Published online Oct 26, 2016. doi: 10.4330/wjc.v8.i10.615
Peer-review started: April 26, 2016
First decision: May 17, 2016
Revised: August 5, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: October 26, 2016
Processing time: 183 Days and 19.3 Hours
To study whether remote ischemic preconditioning (RIPC) has an impact on clinical outcomes, such as post-operative atrial fibrillation (POAF).
This was a prospective, single-center, single-blinded, randomized controlled study. One hundred and two patients were randomized to receive RIPC (3 cycles of 5 min ischemia and 5 min reperfusion in the upper arm after induction of anesthesia) or no RIPC (control). Primary outcome was POAF lasting for five minutes or longer during the first seven days after surgery. Secondary outcomes included length of hospital stay, incidence of inpatient mortality, myocardial infarction, and stroke.
POAF occurred at a rate of 54% in the RIPC group and 41.2% in the control group (P = 0.23). No statistically significant differences were noted in secondary outcomes between the two groups.
This is the first study in the United States to suggest that RIPC does not reduce POAF in patients with elective or urgent cardiac surgery. There were no differences in adverse effects in either group. Further studies are required to assess the relationship between RIPC and POAF.
Core tip: This is the first study in the United States to suggest that remote ischemic preconditioning does not reduce post-operative atrial fibrillation in patients with elective or urgent cardiac surgery.
- Citation: Lotfi AS, Eftekhari H, Atreya AR, Kashikar A, Sivalingam SK, Giannoni M, Visintainer P, Engelman D. Randomized controlled trial of remote ischemic preconditioning and atrial fibrillation in patients undergoing cardiac surgery. World J Cardiol 2016; 8(10): 615-622
- URL: https://www.wjgnet.com/1949-8462/full/v8/i10/615.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i10.615
Post-operative atrial fibrillation (POAF) is the most common arrhythmia after coronary artery bypass grafting (CABG)[1]. Despite improvement in medical therapy, surgical technique, and anesthesia, POAF occurs in 25%-40% of patients undergoing CABG and valve surgery[1-4]. POAF remains challenging to prevent, treat, or cure[5], and contributes to increased short and long term mortality[6,7], stroke[8], an increase in length of hospital stay[9], intensive care unit readmission, and treatment costs[10]. Some factors associated with POAF include a patient’s preoperative status, age, and preexisting electrocardiogram abnormalities[11]. Intra-operative stress also plays a key role, due to occurrence of reperfusion, inflammation, oxidative stress, and/or hemostasis[12-14]. Most POAF episodes occur within the first 6 d following cardiac surgery, with the peak incidence on the second or third post-operative day, coinciding with the peak of systemic inflammation caused by surgery and with atrial stretch[8].
Remote ischemic preconditioning (RIPC) is one strategy that has shown a myocardial protective effect during CABG and heart valve surgery[15-17]. It was first described in 1986 in a dog model, where RIPC provided a protective effect on the myocardium that was later subjected to a sustained bout of ischemia[18]. RIPC was shown to reduce the incidence of ischemic reperfusion ventricular arrhythmias[19]. There is evidence that RIPC can preserve mitochondrial function and influences myocardial microRNA expression of the right atrium, potentially decreasing the incidence POAF after CABG surgery[20,21]. In addition, the efficacy of preconditioning to reduce myocardial injury in cardiac surgery and percutaneous coronary interventions has also been demonstrated[22].
There is growing evidence that AF is associated with increased inflammation[23,24], and Jannati et al[25], demonstrated that myocardial ischemic preconditioning by aortic cross-clamping in patients undergoing CABG reduced the incidence of POAF.
Currently, there is no optimal preconditioning protocol or tool being utilized during cardiac surgery and aortic cross-clamping may increase the risk of embolic stroke, particularly in elderly patients[26]. We conducted a randomized clinical trial to assess if RIPC can reduce POAF after CABG, with or without concomitant valve surgery or valve surgery alone.
This study was a prospective, single-center, single-blinded, randomized controlled trial. The trial was registered with http://www.clinicaltrials.gov (NCT01500369).
Patients who were undergoing non-emergent cardiac surgery were screened and recruited from the cardiac surgical service.
Eligible patients were adults greater than 18 years old who were referred for elective or urgent CABG, with or without valve surgery, or valve surgery alone between April 2011 and October 2013.
Exclusion criteria included any preoperative rhythm detected other than a sinus rhythm, a history of AF, New York Heart Association IV congestive heart failure, cardiogenic shock, emergent CABG and/or valve surgery, bleeding diathesis, patients taking K(+) ATPase channel blockers (sulphonylureas), and women of child-bearing age. Patients were contacted by the primary investigator or a cardiology fellow to explain the study and obtain consent. This occurred during the 24-h period after undergoing cardiac catheterization (urgent care patients) or during a pre-op office visit (elective surgery patients). Patients who were interested gave written informed consent. Trial approval was obtained from the Institutional Review Board and the study is registered at http://www.clinicaltrials.gov; identifier NCT01500369. Upon consent, participants were randomized during the pre-operative period to either the treatment or control group.
Patient blinding: Patients were randomly assigned to a treatment strategy (RIPC/no RIPC) in the operative room during the 45-min pre-operation period. Randomization occurred after patients were anesthetized; thus, patients were unaware of their treatment assignment.
Physician blinding: Since randomization and the RIPC procedure were conducted preoperatively, we expect that the surgeons were unaware of patient treatment assignment, and an effort was made to prevent surgeon knowledge of which group was selected.
The randomization schedule was developed by the institution’s statistical core facility and patients were randomized according to a computer-generated randomization procedure. Patients were randomized using blocks in sizes 4 and 6, administered in a random fashion.
Consecutively-numbered envelopes were created and populated with a patient identification and the treatment assignment, based on the random block. The envelopes were kept in a locked cabinet. When an eligible patient was identified, consented, and moved to the pre-operative area, the staff member would select the next envelope in the consecutive list and give it to the research nurse. The research nurse would open the envelope and proceed as indicated on the enclosed form.
For all study participants, anesthesia was induced with intravenous propofol (0.5-2 mg/kg), midazolam (0.04-0.05 mg/kg), fentanyl (1-5 μg/kg), and rocuronium (0.6-1 mg/kg), and maintained with isoflurane. On-pump surgical revascularization was achieved through a median sternotomy. The internal thoracic arteries, radial arteries, and saphenous veins were used as grafts. Heparin was administered to achieve an activated clotting time longer than 400 s. Standard non-pulsatile cardiopulmonary bypass with a membrane oxygenator was used with an ascending-aortic and two-stage venous cannulation. During cardiopulmonary bypass, moderate hemodilution with a hematocrit of approximately 25% and mild systemic hypothermia (32 °C) were maintained. Retrograde warm blood cardioplegia was used for all distal anastomoses. Proximal anastomoses were constructed with partial side clamping of the ascending aorta. Bypass graft flow was assessed with an ultrasonic transit time-flow measurement probe. After reperfusion and weaning from cardiopulmonary bypass, protamine was administered for heparin reversal. For hemodynamic support, inotropes and/or vasopressors were infused as required.
RIPC, for those in the study arm, took place after induction of anesthesia and prior to skin incision during which time the patient was prepped, draped, and prepared for surgery using the following protocol.
Treatment group: Patients in the treatment group received 3 sequential sphygmomanometer cuff inflations on their right upper arm after induction of anesthesia in the operating room. The cuff was inflated to 200 mmHg for five minutes each occasion, with a period of five minutes deflation between inflations. The entire RIPC phase lasted 30 min.
Control group: Patients in the control group had the sphygmomanometer cuff placed on their right upper arm, but the cuff was not inflated. Similar to patients in the treatment group, patients in the control group had to undergo the same 30 min delay before the initiation of a skin incision.
Primary outcome: The primary outcome was POAF lasting for five minutes or longer during the first seven days after surgery. This outcome was assessed by using patient’s hospital records as well as the Society of Thoracic Surgery (STS) database which records outcomes up until 30 d after surgery.
Secondary outcomes: Secondary outcomes such as length of hospital stay, inpatient death, myocardial infarction (MI), and stroke were recorded during the study follow-up period. Additionally, using the STS definitions for perioperative outcomes (Table 1), the 30-d death, MI, stroke, and readmission were obtained from the institutional STS database.
| Outcomes | Definition |
| Stroke | If the patient had a central neurological deficit persisting postoperatively for > 72 h |
| Peri-operative MI | 0-24 h post-operative: The CK-MB (or CK if MB not available) must be greater than or equal to 5 times the upper limit of normal, with or without new Q waves present in two or more contiguous ECG leads No symptoms required > 24 h post-operative: Indicate the presence of a peri-operative MI (> 24 h post-op) as documented by at least one of the following criteria: (1) Evolutionary ST-segment elevations (2) Development of new Q-waves in two or more contiguous ECG leads (3) New or presumably new LBBB pattern on the ECG (4) The CK-MB (or CK if MB not available) must be greater than or equal to 3 times the upper limit of normal |
Adverse outcomes: Adverse events were documented after the initiation of the protocol.
Treatment and control groups were compared on baseline characteristics to identify whether randomization was successful. Continuous variables were compared using 2-sample t tests or the non-parametric equivalent (Wilcoxon rank-sum test), while categorical variables were compared using Pearson χ2 or Fisher’s exact test. For dichotomous outcomes, logistic regression was used to adjust for group imbalances, when necessary. To examine whether treatment assignment influenced time to first occurrence of POAF, a log-rank test of the Kaplan-Meier survival functions was conducted.
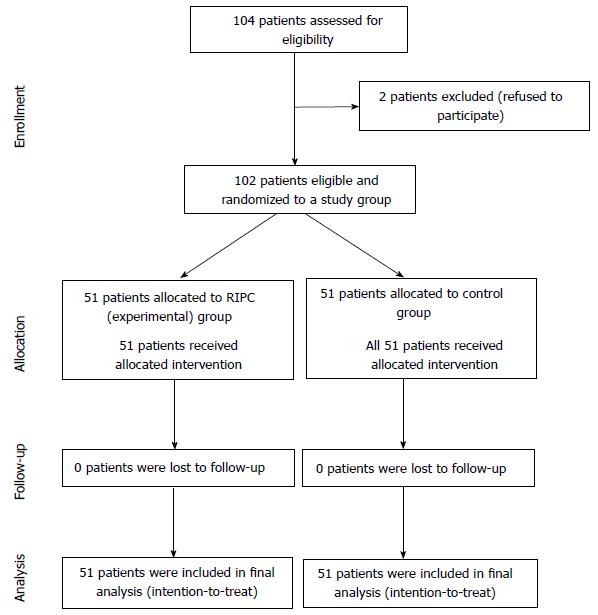
A total of 102 patients were randomized between April 2011 and September 2013 (Figure 1). Sixty-nine point nine percent of the patients were males and 89% were Caucasian (Table 2). The mean age of patients in the RIPC and control group was 69.4 and 68.9 years, respectively. With the exception of diabetes mellitus, the two groups were balanced with respect to baseline characteristics. Study groups were also well balanced with respect to medication use including beta blocker and HMG-CoA reductase inhibitors (statins). 46% of the patients presented with acute coronary syndrome and 23.5% presented with stable angina and were well matched (Table 3).
| Characteristic | Control (n = 51) | RIPC (n = 51) | P |
| Demographics | |||
| Mean age (± SD) | 68.9 (± 9.8) | 69.4 (± 9.9) | 0.77 |
| Male % (n) | 62.8 (32) | 76.5 (39) | 0.20 |
| Caucasian % (n) | 88.2 (45) | 90.2 (46) | 1.00 |
| Mean BMI (± SD) | 30.4 (± 7.6) | 28.4 (± 5.2) | 0.13 |
| Co-morbidities | |||
| Diabetes mellitus % (n) | 39.2 (20) | 62.7 (32) | 0.029 |
| Hypertension % (n) | 84.3 (43) | 82.4 (42) | 1.00 |
| Dyslipidemia % (n) | 90.2 (46) | 90.2 (46) | 1.00 |
| Heart failure % (n) | 21.6 (11) | 23.5 (12) | 1.00 |
| Atrial fibrillation % (n) | 0.0 (0) | 0.0 (0) | NA |
| AICD % (n) | 0.0 (0) | 2.0 (1) | 1.00 |
| CVA % (n) | 3.9 (2) | 5.9 (3) | 1.00 |
| TIA % (n) | 2.0 (1) | 7.8 (4) | 0.36 |
| PAD % (n) | 9.8 (5) | 21.6 (11) | 0.17 |
| CKD % (n) | 23.5 (12) | 23.5 (12) | 1.00 |
| Dialysis % (n) | 2.0 (1) | 2.0 (1) | 1.00 |
| Mean creatinine (± SD) | 1.2 (± 1.1) | 1.2 (± 0.7) | 0.89 |
| COPD % (n) | 5.9 (3) | 0.0 (0) | 0.24 |
| Tobacco use % (n) | 17.6 (9) | 27.5 (14) | 0.34 |
| Characteristic | Control (n = 51) | RIPC (n = 51) | P |
| Medications % (n) | |||
| Alpha blockers | 7.8 (4) | 2.0 (1) | 0.36 |
| Beta blockers | 78.4 (40) | 80.4 (41) | 1.00 |
| ACE-inhibitors | 37.3 (19) | 41.2 (21) | 0.84 |
| Aspirin | 90.2 (46) | 90.2 (46) | 1.00 |
| Statins | 84.3 (43) | 86.3 (44) | 1.00 |
| Clinical presentation % (n) | |||
| Stable angina | 23.5 (12) | 23.5 (12) | 1.00 |
| Unstable angina | 25.5 (13) | 25.5 (13) | 1.00 |
| Positive stress test | 27.5 (14) | 25.5 (13) | 1.00 |
| Non-STEMI | 19.6 (10) | 17.6 (9) | 1.00 |
| STEMI | 0.0 (0) | 2.0 (1) | 1.00 |
| Valve without CAD | 17.6 (9) | 27.5 (14) | 0.34 |
POAF occurred at the rate of 54.0% in the RIPC group and 41.2% in the control group (P = 0.23). Expressed as a difference in proportions, the percent of patients experiencing POAF was 12.8% higher in the RIPC group compared with the usual care group (95%CI: -6.5%-32.1%). Although the presence of diabetes was significantly higher in the RIPC group, it was not associated with any outcome, and consequently, adjusting for diabetes in logistic regression models did not materially change the univariable results.
No post-operative MIs occurred in the RIPC group while 3.9% did in the control group, although this difference was not statistically significant (P = 0.50) (Table 4). There were only two deaths and two strokes for the entire study group and both occurred in the RIPC group. The 30-d readmission rates demonstrated no statistically significant difference between the two groups. The length of stay, left ventricular ejection fraction, and cross-clamp time demonstrated no significant difference between the control and RIPC groups.
| Characteristic | Control (n = 51) | RIPC (n = 51) | P |
| Primary endpoint | |||
| POAF % (n) | 41.2 (21) | 54.0 (27) | 0.23 |
| Secondary endpoints | |||
| Other arrhythmia % (n) | 13.7 (7) | 11.8 (6) | 1.00 |
| MI % (n) | 3.9 (2) | 0.0 (0) | 0.50 |
| Stroke % (n) | 0.0 (0) | 3.9 (2) | 0.24 |
| Mean EF (± SD) | 53.1 (± 14.8) | 50.5 (± 16.9) | 0.43 |
| Bleeding % (n) | 21.6 (11) | 28.0 (14) | 0.50 |
| Mean cross-clamp time (± SD) | 88.7 (± 44.8) | 93.0 (± 38.5) | 0.61 |
| In-hospital mortality % (n) | 0 (0) | 3.9 (2) | 0.50 |
| 30-d mortality (after discharge) % (n) | 0 (0) | 0 (0) | 1.0 |
| 30-d readmission % (n) | 11.8 (6) | 16.3 (8) | 0.57 |
| Mean LOS (± SD) | 13.7 (± 7.8) | 14.0 (± 7.7) | 0.87 |
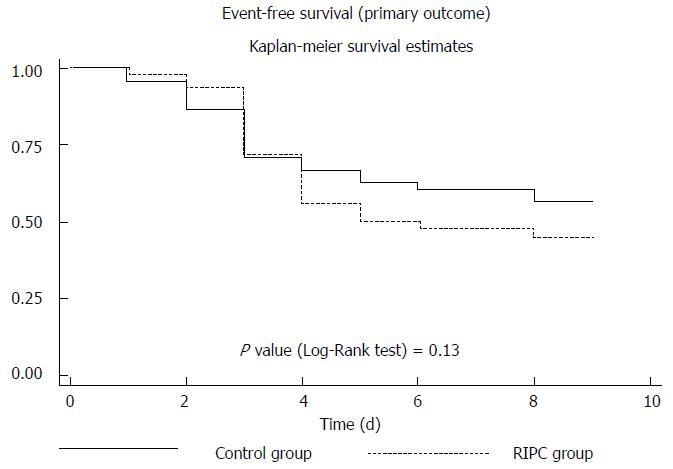
The event rate for POAF, based on Kaplan-Meier analysis, was not significantly different between the RIPC and control group (P = 0.13) (Figure 2). No adverse events related to RIPC occurred.
In our study that assessed the effect of RIPC on clinical outcomes in patients undergoing elective or urgent cardiac surgery, we found that RIPC did not reduce POAF. In addition, there were no statistically significant differences in secondary outcomes, including post-operative MI and stroke and no adverse events were reported with RIPC.
The Effect of Remote Ischemic Preconditioning on Clinical Outcomes in CABG Surgery (ERICCA) study randomized 1216 patients who underwent CABG to RIPC vs control and demonstrated that at one year there was no statistically significant difference in the primary clinical outcome (cardiovascular clinical death, MI, stroke and coronary revascularization)[27]; no data regarding POAF were provided. Previous studies to date have largely evaluated the impact of RIPC on surrogate markers of clinical outcomes. RIPC has been evaluated in patients undergoing percutaneous coronary intervention to reduce myocardial injury[28], reduce contrast-induced nephropathy[29], and myocardial salvage in ST-segment elevation MI[30]. Specifically, in patients undergoing cardiac surgery, RIPC has been known to decrease myocardial injury measured by cardiac troponin release[31,32]. At the same time, several other trials have failed to show improvement in surrogate outcomes with the implementation of RIPC[33,34], and this can be attributed to variable protocols, medications, surgical, and anesthetic regimens. It is also difficult to draw any conclusions regarding clinical outcomes from these studies as they were included only as secondary outcomes, often under-powered and had varying definitions of clinical outcomes[35]. Thus, no trials have been published demonstrating that RIPC significantly reduced clinical endpoints in patients undergoing cardiac surgery[36].
The rate of POAF in our study was higher than expected in both groups, which could be related to the small sample size and the presenting co-morbidities. Additionally, the absolute numbers of secondary outcomes recorded were quite small and therefore, are only exploratory at this stage. The unreliability of studies with small study samples is well-known[37,38]. Even if significant results had emerged from our study, regardless of direction of effect, we would caution against the over-interpretation of results, since small studies often produce large effects that frequently defy replication[39]. To our knowledge, this is the first study undertaken in the United States to assess the relationship of RIPC with POAF. Although this small study found no significant association of RIPC with clinical outcomes, it serves as an addition to the sparse literature on RIPC and clinical outcomes and would be of value when additional small studies are published. Meta-analyses of randomized controlled studies could yield a more accurate estimation of the true relationship between RIPC and POAF by combining patients and increasing sample power.
There were several limitations to our study that may have contributed to it not resulting in a positive finding. First, the study was halted prematurely, due to the lack of financial support to continue recruitment, which led to a study with less power than intended. However, given a control POAF rate of 50% (as seen in this population), the study still had 70% power to detect a 25% percentage points difference. Second, there was a significantly higher percentage of patients with diabetes mellitus in the RIPC arm, which may have masked the beneficial effect of RIPC[40]. However, this is unlikely to have significantly confounded the results as there was no change in the relationship of RIPC with outcomes even after adjustment using logistic regression analysis. Third, there is some recent evidence that patients given propofol may not gain protection from RIPC[41,42], possibly related to its structure being similar to that of phenol-based radical scavengers. This study was started prior to the publication of the study by Kottenberg et al[41], and in our study, propofol was used for the induction of anesthesia, not for maintenance. As with the majority of RIPC studies[35], we performed 3 cycles of RIPC, and in future trials it may be necessary to perform more than 3 cycles of blood pressure cuff inflation to provide clinical benefit. A final limitation is that warm cardioplegia has demonstrated a reduction in myocardial injury as compared to cold cardioplegia with similar clinical events[43,44]. Given that all our patients received warm cardioplegia, this could have masked the benefit of RIPC.
Despite the fact that the results of this study suggest that there is no beneficial effect of RIPC on reducing POAF, RIPC still holds promise in improving clinical outcomes, based on “proof-of-concept” studies using cardiac biomarkers as primary endpoints[31,45,46] and, due to the fact it is a simple, safe, non-invasive, and inexpensive intervention. Although it has been challenging to identify which groups of patients benefit from RIPC, further evaluation of RIPC to decrease post-operative events with carefully planned and funded studies with adequate power is warranted. Additionally, meta-analysis of small randomized controlled studies may also be useful in studying the relationship of RIPC and clinical outcomes, including POAF.
Remote ischemic preconditioning (RIPC) has been demonstrated to reduce perioperative myocardial injury following cardiac surgery (coronary artery bypass, with or without valve surgery).
It is unknown whether it has an impact on clinical outcomes, such as post-operative atrial fibrillation, peri-operative myocardial infarction and stroke.
This is the first study in the United States evaluating these clinical outcomes following the use of RIPC with cardiac surgery.
Although this study did not suggest a clinically significant benefit with the use of RIPC, future meta-analyses of small randomized controlled studies may be useful in studying its relationship with clinical outcomes.
RIPC is a strategy in which brief episodes of non-lethal ischemia and reperfusion are applied to the arm or leg in order to achieve myocardial protection from ischemic events.
This is an interesting manuscript about the effect of (PIPC on clinical outcomes such as post-operative atrial fibrillation (POAF), myocardial infarction, stroke, and mortality in 102 patients undergoing cardiac surgery. The data demonstrated that PIPC did not reduce POAF. In addition, there were no significant differences in post-operative myocardial infarction, stroke, and mortality between RIPC group and control group. Therefore, the authors have suggested that further evaluations of RIPC are required to decrease post-operative events.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chu D, Firstenberg MS, Kettering K, Ueda H S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Lauer MS, Eagle KA, Buckley MJ, DeSanctis RW. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989;31:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Mitchell LB. Prophylactic therapy to prevent atrial arrhythmia after cardiac surgery. Curr Opin Cardiol. 2007;22:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Lim E, Barlow CW, Hosseinpour AR, Wisbey C, Wilson K, Pidgeon W, Charman S, Barlow JB, Wells FC. Influence of atrial fibrillation on outcome following mitral valve repair. Circulation. 2001;104:I59-I63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Tanawuttiwat T, O’Neill BP, Cohen MG, Chinthakanan O, Heldman AW, Martinez CA, Alfonso CE, Mitrani RD, Macon CJ, Carrillo RG. New-onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol. 2014;63:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Van Wagoner DR. Recent insights into the pathophysiology of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2007;19:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, Cantore C, Biglioli P, Sala A. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 491] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 8. | Kaireviciute D, Aidietis A, Lip GY. Atrial fibrillation following cardiac surgery: clinical features and preventative strategies. Eur Heart J. 2009;30:410-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Lotfi A, Wartak S, Sethi P, Garb J, Giugliano GR. Postoperative atrial fibrillation is not associated with an increase risk of stroke or the type and number of grafts: a single-center retrospective analysis. Clin Cardiol. 2011;34:787-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 872] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 11. | Rodrigo R, Cereceda M, Castillo R, Asenjo R, Zamorano J, Araya J, Castillo-Koch R, Espinoza J, Larraín E. Prevention of atrial fibrillation following cardiac surgery: basis for a novel therapeutic strategy based on non-hypoxic myocardial preconditioning. Pharmacol Ther. 2008;118:104-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, Browner WS. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Zaman AG, Archbold RA, Helft G, Paul EA, Curzen NP, Mills PG. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation. 2000;101:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Patel P, Dokainish H, Tsai P, Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 16. | D’Ascenzo F, Cavallero E, Moretti C, Omedè P, Sciuto F, Rahman IA, Bonser RS, Yunseok J, Wagner R, Freiberger T. Remote ischaemic preconditioning in coronary artery bypass surgery: a meta-analysis. Heart. 2012;98:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Xie JJ, Liao XL, Chen WG, Huang DD, Chang FJ, Chen W, Luo ZL, Wang ZP, Ou JS. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart. 2012;98:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5541] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 19. | Dow J, Bhandari A, Simkhovich BZ, Hale SL, Kloner RA. The effect of acute versus delayed remote ischemic preconditioning on reperfusion induced ventricular arrhythmias. J Cardiovasc Electrophysiol. 2012;23:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Slagsvold KH, Rognmo O, Høydal M, Wisløff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microRNA expression in atrial myocardium during coronary bypass surgery. Circ Res. 2014;114:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Krogstad LE, Slagsvold KH, Wahba A. Remote ischemic preconditioning and incidence of postoperative atrial fibrillation. Scand Cardiovasc J. 2015;49:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Rezkalla SH, Kloner RA. Preconditioning in humans. Heart Fail Rev. 2007;12:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 643] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 24. | Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Asberg S. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Jannati M, Kojuri J. Ischemic preconditioning and atrial fibrillation after coronary artery bypass grafting surgery. Iranian Cardiovascular Research Journal. 2008;2:4. |
| 26. | Karthik S, Grayson AD, Oo AY, Fabri BM. A survey of current myocardial protection practices during coronary artery bypass grafting. Ann R Coll Surg Engl. 2004;86:413-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 28. | Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 29. | Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T, Benzing T, Erdmann E, Burst V. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation. 2012;126:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Hausenloy DJ. Conditioning the heart to prevent myocardial reperfusion injury during PPCI. Eur Heart J Acute Cardiovasc Care. 2012;1:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 376] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 32. | Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;101:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Lavi S, D’Alfonso S, Diamantouros P, Camuglia A, Garg P, Teefy P, Jablonsky G, Sridhar K, Lavi R. Remote ischemic postconditioning during percutaneous coronary interventions: remote ischemic postconditioning-percutaneous coronary intervention randomized trial. Circ Cardiovasc Interv. 2014;7:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Zografos TA, Katritsis GD, Tsiafoutis I, Bourboulis N, Katsivas A, Katritsis DG. Effect of one-cycle remote ischemic preconditioning to reduce myocardial injury during percutaneous coronary intervention. Am J Cardiol. 2014;113:2013-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol. 2012;24:42-48. [PubMed] |
| 36. | Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, Bahk JH, Sim JY, Choi IC, Jeon Y. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J. 2014;35:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1276] [Cited by in RCA: 1119] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 38. | Wittes J, Brittain E. The role of internal pilot studies in increasing the efficiency of clinical trials. Stat Med. 1990;9:65-71; discussion 71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 293] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 39. | Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4056] [Cited by in RCA: 4256] [Article Influence: 354.7] [Reference Citation Analysis (0)] |
| 40. | Jensen RV, Zachara NE, Nielsen PH, Kimose HH, Kristiansen SB, Bøtker HE. Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc Res. 2013;97:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol - a clinical trial. Acta Anaesthesiol Scand. 2012;56:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 42. | Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;147:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 43. | Fan Y, Zhang AM, Xiao YB, Weng YG, Hetzer R. Warm versus cold cardioplegia for heart surgery: a meta-analysis. Eur J Cardiothorac Surg. 2010;37:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Fremes SE, Tamariz MG, Abramov D, Christakis GT, Sever JY, Sykora K, Goldman BS, Feindel CM, Lichtenstein SV. Late results of the Warm Heart Trial: the influence of nonfatal cardiac events on late survival. Circulation. 2000;102:III339-III345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 763] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 46. | Bell RM, White SK, Yellon DM. Remote ischaemic conditioning: building evidence of efficacy. Eur Heart J. 2014;35:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |