, Kellum JM, Sugerman HJ. Normal anatomy and complications after gastric bypass surgery: helical CT findings. Radiology. 2004;231:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Karcz WK, Kuesters S, Marjanovic G, Suesslin D, Kotter E, Thomusch O, Hopt UT, Felmerer G, Langer M, Baumann T. 3D-MSCT gastric pouch volumetry in bariatric surgery-preliminary clinical results. Obes Surg. 2009;19:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lehnert B, Moshiri M, Osman S, Khandelwal S, Elojeimy S, Bhargava P, Katz DS. Imaging of complications of common bariatric surgical procedures. Radiol Clin North Am. 2014;52:1071-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Dallal RM, Bailey L, Nahmias N. Back to basics--clinical diagnosis in bariatric surgery. Routine drains and upper GI series are unnecessary. Surg Endosc. 2007;21:2268-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Carter JT, Tafreshian S, Campos GM, Tiwari U, Herbella F, Cello JP, Patti MG, Rogers SJ, Posselt AM. Routine upper GI series after gastric bypass does not reliably identify anastomotic leaks or predict stricture formation. Surg Endosc. 2007;21:2172-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Fernandez AZ Jr, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, Sugerman HJ. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc. 2004;18:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 826] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 21. | Carucci LR, Turner MA. Radiologic evaluation following Roux-en-Y gastric bypass surgery for morbid obesity. Eur J Radiol. 2005;53:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, Rosenthal R, Murr MM. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Marshall JS, Srivastava A, Gupta SK, Rossi TR, DeBord JR. Roux-en-Y gastric bypass leak complications. Arch Surg. 2003;138:520-523; discussion 523-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 448] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Carucci LR, Turner MA, Conklin RC, DeMaria EJ, Kellum JM, Sugerman HJ. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology. 2006;238:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |