Copyright
©2014 Baishideng Publishing Group Inc.
World J Radiol. Sep 28, 2014; 6(9): 677-692
Published online Sep 28, 2014. doi: 10.4329/wjr.v6.i9.677
Published online Sep 28, 2014. doi: 10.4329/wjr.v6.i9.677
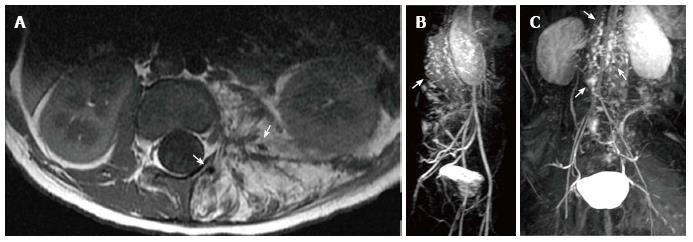
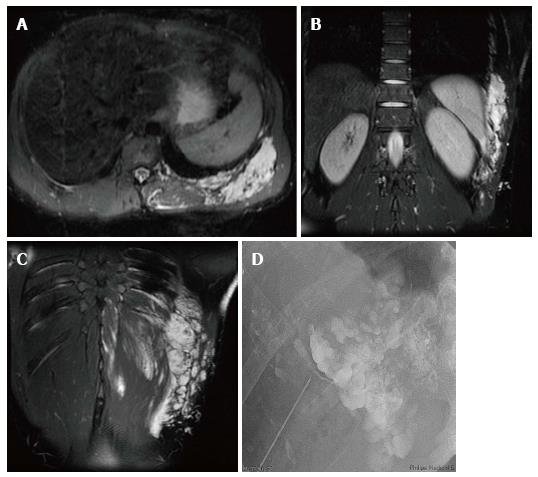
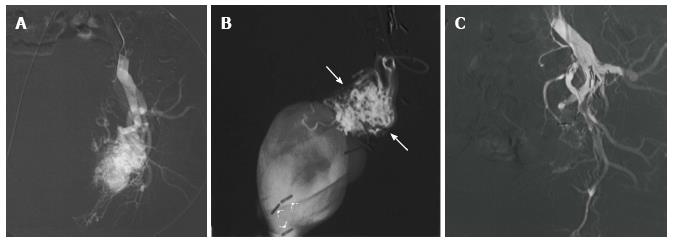
Figure 1 Kaposiform hemangioendothelioma.
A: Magnetic resonance (MR) of the retroperitoneum demonstrating infiltrative tumor with fat and interspersed signal void (arrows) consistent with high flow arterial signal; B, C: MR angiogram demonstrating arteriovenous shunting within the tumor (arrows).
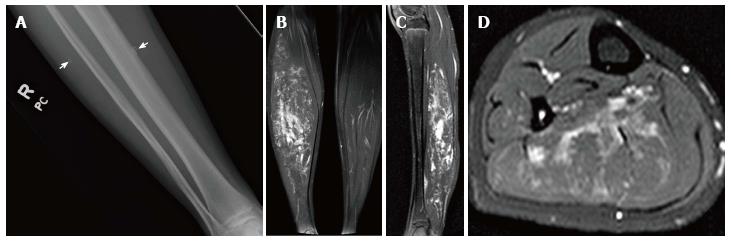
Figure 2 Low flow extratruncular venous malformation.
Radiograph of the right lower leg (A) demonstrates phleboliths within the soft tissues (arrows). T2-weighted magnetic resonance images in the coronal (B), sagittal (C), and axial (D) planes demonstrate hyperintense signal within the gastrocnemius muscle due to infiltrative low flow extratruncular venous malformation.
Figure 3 Three-month-old child with CLOVES syndrome.
Radiograph of the chest and abdomen (A) demonstrates large soft tissue mass within the left chest and upper abdominal wall. Radiograph of the foot (B) demonstrates overgrowth of the third and fourth digits. Fat suppressed T2 weighted magnetic resonance (MR) images in axial (C), coronal (D) and sagittal (E) planes show the large soft tissue mass within the chest wall contains several loculations, some of which demonstrate hypointense fluid-fluid levels due to hemorrhage. T1 weighted MR image in the axial plane (F) confirms lipomatous overgrowth admixed with muscle.
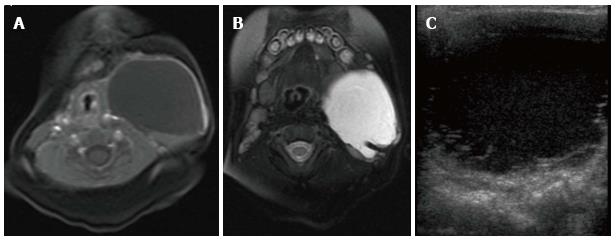
Figure 4 Cervicothoracic macrocystic lymphatic malformation.
T1- (A) and T2-weighted (B) fat suppressed magnetic resonance images (MRI) demonstrate a macrocyst within the left neck that is predominantly hypointense on T1-weighted image and hyperintense on T2-weighted image. Trace blood products layering within the posterior aspect of the cyst are hyperintense on the T1 weighted image and hypointense on the T2 weighted image. Transverse ultrasound (C) demonstrates a predominantly anechoic macrocyst with layering low-level echoes, corresponding to blood products seen on MRI.
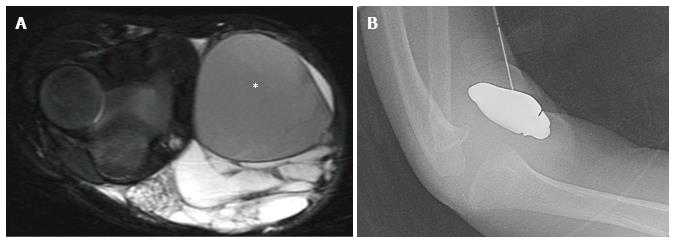
Figure 5 Macrocystic lymphatic malformation with hemorrhage.
Axial fat suppressed T2 weighted magnetic resonance image of the elbow (A) demonstrates a predominantly hyperintense malformation with hypointense hemorrhage in a loculation (asterisk). Direct access to the malformation is obtained with a 22-gauge needle for sclerosis (B), subsequently performed with doxycycline.
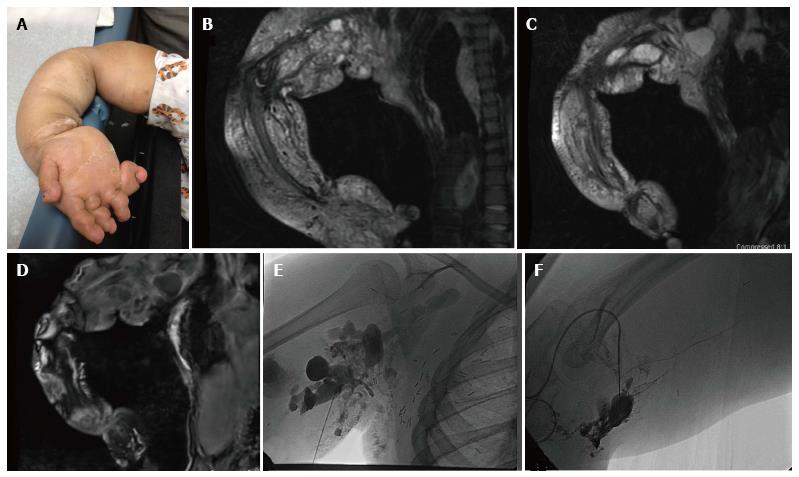
Figure 6 Ten-year-old girl with CLOVES syndrome.
Photograph (A) demonstrates right upper extremity overgrowth. Pre-treatment coronal MR images of the right upper extremity (B, C, D) demonstrate a large, combined macro/microcystic lymphatic malformation with venous lakes in the axilla and evidence of lipomatous overgrowth. Angiographic images (E, F) demonstrate direct puncture of the malformation followed by sclerosis with doxycycline.
Figure 7 Infiltrative extratruncular low flow vascular malformation of the right leg.
Coronal (A) and axial (B) T2-weighted magnetic resonance images demonstrate a high signal intensity infiltrative lesion (arrows). Venography (C) demonstrates filling of the malformation with outflow communication to the femoral vein (arrow). Sclerosis was subsequently performed utilizing 3% STS opacified with contrast (D) with compression of the outflow vein using metal forceps.
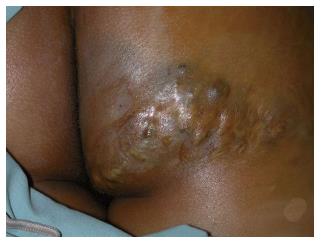
Figure 8 Superficial low flow venous malformation.
This soft compressible mass with nodular purple skin discoloration in the buttock is typical of a superficial low flow venous malformation.
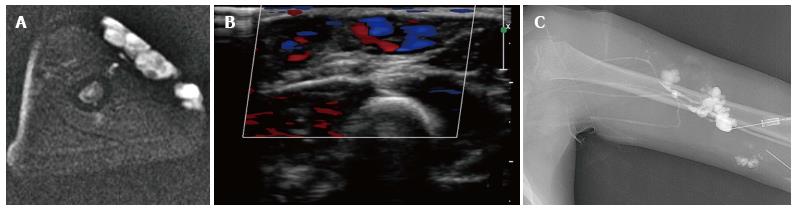
Figure 9 Foaming the sclerosant increases the surface contact of the foam micelles with the endothelium of the malformation.
Axial T2 magnetic resonance demonstrating high signal intensity subcutaneous low flow venous malformation of the left upper arm (A). The lower signal small round structure likely represents a phlebolith. Color Doppler ultrasound demonstrates low flow signal in the venous malformation (B). Venography prior to sclerotherapy with tourniquet in place on upper arm demonstrates the venous malformation with no filling of normal deep venous drainage (C). A few faint radio-opaque phleboliths are seen in the venographic image.
Figure 10 Foam sclerotherapy is ideal for treatment of low flow venous vascular malformations.
Axial and coronal T2 weighted magnetic resonance images demonstrate a low flow venous malformation of the chest and upper abdominal wall (A, B, C). Direct access venography with a 22-gauge needle shows foamed STS opacified with contrast filling the malformation (D).
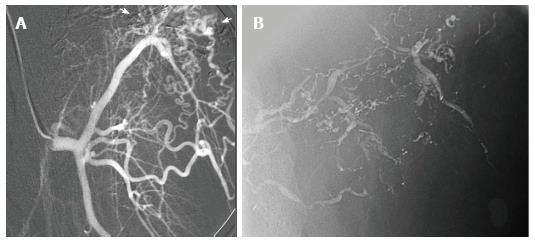
Figure 11 High flow arteriovenous malformation of second digit.
This patient scheduled for amputation due to intractable pain under went alcohol embolization as a last resort prior to surgery. Digital arteriography with a microcatheter in the lateral digital artery with the tip at the level of the middle phalanx demonstrating filling of the nidus of the malformation (A, B, C). Following embolizaton, there is stasis of flow in the malformation (D). The medial digital artery remained patent with preservation of arterial flow to the digit.
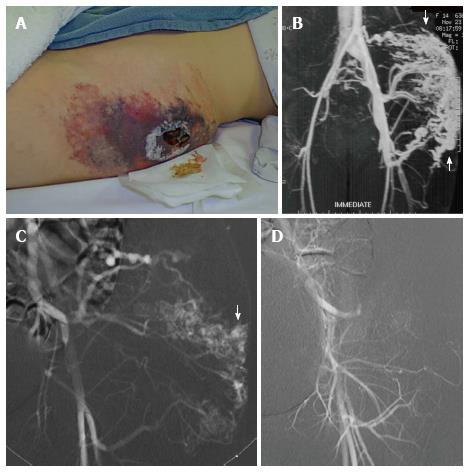
Figure 12 Superficial lesions may present as a warm painless mass with palpable bruit and associated dilated veins.
High flow arteriovenous malformation with extensive skin breakdown in the region of this superficial malformation (A). Magnetic resonance angiogram demonstrates a high flow arteriovenous malformation with arteriovenous shunting (arrow) (B). Left internal iliac arteriogram demonstrating filling of the nidus of the malformation (arrow) (C). Arteriography following alcohol embolization shows eradication of the nidus of the malformation (D). As the malformation was located in subcutaneous fat, it was resected en block and skin grafts were created to bridge the area of skin breakdown.
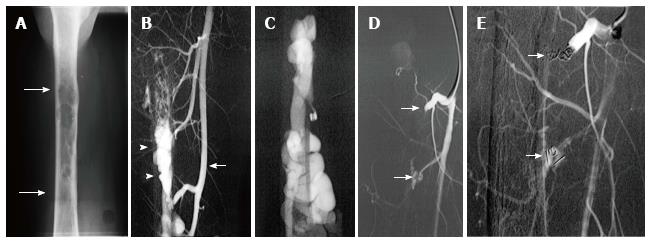
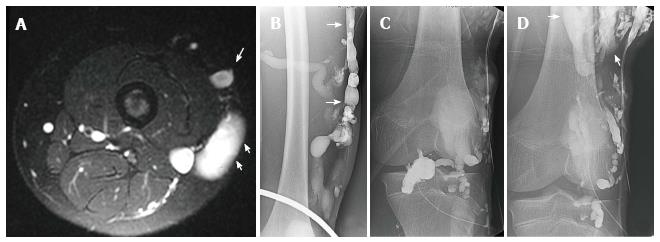
Figure 13 Use of detachable coils, released only when satisfactory placement is achieved, may increase the safety of the procedure.
Radiograph of the mid-diaphysis of the femur demonstrates a region of endosteal erosion (arrows) (A). Arteriography demonstrates a multifistulous malformation with arterial supply from two muscular branches of the superficial femoral artery (arrow) with early filling of intraosseous venous drainage (arrowheads) (B, C). Occlusion of the feeding arteries was accomplished with large coils (arrow) eliminating the fistulous communications (D, E).
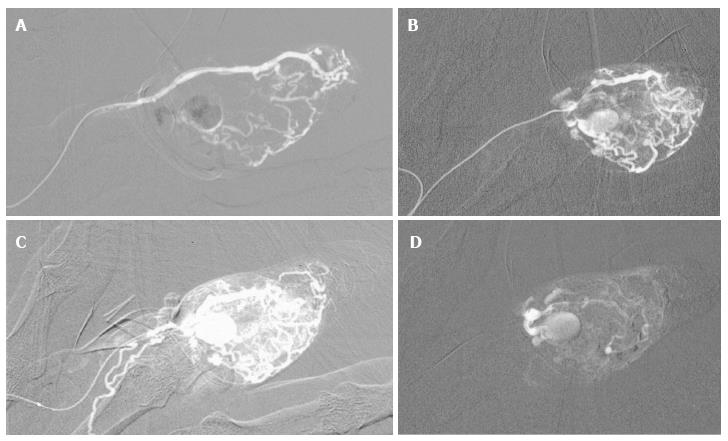
Figure 14 Particulate agents do not generally provide complete occlusion, and recanalization may occur.
High flow arteriovenous malformation with nidus and venous aneurysm originating from branches of the left internal iliac artery (A). Following superselective catheterization of the feeding branch of the inferior gluteal artery there is demonstration of the nidus and venous aneurysm (arrow) (B). Following particulate embolization with PVA, there is occlusion of the nidus. Surgical clips are seen overlying internal iliac artery branches from previous unsuccessful attempts at surgical treatment (C).
Figure 15 Arteriogram of high flow arteriovenous malformation.
A: Arteriogram demonstrates nidus (arrows) of a high flow arteriovenous malformation of the abdominal wall, supplied in part by a muscular branch of the circumflex femoral artery; B: The lesion was treated twice, initially with Onyx, then with n-BCA glue, seen within the arterial feeders of the malformation on post-embolization imaging.
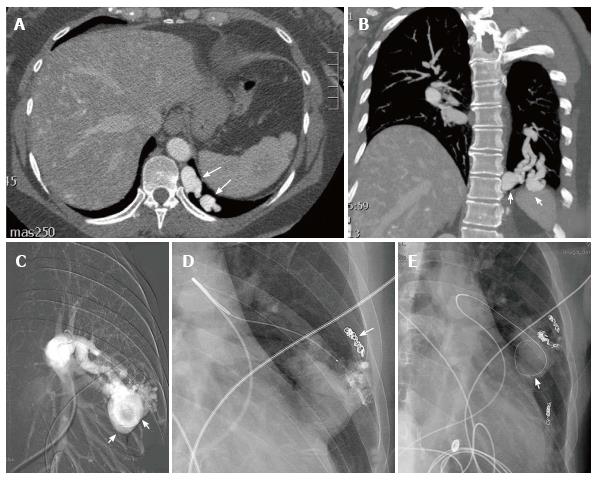
Figure 16 This patient with hereditary hemorrhagic telangiectasia presented with an abnormal chest radiograph.
A computed tomography angiogram was performed for further evaluation. Axial CTA demonstrates a left lower lobe high flow vascular malformation (arrows) (A). Coronal reconstruction demonstrates several branches of the left lower lobe pulmonary artery feeding the malformation (arrows) (B). Sequential coil embolization of pulmonary arterial branches feeding the malformation was performed (arrow) (C). A framing coil was then placed in the venous aneurysm (D) followed by coil occlusion of the venous aneurysm and each of the remaining pulmonary arterial feeding branches (arrow) (E).
Figure 17 Venogram of low flow venous malformation in Parkes Weber Syndrome.
Right lower extremity venogram demonstrates extensive low flow venous malformation in a patient with the Parkes Weber syndrome (A). Right lower extremity arteriogram demonstrating tibial artery shunting to the venous malformation (arrows) (B, C).
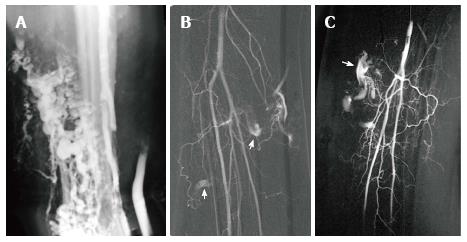
Figure 18 Klippel Trenaunay Syndrome.
Axial T2 weighted fat suppressed magnetic resonance image of the thigh (A) in a patient with Klippel Trenaunay syndrome demonstrates a lateral embryonic vein (arrows) and venous malformation (short arrows). Lower extremity venogram demonstrates the lateral embryonic vein (arrows) (B). Direct puncture venography prior to alcohol sclerotherapy demonstrates progressive filling of the low flow truncular venous malformation (arrows) (C, D).
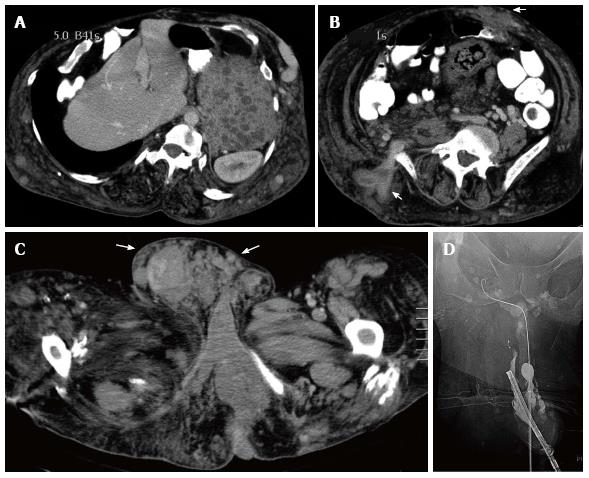
Figure 19 Klippel Trenaunay Syndrome.
CT scan of the abdomen in a patient with the Klippel Trenaunay syndrome demonstrating extensive venous malformation in the abdominal wall (arrows) as well as venous malformations in the spleen (asterisk) (A, B). CT scan of the pelvis demonstrating extensive venous malformation in the inguinal canal and scrotum (arrows) (C). Sclerotherapy was performed through the dorsal vein of the penis to treat intractable urethral hemorrhage (D).
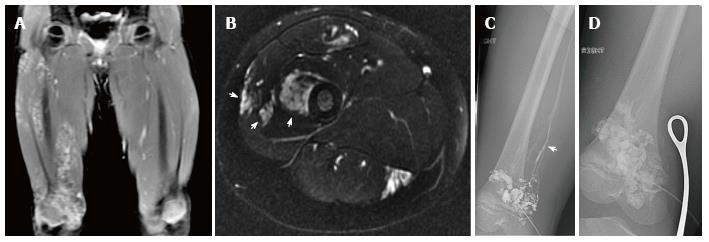
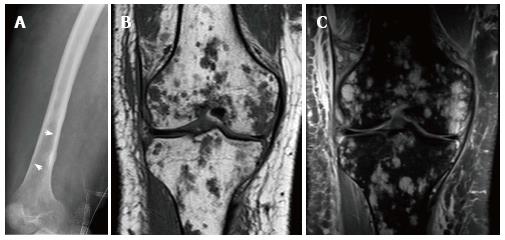
Figure 20 21-year-old man, who presented with pain and swelling of his leg.
Radiograph of the femur demonstrates cortical erosion of the distal femur (arrows) (A). T1 weighted coronal magnetic resonance (MR) of the knee demonstrates innumerable focal lesions in the tibia and femur (B). These lesions are high signal intensity on T2 weighted coronal MR (C), however no contrast enhancement was seen within the lesions. Biopsy of the femur demonstrated a lymphatic microcystic lymphatic malformation.
- Citation: Nosher JL, Murillo PG, Liszewski M, Gendel V, Gribbin CE. Vascular anomalies: A pictorial review of nomenclature, diagnosis and treatment. World J Radiol 2014; 6(9): 677-692
- URL: https://www.wjgnet.com/1949-8470/full/v6/i9/677.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i9.677